Involvement of transcription factor activator protein-2α in doxazosin-induced HeLa cell apoptosis1
Introduction
Doxazosin is a long-acting α-1-adrenoceptor antagonist structurally related to prazosin and terazosin. Its antihypertensive effect is produced by a reduction in the smooth muscle tone of peripheral vascular beds resulting in a decrease in total peripheral resistance without significant effect on cardiac output or heart rate. In most comparative trials, doxazosin has proven to be equally effective as the comparator drug in the treatment of mild to moderate hypertension[1]. Doxazosin has a beneficial effect on some of the risk factors associated with hypertension, including elevated serum lipid levels, impaired glucose metabolism, insulin resistance, and left ventricular hypertrophy. Modest decreases in total cholesterol, low density lipoprotein cholesterol, and triglycerides are seen with doxazosin therapy, while small increases in high density lipoprotein cholesterol and a high density lipoprotein cholesterol/total cholesterol ratio are consistently reported[2]. Furthermore, in benign prostatic hyperplasia, the effect of doxazosin in relieving bladder outflow obstruction is produced through a reduction in prostatic tone mediated via α-1-adrenoceptor blockade[3].
Recently, a large number of studies have demonstrated the potential antitumor action of doxazosin. A clinical research study reported that exposure to doxazosin significantly decreased the incidence of prostate cancer[4]. Doxazosin suppress prostate cancer cell growth by inducing apoptosis, and the apoptotic effect of doxazosin appears to be independent of the α-1-adrenoceptor block[5–8]. Keledjian et al demonstrated that doxazosin induced anoikis and inhibits prostate cancer cell invasion, an effect that was antagonized by Bcl-2[9]. Partin et al found that doxazosin mediated prostate cancer apoptosis by initially inducing the expression of transforming growth factor-β1 signaling effectors and subsequently IκBα[10]. Some reports showed that doxazosin increased the activities of caspase-3 and caspase-8 in prostate cancer cells, which suggests that doxazosin induces apoptosis of prostate cancer cells by activating caspase-3 and caspase-8. Some research results implicated Fas-mediated apoptosis as the underlying mechanism of the effect of doxazosin in prostate cells[11,12]. Shaw et al demonstrated that the ability of doxazosin to induce apoptosis in PC-3 prostate cancer cells was in part attributable to the inhibition of protein kinase B/Akt activation[13]. Arencibia et al proposed a novel mechanism of action for doxazosin in prostate cancer cells that implied DNA damage-mediated apoptosis by the downregulation of XRCC5 and PRKDC genes[14].
Furthermore, doxazosin inhibits pituitary tumor cell growth in vitro and in vivo by mechanisms that are in part independent of its α-1-adrenergic receptor-blocking actions and are involved in the downregulation of NF-κB signaling[15].
All of the research reports discussed earlier showed that doxazosin plays antitumor roles by inducing tumor cell apoptosis, especially in prostate cancer. However, it has not been mentioned whether doxazosin has the same pro-apoptotic roles in cervical cancer cells as in prostate cancer cells. Cervical cancer is the most common gynecological malignant disorder worldwide. There are approximately 500 million cases of genital warts per annum worldwide and approximately 450 000 cases of cervical cancer. It has been estimated that approximately 420 of the 1400 women diagnosed with cervical cancer will die within 5 years of the diagnosis[16], so it is crucial to find efficacious therapeutic drugs against cervical cancer.
Activator protein-2α (AP-2α) is a sequence-specific, DNA-binding transcription factor that is a member of the AP-2 family. AP-2 family proteins, consisting of the 5 homologous 52 kDa polypeptides of AP-2α, β, γ, δ, and ε, function in differentiation and development[17–19]. Much interest has recently been focused on findings that alterations in AP-2 activities are also related to the development of malignancies in humans, including breast cancer[20,21], ovarian carcinoma[22], lung carcinoma[23], gliomas[24], testicular carcinoma[25], and melanoma[26]. The present study was initiated to examine the effects of doxazosin on apoptosis in the cervical cancer cell line HeLa cells, then to explore the association of transcription factor AP-2α with doxazosin-induced apoptosis. We demonstrated that doxazosin induced apoptosis in HeLa cells, and transcription factor AP-2α play roles in doxazosin-induced apoptosis. We also showed that the caspase-3 pathway seemed to participate in doxazosin-induced apoptosis through AP-2α.
Materials and methods
Construction of vectors, synthesis of oligonucleotides, and drugs The expression vectors pCMV-Myc–AP-2α were constructed by ligating the full-length cDNA of AP-2α into the pCMV-Myc vector (Clontech, USA). To knock down the transcription of AP-2α, the phosphorothioate antisense oligonucleotide against AP-2α (ODNAP-2α) and the control sense oligonucleotide (COAP-2α) were synthesized according to published data[27]. The sequence of ODNAP-2α was CGTCAATTTCCAAAGCATTTTCATGGATCGG, and of COAP-2α was CAAAGTCTTGCATTATTCGGTCATAAT GGCC. Doxazosin mesylate was purchased from Sigma (St Louis, MO, USA).
Cell culture and transient transfections The HeLa cells were cultured in 10% fetal calf serum/Dulbecco’s modified Eagle’s medium (Gibco, USA) supplemented with penicillin and streptomycin in a humidified atmosphere at 37 ℃ and 5% CO2, and were transfected at 80% confluence using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
RNA extraction and relative quantitative real-time RT–PCR Total RNA was extracted from the HeLa cells using TRIzol reagent (Invitrogen, USA) according to the manufacturer’s instructions. For RT–PCR, 2 µg of total RNA was subjected to a reverse transcription step using Promega reagents (USA). Real-time PCR quantification was then performed using a SYBR green PCR master mix (Invitrogen, USA) with a 7900 HT fast real-time PCR system (AB, USA). For each sample, the mRNA levels of the target genes were corrected for the β-actin mRNA levels. The PCR primers were 5'-GCTGGGCACTGTAGGTCAATC-3' (AP-2α sense) and 5'-TTCAGGCTGTAGGGGTCGTT-3' (AP-2α antisense), 5'-GCTCTGGTTTTCGGTGGGT-3' (caspase-3 sense) and 5'-GAGTCCATTGATTCGCTTCCA-3' (caspase-3 antisense), and 5'-GGCGGCAACACCATGT-ACCCT-3' (β-actin sense) and 5'-AGGGGCCGGACTCGTCATACT-3' (β-actin antisense).
Western blot analysis The HeLa cells were lysed in RIPA buffer with protease inhibitors. The protein concentration was determined using the Bradford method with a Bio-Rad protein assay reagent. Fifty micrograms of proteins were subjected to electrophoresis on 12% SDS–PAGE and were then immunoblotted with an AP-2α (3B5) monoclonal antibody (Santa Cruz, Santa Cruz, CA, USA) and caspase-3 polyclonal antibodies (Santa Cruz, USA). Immunodetection was performed with an enhanced chemiluminescence (ECL) detection kit (GE Healthcare, UK).
DNA fragmentation assay DNA ladder formation was detected by gel electrophoresis. In brief, 5×107 HeLa cells were incubated in 0.5 mL TBE buffer containing 0.25% Nonidet and 0.1 mg/mL RNase A at 37 ℃ for 30 min and for an additional 30 min with proteinase K (1 mg/mL). DNA samples (equivalent to 1×106 cells) were electrophoresed at 100 V in 1.5% horizontal agarose gels and visualized under UV after staining with ethidium bromide (0.5 µg/mL).
Hoechst 33258 staining Apoptotic morphological changes in the nuclear chromatin of cells were detected by Hoechst 33258 staining. HeLa cells seeded on sterile cover glasses were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 10 min and then incubated with 50 µmol/L Hoechst 33258 staining solution for 10 min. After 3 washes with PBS, the cells were viewed under a fluorescence microscope (Zeiss Axioskop 2, Germany).
Flow cytometric assessment of apoptosis Apoptotic and total dead cells were determined by the Annexin V–fluorescein-isothiocyanate (FITC)/propidium iodide (PI) detection kit (Jingmei Biotech, China). In brief, the cultured HeLa cells (1×105 cells) were washed with 1× Annexin V binding buffer and stained with Annexin V (5 µL) and PI (10 µL) for 15 min at room temperature in the dark. After the addition of 400 µL binding buffer, the cells were analyzed by flow cytometry (Becton Dickinson FACSCalibur, USA).
Caspase-3 activity assay The activity of caspase-3 was determined using the caspase-3 activity kit (Beyotime Institute of Biotechnology, China). To evaluate the activity of caspase-3, cell lysates were prepared after their respective treatment with various designated treatments. Assays were performed on 96-well microtitre plates by incubating 10 µL protein of cell lysate per sample in 80 µL reaction buffer (1% NP-40, 20 mmol/L Tris-HCl [pH 7.5], 137 mmol/L NaCl, and 10% glycerol) containing 10 µL caspase-3 substrate (2 mmol/L Ac-DEVD-pNA). The lysates were incubated at 37 ℃ for 4 h. The samples were measured with an ELISA reader at an absorbance of 405 nm. The detailed analysis procedure was according to the manufacturer’s protocol. All the experiments were carried out in triplicate.
Statistic analysis Statistical analysis was performed using SPSS version 13.0 software (SPSS, Chicago, IL, USA). Data are presented as mean±SD. Differences were analyzed by ANOVA. Statistical significance differences were considered when P<0.05 or P<0.01.
Results
Doxazosin induces apoptosis in HeLa cells in a dose-dependent manner To explore the effects of doxazosin on HeLa cell apoptosis, we tested apoptosis by the DNA ladder formation assay, Hoechst 33258 staining, and Annexin V–FITC/PI methods. After the HeLa cells were exposed to various dose of doxazosin for 16 h, DNA laddering was observed, especially when exposed to 60 µmol/L doxazosin, indicating the occurrence of apoptosis at these concentrations (Figure 1A). Furthermore, as shown in Figure 1B, the cells treated with 10−60 µmol/L doxazosin for 16 h showed nuclear morphological changes typical of apoptosis with Hoechst 33258 staining, that is, nuclei fragmentation with condensed chromatin, while almost no apoptotic nuclei were observed in the untreated cells; the majority of cells had uniformly stained nuclei. Figure 1C shows that when exposed to 0~60 µmol/L doxazosin for 16 h, the proportion of apoptosis cells increased from 4.39% to 14.25%, and the total cell death increased from 8.41% to 21.2%, as determined by the flow cytometric analysis with Annexin V–FITC/PI double staining. These results of the 3 assays provide substantial evidence that doxazosin induces HeLa cell apoptosis in a dose-dependent manner.
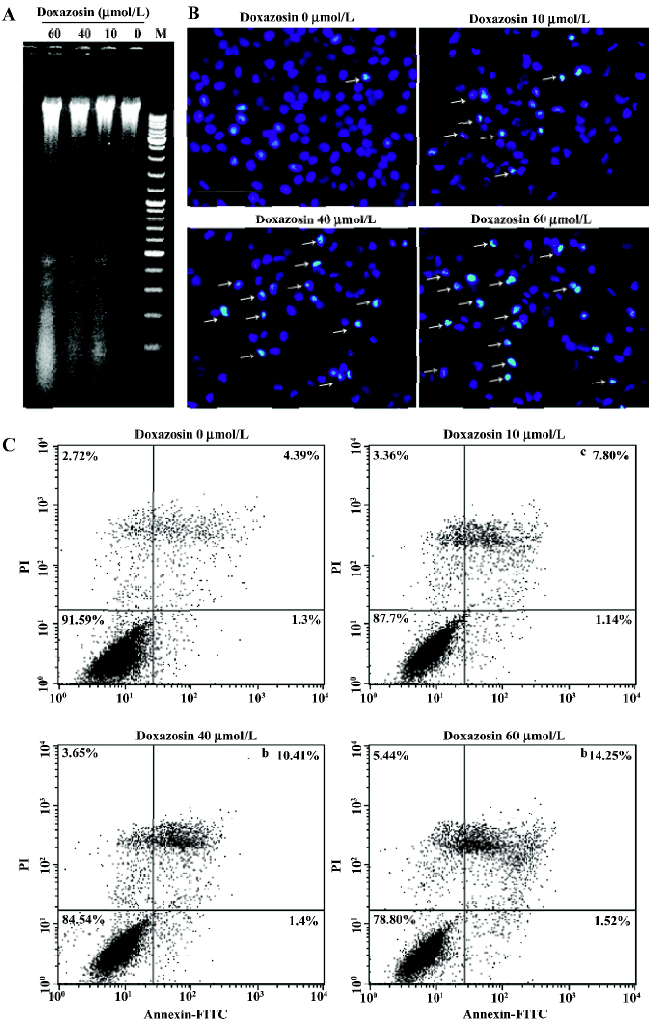
AP-2α participates in doxazosin-induced apoptosis in HeLa cells To detect whether the pro-apoptotic effects of doxazosin are associated with AP-2α, the transfections with AP-2α overexpression constructs and the AP-2α antisense oligonucleotide were performed respectively, then the apoptotic effects were assessed by flow cytometric analysis. As shown in Figure 2, the total cell death and apoptosis, especially late apoptosis, were markedly increased with the overexpression of AP-2α or knocking down of AP-2α when treated with 60 µmol/L doxazosin, compared with the absence of doxazosin (Figure 1C). Transfection with AP-2α resulted in apoptosis, which increased from 18.47% to 26.82%, and the total cell death increased from 62.05% to 71.24% with the presence of 60 µmol/L doxazosin, compared with transfection with the empty vectors,. However, transfection with the AP-2α antisense oligonucleotide resulted in apoptosis, which decreased from 19.70% to 13.70%, and the total cell death decreased from 29.59% to 23.29%, compared with the sense oligonucleotide. The above results suggest that AP-2α can induce apoptosis in HeLa cells, and the antisense AP-2α can partly block doxazosin-induced apoptosis.

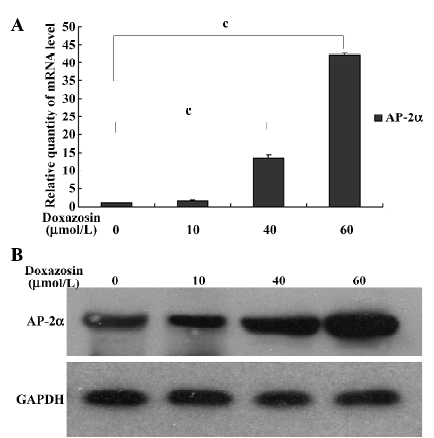
Doxazosin upregulates the expression of AP-2α in HeLa cells To further confirm that AP-2α is associated with doxazosin-induced HeLa cell apoptosis, we detected the effects of doxazosin on AP-2α expression. The AP-2α mRNA and protein levels were increased by doxazosin in a dose-dependent manner in the HeLa cells, as shown by relative quantitative real-time RT–PCR (Figure 3A) and Western blotting (Figure 3B), respectively. Together with the results of apoptosis assays, this suggests that the pro-apoptotic effect of doxazosin on HeLa cells is associated with AP-2α.
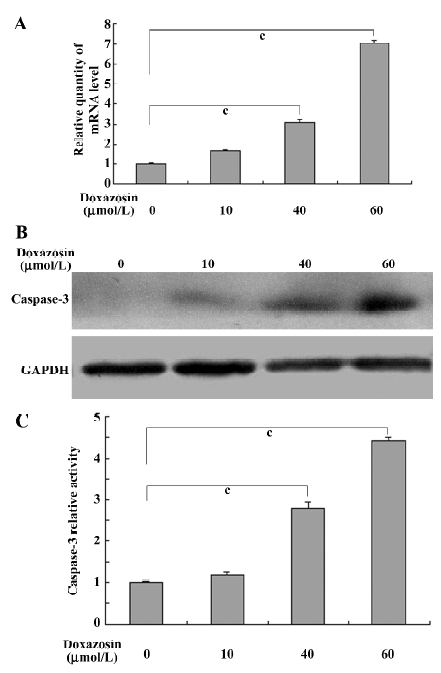
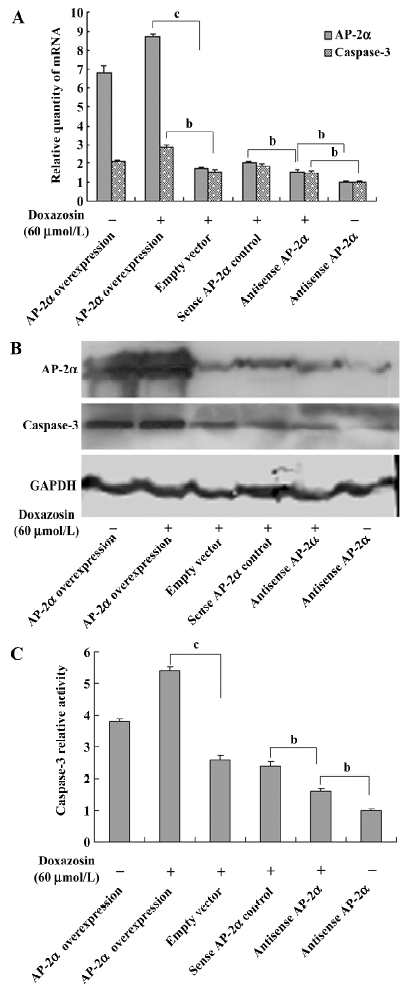
Doxazosin affects the caspase-3 pathway through AP-2 in HeLa cells A previous study reported that doxazosin induces apoptosis of prostate cancer cells by activating caspase-3[11], so we determined whether the caspase-3 pathway participates in doxazosin-induced apoptosis through AP-2α in HeLa cells. The expression of caspase-3 was detected. As shown in Figure 4A and 4B, the mRNA and protein levels of caspase-3 in the HeLa cells were increased in a dose-dependent manner when exposed to 0−60 µmol/L doxazosin, suggesting that doxazosin upregulates the expression of caspase-3. The effects of AP-2α on caspase-3 were detected with the presence of 60 µmol/L doxazosin, Figure 5A shows that the overexpression of AP-2α resulted in the concomitant increase of caspase-3 mRNA, whereas the antisense AP-2α in part abolished the increased effect of doxazosin on caspase-3 mRNA. The trend of protein levels was similar to that of the mRNA levels (Figure 5B). Because caspase-3 plays an important role during apoptosis, generally only in its active form, we further detected the effects of doxazosin and AP-2α on caspase-3 activity. The activity of caspase-3, analyzed by measuring the levels of p-nitroanilide cleaved from the substrate N-Ac-DEVD-pNA, was increased by doxazosin in a similar dose-dependent manner to that of the mRNA and Western blotting results (Figure 4C). As presented in Figure 5C, the HeLa cells transfected with AP-2α demonstrated significant increases in caspase-3 activity compared with that of the empty vector group; however, the antisense AP-2α decreased the caspase-3 activity compared with sense AP-2α. These results suggest that the caspase-3 pathway was associated with doxazosin-induced apoptosis through AP-2α.
Discussion
Despite the association of AP-2 with several human malignancies[20–26], very little is known about the status of AP-2 in cervical cancer cells. Here we investigated the effects of α-1-adrenoceptor antagonist doxazosin on AP-2α mRNA and protein levels in cervical cancer cells. We found that doxazosin upregulates the expression of AP-2α in a dose-dependent manner. We also found that doxazosin induces apoptosis in HeLa cells in a dose-dependent manner by a DNA fragmentation assay, flow cytometric assessment, and Hoechst 33258 staining. We found that the pro-apoptotic effects of doxazosin seemed to be associated with AP-2α. the overexpression of AP-2α resulted in increased apoptosis, whereas apoptosis was decreased when knocking down AP-2α. This is the first report to demonstrate that AP-2α participates in doxazosin-induced apoptosis in HeLa cells.
Studies have demonstrated AP-2α acts as a tumor suppressor by inducing apoptosis in melanomas and mammary carcinomas. Wajapeyee et al found that AP-2α inhibits the growth of cancer cells of different types, including H460 (lung carcinoma cell line), HT180 (fibrosarcoma cell line), HCT116 (colon cancer cell line), U2OS (osteosarcoma cell line), and SW480 (colon cancer cell line) by inducing cell cycle arrest and apoptosis[28]. They also found that blocking the endogenous AP-2α by small interfering RNA in human breast cancer cells leads to decreased apoptosis[29], and AP-2α induces apoptosis by downregulating Bcl-2 and utilizing a Bax/cytochrome c/Apaf1 (apoptosis protease activation factor-1)/caspase 9-dependent mitochondrial pathway[30]. These research reports provide evidence that supports our results.
Caspases, a family of cysteine acid proteases, are crucial mediators of apoptosis, and include caspases-1 to -14. Among them, caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins and is essential for normal brain development and in other apoptotic scenarios in a remarkable tissue-, cell type-, or death stimulus-specific manner. Caspase-3 is also required for some typical hallmarks of apoptosis and is indispensable for apoptotic chromatin condensation and DNA fragmentation in all cell types examined[31]. A previous study reported that doxazosin induces apoptosis of prostate cancer cells by activating caspase-3[11]. Our results implicated the caspase-3 pathway as the underlying mechanism of doxazosin-induced apoptosis through AP-2α in cervical cancer cells, as displayed in Figures 4 and 5.
The present results seem to implicate the potentially complicated interaction between AP-2α and doxazosin. When the HeLa cells were treated with 0−60 µmol/L doxazosin, 60 µmol/L doxazosin induced approximately a 3-fold increase in late apoptosis from 4.39% to 14.25% (Figure 1C), but when AP-2α was overexpressed, the treatment of the HeLa cells with 60 µmol/L doxazosin only resulted in a 1.3-fold increase in late apoptosis from 16.1% to 20.91% (Figure 2). These results showed that the overexpression of AP-2α partly inhibited doxazosin-induced apoptosis. Similar trends were observed in the expression and activity of caspase-3. When AP-2α was not overexpressed, the treatment of the HeLa cells with 60 µmol/L doxazosin induced significant increases in the expression and activity of caspase-3 (Figure 4); however, the overexpression of AP-2α with 60 µmol/L doxazosin induced increases in the expression and activity of caspase-3 that were non-significant (Figure 5). Here, we hypothesized a potential mechanism in which doxazosin induces apoptosis in HeLa cells through AP-2α. AP-2α is a transcription factor that regulates multiple genes; our study found that there was a complicated interaction between AP-2α and doxazosin. The appropriate concentrations of doxazosin upregulated AP-2α in a certain range and then induced apoptosis through the actions of the upregulated AP-2α on caspase-3. However, when AP-2α was overexpressed, the over-threshold of AP-2α would probably inhibit the doxazosin-induced apoptosis in a negative feedback mechanism. Other genes controlled by AP-2α are probably involved in this pathway; however, these need to be further explored in future studies.
Our results demonstrate that doxazosin induces apoptosis of cervical cancer cells, and suggest at least a partial role for AP-2α in doxazosin-induced cervical cancer cell apoptosis. These results provide new insights for the use of doxazosin in the therapy of cervical cancer, and that the use of AP-2α should be explored as a therapeutic target.
References
- Black HR. Doxazosin as combination therapy for patients with stage 1 and stage 2 hypertension. J Cardiovasc Pharmacol 2003;41:866-9.
- Pool JL. Effects of doxazosin on serum lipids: a review of the clinical data and molecular basis for altered lipid metabolism. Am Heart J 1991;121:251-9.
- Lowe F. α-1-Adrenoceptor blockade in the treatment of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis 1999;2:110-9.
- Harris AM, Warner BW, Wilson JM, Becker A, Rowland RG, Conner W, et al. Effect of α1-adrenoceptor antagonist exposure on prostate cancer incidence: an observational cohort study. J Urol 2007;178:2176-80.
- Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by α1-adrenoceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res 2000;60:4550-5.
- Kyprianou N, Chon J, Benning CM. Effects of α (1)-adrenoceptor (α(1)-AR) antagonists on cell proliferation and apoptosis in the prostate: therapeutic implications in prostatic disease. Prostate 2000;9 Suppl:42-6.
- Benning CM, Kyprianou N. Quinazoline-derived α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via an α1-adrenoceptor-independent action. Cancer Res 2002;62:597-602.
- Tahmatzopoulos A, Sheng S, Kyprianou N. Maspin sensitizes prostate cancer cells to doxazosin-induced apoptosis. Oncogene 2005;24:5375-83.
- Keledjian K, Kyprianou N. Anoikis induction by quinazoline-based α1-adrenoceptor antagonists in prostate cancer cells: antagonistic effect of bcl-2. J Urol 2003;169:1150-6.
- Partin JV, Anglin IE, Kyprianou N. Quinazoline-based α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-β signalling and IκBα induction. Br J Cancer 2003;88:1615-21.
- Walden PD, Globina Y, Nieder A. Induction of anoikis by doxazosin in prostate cancer cells is associated with activation of caspase-3 and a reduction of focal adhesion kinase. Urol Res 2004;32:261-5.
- Garrison JB, Kyprianou N. Doxazosin induces apoptosis of benign and malignant prostate cells via a death receptor-mediated pathway. Cancer Res 2006;66:464-72.
- Shaw YJ, Yang YT, Garrison JB, Kyprianou N, Chen CS. Pharmacological exploitation of the α1-adrenoreceptor antagonist doxazosin to develop a novel class of antitumor agents that block intracellular protein kinase B/Akt activation. J Med Chem 2004;47:4453-62.
- Arencibia JM, Del Rio M, Bonnin A, Lopes R, Lemoine NR, Lopez-Barahona M. Doxazosin induces apoptosis in LNCaP prostate cancer cell line through DNA binding and DNA-dependent protein kinase down-regulation. Int J Oncol 2005;27:1617-23.
- Fernando MA, Heaney AP. α1-Adrenergic receptor antagonists: novel therapy for pituitary adenomas. Mol Endocrinol 2005;19:3085-96.
- Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. Cmaj 2001;164:1017-25.
- Zhang J, Hagopian-Donaldson S, Serbedzija G, Elsemore J, Plehn-Dujowich D, McMahon AP, et al. Neural tube, skeletal and body wall defects in mice lacking transcription factor AP-2. Nature 1996;381:238-41.
- Holzschuh J, Barrallo-Gimeno A, Ettl AK, Durr K, Knapik EW, Driever W. Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development 2003;130:5741-54.
- Knight RD, Javidan Y, Zhang T, Nelson S, Schilling TF. AP2-dependent signals from the ectoderm regulate craniofacial development in the zebrafish embryo. Development 2005;132:3127-38.
- Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L, Carter D, et al. Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer Res 1998;58:5466-72.
- Pellikainen J, Naukkarinen A, Ropponen K, Rummukainen J, Kataja V, Kellokoski J, et al. Expression of HER2 and its association with AP-2 in breast cancer. Eur J Cancer 2004;40:1485-95.
- Odegaard E, Staff AC, Kaern J, Florenes VA, Kopolovic J, Trope CG, et al. The AP-2γ transcription factor is upregulated in advanced-stage ovarian carcinoma. Gynecol Oncol 2006;100:462-8.
- Khattar NH, Lele SM, Kaetzel CS. Down-regulation of the polymeric immunoglobulin receptor in non-small cell lung carcinoma: correlation with dysregulated expression of the transcription factors USF and AP2. J Biomed Sci 2005;12:65-77.
- Heimberger AB, McGary EC, Suki D, Ruiz M, Wang H, Fuller GN, et al. Loss of the AP-2α transcription factor is associated with the grade of human gliomas. Clin Cancer Res 2005;11:267-72.
- Hoei-Hansen CE, Nielsen JE, Almstrup K, Sonne SB, Graem N, Skakkebaek NE, et al. Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin Cancer Res 2004;10:8521-30.
- Tellez C, McCarty M, Ruiz M, Bar-Eli M. Loss of activator protein-2α results in overexpression of protease-activated receptor-1 and correlates with the malignant phenotype of human melanoma. J Biol Chem 2003; 278: 46 632–42.
- Fan WH, Karnovsky MJ. Increased MMP-2 expression in connective tissue growth factor over-expression vascular smooth muscle cells. J Biol Chem 2002;277:9800-5.
- Wajapeyee N, Somasundaram K. Cell cycle arrest and apoptosis induction by activator protein 2α (AP-2α) and the role of p53 and p21WAF1/CIP1 in AP-2α-mediated growth inhibition. J Biol Chem 2003; 278: 52 093–101.
- Wajapeyee N, Raut CG, Somasundaram K. Activator protein 2α status determines the chemosensitivity of cancer cells: implications in cancer chemotherapy. Cancer Res 2005;65:8628-34.
- Wajapeyee N, Britto R, Ravishankar HM, Somasundaram K. Apoptosis induction by activator protein 2α involves transcriptional repression of Bcl-2. J Biol Chem 2006; 281: 16 207–19.
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ 1999;6:99-104.
