Acetamide-45 inhibited hyperresponsiveness and airway inflammation in mice partly depending on phosphodiesterase activity suppression
Introduction
Asthma is recognized as a common disease of the airway characterized by local and systemic allergic inflammation and reversible airway obstruction[1]. The hallmarks of allergic asthma include airway hyperresponsiveness (AHR), chronic pulmonary inflammation with infiltration of eosinophils into the bronchial wall and lumen, and mucus hypersecretion in the airways[2,3,4]. Eosinophils are generally considered putative effector cells in asthma through the release of their granule-stored cationic proteins, which are consistently increased in number during an asthmatic episode[5]. The predominance of Th2 cells and associated Th2-type cytokines play critical roles in the inflammation in asthma. Previous studies have shown the presence of CD4+ T cells producing IL-4, IL-5, and IL-13 in bronchoalveolar lavage fluid and in airway epithelial biopsies of asthmatics. IL-4 and IL-13 have been implicated in multiple pathologies of asthma, including airway eosinophilia, goblet cell hyperplasia, the development of AHR, and lung remodeling[6,7].
Phosphodiesterases (PDE) are a group of enzymes that hydrolyze cAMP and cGMP and are second messengers mediating the physiologic responses to their respective inactive 5′ mononucleotides. At least 11 families of PDE are known to exist based on a variety of criteria including substrate specificity, inhibitor potency, enzyme kinetics and amino acid sequences[8]. cAMP-PDE such as the PDE4 isozyme plays a particularly important role in inflammatory and immunomodulatory cells and is the predominant PDE in inflammatory cells including mast cells, eosinophils, neutrophils, T cells, macrophages and structural cells, such as sensory nerves and epithelial cells[9]. Inhibition of cAMP-PDE activity allows cAMP to increase in cells, which results in a variety of anti-inflammatory effects. Animal studies have shown that selective PDE4 inhibitors possess inhibitory effects on eosinophil recruitment, cytokine and chemokine release, and AHR[10]. Therefore, inhibition of cAMP-PDE has been important as a significant pharmacological target in respiratory disease. The second generation PDE4 inhibitors, cilomilast and roflumilast, have reached clinical trial stage and have some demonstrable beneficial effects in asthma[11].
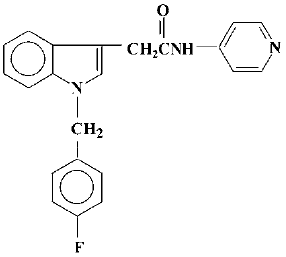
A series of N-(pyridin-4-yl)-(indol-3-yl) alkylamides (44-84) have been prepared in the search for novel anti-allergic drugs among which acetamide-45 was considered to be a potential agent (Figure 1). Initial studies showed that acetamide-45 inhibited IL-4 and IL-5 biosynthesis and histamine release[12]. We have previously reported that acetamide-45 has an inhibitory effect on histamine- and methacholine-induced contractions of isolated guinea pig trachea, and that it can inhibit PDE4 activity[13,14]. Acetamide-45 also displayed anti-inflammatory effects in an allergic model of rat[15]. However, little is known about its effects on eosinophil infiltration in airway and lung function in asthmatic mice. There have been no reports regarding the effect of acetamide-45 on AHR in any animal model of asthma.
In the present study, we used a mouse model of chronic asthma, which replicates many features of the human disease, to investigate inhibition of the airway lesions by acetamide-45 compared with glucocorticoids. The purpose of the present study is to determine whether acetamide-45 could suppress allergic-induced AHR and airway inflammation in mice. The study was conducted on mice sensitized and challenged with ovalbumin (Ova) and the whole body plethysmography was carried out to assess the AHR. The bronchoalveolar lavage (BAL) histopathology were examined. It was found that acetamide-45 significantly inhibited the enhanced AHR and eosinophil recruitment in airways with elimination of cAMP-PDE activity in lung tissue. High IL-4 and IL-5 in BALF in asthmatic mice were markedly decreased. Our results indicate that the agents have a potential role in inflammatory disease.
Materials and methods
Mice 8–10-week-old male Balb/c mice weighing 20–25 g were purchased from the Laboratory Animal Center of the Medical School of Zhejiang University (certificate no. 220010401, conferred by the Zhejiang Medical Laboratory Animal Administration Committee).
Asthma model and drug administration Thirty-six mice were divided into 6 groups. Ova group: mice were sensitized at d 0 and d 14 by ip injection of ovalbumin (Ova) mixed with aluminum. At d 24, 25, and 26, mice were challenged by aerosolized 1% Ova in saline for 40 min. Ace3 group: mice were sensitized and challenged by Ova in the same way as the Ova group with acetamide-45 at 3 mg/kg 1 h before Ova challenge. Ace10 group: mice were sensitized and challenged by Ova in the same way as the Ova group with acetamide-45 at 10 mg/kg 1 h before challenge. Ace30 group: mice were sensitized and challenged by Ova in the same way as the Ova group with acetamide-45 at 30 mg/kg 1 h before challenge. Dxm group: mice were sensitized and challenged by Ova in the same way as the Ova group with dexamethasone (Jiangsu, China) at 0.5 mg/kg 1 h before challenge. Saline group: mice were sensitized at d 0 and d 14 by ip injection of saline. At d 24, 25, and 26, mice were challenged by aerosolized saline for 40 min. Acetamide-45 was kindly provided by the Department of Organic Chemistry and Medical Chemistry, Faculty of Pharmacy (Nantes University, France).
BAL of the lungs At d 28, BAL was carried out using 1 mL of phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS). Total BAL cellularity was determined with the use of a hemocytometer. Cytospin slides were fixed and stained by Wright-Giemsa staining. Differential cell counts were based on counts of 200–300 cells using standard morphological criteria to classify individual leukocyte populations.
Pulmonary histology After BAL was carried out, the lungs were inflated with 1 mL of 10% neutral-buffered formalin via a tracheostomy tube. After instillation of a fixative, the trachea was ligated, and the lung was excised and fixed in formalin for 24 h at 4 °C. These tissues were then embedded in paraffin, cut in 4 μm frontal sections, mounted onto slides, and stained with hematoxylin and eosin (H&E). The severity of inflammatory cell infiltration in lung was evaluated by a 5 point scoring system[16]. Briefly, the scoring system was: 0, no cells; 1, a few cells; 2, a ring of cells one cell layer deep; 3, a ring of cells 2–4 cells deep; 4, a ring of cells >4 cells deep. Scoring of inflammatory cells was carried out in at least 3 different fields for each lung section.
Airway hyperresponsiveness in response to methacholine Airway responsiveness to methacholine (MeCh) was assessed by whole body plethysmography (Buxco Electronics, Troy, NY, USA). Mice were exposed for 3 min to aerosolized saline and subsequently to increasing concentrations of aerosolized MeCh (0, 3.125, 6.25, 12.5, 25, 50, and 100 mg/mL in isotonic saline) dissolved in isotonic saline. Following each nebulization, enhanced pause (Penh) were recorded for 3 min. The Penh values measured during each 3 min sequence were averaged and expressed for each dose of MeCh. Penh data were plotted as the change from baseline per dose.
Cytokine assay IL-4, IL-5 and IFN-γ levels in BAL were determined using the mouse IL-4, IFN-γ enzyme-linked immunosorbent assay (ELISA) kits (Jingmei BioTech, Shenzhen, China), as per the manufacturer’s instructions. The sensitivity for each cytokine was 2.0 pg/mL for IL-4, IL-5 and IFN-γ.
PDE4 activity assay Frozen 25 mg pieces of lung tissue were homogenized and cAMP-PDE activity was determined by high performance liquid chromatography (HPLC) as described previously[16].
Statistical analysis Data are expressed as mean±SEM. GraphPad Prism (GraphPad Software Inc, San Diego, CA, USA) was used for statistical analysis. Differences between groups were tested using ANOVA or the Mann-Whitney test. P-values of less than 0.05 were considered significant.
Results
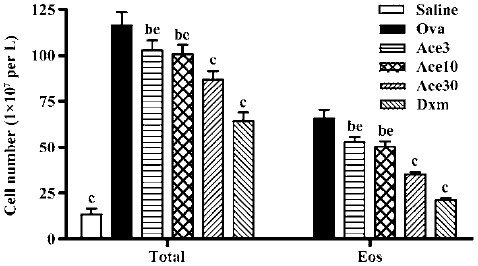
Acetamide-45 decreased total leukocytes and eosinophils in the BALF of allergic mice To determine whether acetamide-45 influences the inflammatory cells accumulation in airways responding to allergen challenge, BALF was recovered. Total cells of BALF were counted and differentiated using morphological criteria. As shown in Figure 2, the total number of leukocytes and eosinophils in the Ova group was significantly greater than that in the Saline group (116±7.0 vs 13.5±3.0, P<0.01; 66±5.0 vs 0.0±0.0, P<0.01). Acetamide-45 displayed inhibitory potency on inflammatory cells in a dose-dependent manner. The mice treated with actamide-45 at 3 mg/kg showed a deceased total number of leukocytes in BALF, compared with allergic mice (103±5.3 vs 116±7.0, P<0.05). The decrease of heightened total number of leukocytes in mice treated with actamide-45 at 30 mg/kg was greater than that in mice treated with actamide-45 at 3 mg/kg and 10 mg/kg (103±5.3 vs 87±4.8, P<0.05; 100±5.0 vs 87±4.8, P<0.05). The increase of eosinophils was remarkably reduced by acetamide-45, which accounted for the elevation of the total number of leukocytes. Dxm exhibited significant suppressive effects on the recruitment of inflammatory cells. No significant difference in neutrophil and lymphocyte was observed between the 6 groups.
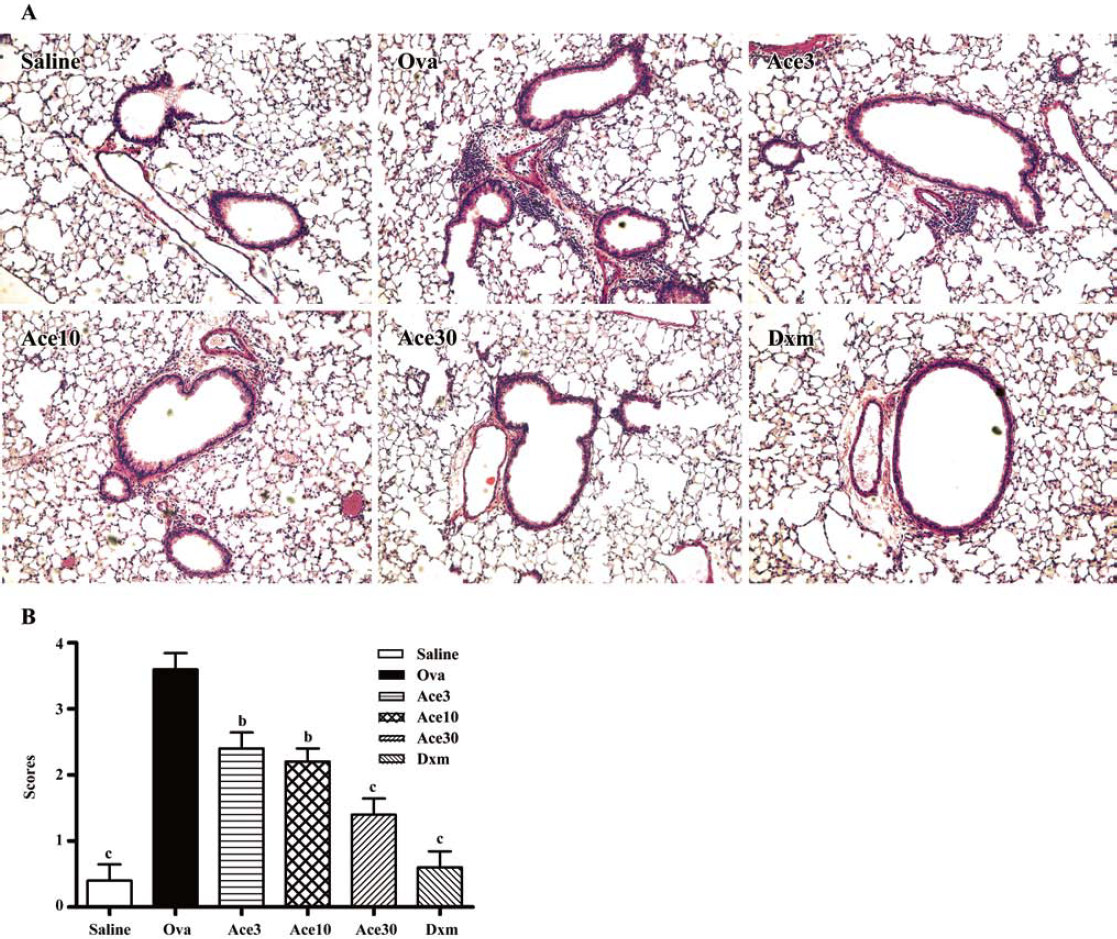
Acetamide-45 reduced Ova-induced inflammatory infiltration in lung tissue of mice To examine inhibition of inflammation in lung tissue by acetamide-45, we observed the pulmonary pathology stained with H&E in the mice of all groups (Figure 3a). Compared with mice treated with saline, the mice exposure to Ova exhibited the development of a characteristic peribronchial and perivascular airway tissue infiltration. In comparison with mice in the Ova group, the mice given acetamide-45 at various doses exhibited obviously reduced inflammation in the lung tissue (Figure 3b). Ova-induced inflammatory infiltration was decreased in the mice given Dxm.
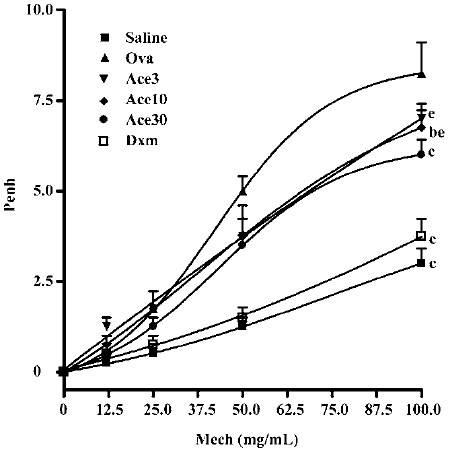
Acetamide-45 decreased Ova-induced AHR in response to methocholine To determine whether acetamide-45 improves pulmonary function of allergic mice after challenge with Ova, whole body plethysmography was carried out to examine the AHR in response to MeCh (Figure 4). Compared with control animals in the saline group, mice in the Ova group developed higher Penh in response to MeCh at the dose of 50 and 100 mg/mL (5.0±0.41 vs 1.25±0.25, P<0.01; 8.3±0.85 vs 3.0±0.41, P<0.01). Administration of acetamide-45 resulted in significant decrease of enhanced AHR in allergic mice at a dose-dependent manner. Compared with mice in the Ova group, the mice in the Ace10 group exhibited a reduction of enhanced AHR responding to MeCh at 100 mg/mL (6.75±0.48 vs 8.3±0.85, P<0.05), and the mice in the Ace30 group showed a decrease of enhanced AHR responding to MeCh at 50 and 100 mg/mL (3.5±0.29 vs 5.0±0.41, P<0.01; 6.0±0.41 vs 8.3±0.85, P<0.01). The decrease of enhanced AHR in the Ace30 group is greater than that in the Ace3 or Ace10 groups (P<0.05) in response to MeCh. Administration of Dxm also significantly prevented the enhancement of AHR in response to MeCh at 50 and 100 mg/mL.
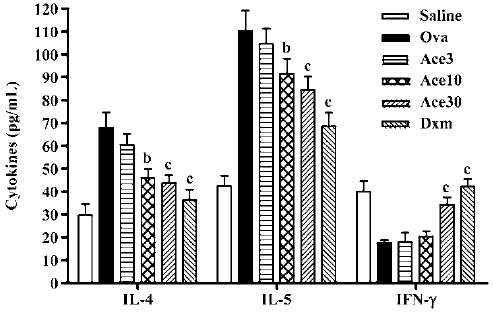
Acetamide-45 decreased Ova-induced enhancement of IL-4 and IL-5 levels in BALF of mice To evaluate the effect of acetamide-45 on the pulmonary immune response in allergic mice, the concentrations of IL-4, IL-5 and IFN-γ in BALF were measured (Figure 5). The IL-4 and IL-5 levels in the Ova group were significantly increased in comparison with the Saline group (67.8±7.01 vs 29.8±4.71, P<0.01; 110±8.9 vs 42.5±4.52, P<0.01). Though there was no change of elevated levels of IL-4 and IL-5 in mice of Ace3, the increases of both cytokines were prevented in mice of Ace10 group. A greater inhibitory effect on increased IL-4 and IL-5 levels was shown in mice of the Ace30 group. Downregulation of elevations of those two cytokines was markedly observed in mice of the Dxm group. The IFN-γ level in the Ova group was less than that in the Saline group; however, acetamide-45 did not affect the IFN-γ level at 3 mg/kg and 10 mg/kg. Administration of Dxm and acetamide-45 at 30 mg/kg increased the reduced IFN-γ level in allergic mice.
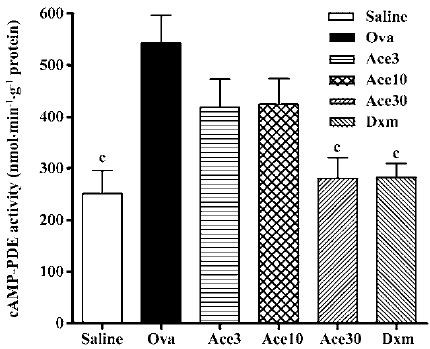
Acetamide-45 inhibited cAMP-PDE activity in lung tissue of allergic mice The effect of acetamide-45 on cAMP-PDE activity in the lung of allergic mice was examined 48 h after the last Ova challenge. As shown in Figure 6, the cAMP-PDE activity of lung in the Ova group was enhanced from 252±44 nmol/g per min to 542±54 nmol/g per min, compared with that in the Saline group (P<0.01). Acetamide-45 did not affect Ova-induced cAMP-PDE activity at 3 mg/kg and 10 mg/kg. However, increased cAMP-PDE activity was almost eliminated by acetamide-45 at 30 mg/kg (281±40 vs 542±54, P<0.01). Dxm at 0.5 mg/kg also significantly suppressed the increased PDE4 activity (282±27 vs 542±54, P<0.01), the inhibitory effect of which was close to acetamide-45 at 30 mg/kg.
Discussion
In the present study, a mice model of asthma was used to assess the effect of acetamide-45 on airway inflammatory disease. We found that acetamide-45 significantly decreased the total number of leukocytes and eosinophil in the BALF of allergic mice. Pathology analysis documented that administration of acetamide-45 resulted in the reduction of allergic inflammatory infiltration in the lung tissues. The Ova-induced increases of IL-4 and IL-5 in BALF were prevented by the compound. We also showed that acetamide-45 exhibited marked suppression on PDE activity in the lungs of allergic mice, which suggests that inhibition of PDE may be responsible for those effects. Collectively, all of these results demonstrate that acetamide-45 is a potent anti-inflammatory agent in pulmonary disease.
In our previous study, we reported that acetamide-45 could suppress the cAMP-PDE activity in a transfected yeast[14]. We also observed that Ova-induced cAMP-PDE activity in the lungs of rats was decreased by acetamide-45[15]. The cAMP-PDE activity in lung tissue was examined in the present study and we found an obvious increase of cAMP-PDE activity in allergic mice. Increased PDE4 activity in allergic mice was almost eliminated by acetamide-45 at 30 mg/kg. Therefore, we supposed that inhibition of cAMP-PDE activity by acetamide-45 in inflammatory and immune cells led to an increase of cAMP contents in these cells, which subsequently influenced the activity of inflammatory and immune cells.
Eosinophilia is a prominent pathological feature of allergic diseases, which is thought to contribute to the airways damage through release of myriad mediators and cytokines[5]. The infiltration of eosinophils into the airways has been linked to the production of IL-5, which is important for eosinophils proliferation, activation, and migration[17]. In the present study, we showed that acetamide-45 inhibited the pulmonary accumulation of eosinophils in mice, which is parallel to the decease of IL-5 in BALF. These results are in agreement with the existing studies that documented suppression of antigen-induced pulmonary eosinophils infiltration and IL-5 production with inhibition of cAMP-PDE in an animal model of asthma. Administration of rolipram, a selective PDE4 inhibitor, significantly prevented eosinophils accumulation in the BALF in a dose-dependent manner in mice[18]. Rolipram also inhibits IL-5 production induced by antigen in an antigen-driven system of splenocytes from Ova-sensitized mice[19]. YM976, another selective inhibitor of PDE4 reduced antigen-induced eosinophils infiltration into the lungs and the amount of IL-5 in the BALF[20]. Our results suggest that acetamide-45 plays a key role in decreasing the infiltration of eosinophils in lung related to the IL-5 pathway.
The episode of allergic asthma is accompanied by a complex series of concurrent inflammatory responses in the lung orchestrated by CD4+ Th2 lymphocytes, in which Th2 cytokines such as IL-4, IL-5, and IL-13 are essential. The effects of dexamethasone are mediated largely via changes in gene transcription. Dexamethasone has been shown to inhibit the transcription of many lymphocyte-derived cytokines, including IL-4, IL-5, and IL-8[21]. The effects of selective PDE4 inhibitors on T cell functions have been extensively studied in an animal model of asthma since the influence of PDE4 to degrade cAMP is predominant in these inflammatory cells. However, the preferential inhibition on Th1 or Th2 response in allergy by PDE4 inhibitors remains controversial. Rolipram appeared to selectively inhibit the proliferation and cytokine generation of Th1 cells in human autoimmune conditions characterized by a Th1 phenotype[22,23]. In contrast, there is some evidence for a preferential inhibition of cytokines in Th2-mediated response. Dexamethasone and rolipram strongly inhibiting the production of IL-5, were less potent against IL-2 and IFN-γ and showed a relatively weak effect against granulocyte-macrophage colony-stimulating factor (GM-CSF), in human peripheral blood lymphocytes stimulated with phytohemagglutinin[24]. IL-13 production in phytohemagglutinin-stimulated human T cells was significantly decreased by PDE4 inhibitor[25]. The preference of PDE4 inhibitors for inhibition of Th1 or Th2 cytokines may depend on the disease model, yet the bases for this observation remain unsettled. In the present study, we showed that acetamide-45 suppressed the allergen-induced elevation of IL-4, and IL-5, but no change of IFN-γ level was observed. This indicated that acetamide-45 inhibited Th2 cells preferentially in allergic diseases.
AHR is defined as the abnormal increase in airflow limitation in response to a provoking stimulus. The exact mechanism that PDE4 inhibitors reduce allergen-induced AHR and inflammation remains unknown. Many studies have shown that PDE4 inhibitors suppress AHR with a decrease of eosinophil infiltration in the lung. Administration of rolipram, a selective PDE4 inhibitor, significantly inhibited AHR and eosinophil accumulation in the BALF in a dose-dependent manner[21]. Treatment with roflumilast or dexamethasone significantly reduced AHR and accumulation of eosinophils and chronic inflammatory cells[26]. We also demonstrated that acetamide-45 attenuated airway resistance with reduced eosinophils infil-tration in an allergic rat model. In addition to eosinophils inflammation, it was reported that predisposing to Th2 also contributed to AHR[2]. Our previous study showed that acetamide-45 decreased on histamine- and methacholine-induced contractions of isolated guinea pig trachea. In general it was suggested that acetamide-45 preventing enhanced AHR in allergic mice was associated with inhibitions of eosinophils recruitment, downregulation of Th2 cytokines production, and decrease of tension of smooth muscle in airways.
In summary, our studies identify the potential of acetamide-45 in allergic inflammation and airway hyperresponsiveness, which in part resulted from inhibition of cAMP-PDE.
Acknowledgement
We thank Prof Guillaume Le BAUT for kindly supplying acetamide-45.
References
- Busse WW, Lemanske RF. Asthma. N Engl J Med 2001;344:350-62.
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 1999;17:255-81.
- Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Invest 1999;104:985-93.
- Adamko D, Odemuyiwa SO, Moqbel R. The eosinophil as a therapeutic target in asthma: beginning of the end, or end of the beginning? Curr Opin Pharmacol 2003;3:227-32.
- Logan MR, Odemuyiwa SO, Moqbel R. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J Allergy Clin Immunol 2003;111:923-32.
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 2003;111:450-63.
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258-61.
- Sanz MJ, Cortijo J, Morcillo EJ. PDE4 inhibitors as new anti-inflammatory drugs: effects on cell trafficking and cell adhesion molecules expression. Pharmacol Ther 2005;106:269-97.
- Torphy TJ. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am J Respir Crit Care Med 1998;157:351-70.
- Fan Chung K. Phosphodiesterase inhibitors in airways disease. Eur J Pharmacol 2006;533:110-7.
- Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet 2005;365:167-75.
- Menciu C, Duflos M, Fouchard F, Le Baut G, Emig P, Achterrath U, et al. New N-(pyridin-4-yl)-(indol-3-yl) acetamides and propanamides as antiallergic agents. J Med Chem 1999;42:638-48.
- Lu YB, Chen Z, Wu M. Acetamide-45 inhibits histamine- and methacholine-induced contraction of isolated guinea pig trachea. Acta Pharmacol Sin 2002;23:152-6.
- Wang K, Chen JQ, Chen Z, Chen JC. Inhibition of human phosphodiesterase 4A expressed in yeast cell GL62 by theophylline, rolipram, and acetamide-45. Acta Pharmacol Sin 2002;23:1013-7.
- Wang K, Shen HH, Chen Z, Chen JC. Inhibitory effect of acetamide-45 on airway inflammation and phosphodiesterase 4 in allergic rats. Acta Pharmacol Sin 2005;26:1492-96.
- Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Antiinflammatory effects of mitogen-activated protein kinase inhibitor U0126 in an asthma mouse model. J Immunol 2004;172:7053-9.
- Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med 1996;183:195-201.
- Kanehiro A, Ikemura T, Makela MJ, Lahn M, Joetham A, Dakhama A, et al. Inhibition of phosphodiesterase 4 attenuates airway hyperresponsiveness and airway inflammation in a model of secondary allergen challenge. Am J Respir Crit Care Med 2001;163:173-84.
- Foissier L, Lonchampt M, Coge F, Canet E. In vitro down-regulation of antigen-induced IL-5 gene expression and protein production by cAMP-specific phosphodiesterase type 4 inhibitor. J Pharmacol Exp Ther 1996;278:1484-90.
- Aoki M, Fukunaga M, Kitagawa M, Hayashi K, Morokata T, Ishikawa G, et al. Effect of a novel anti-inflammatory compound, YM976, on antigen-induced eosinophil infiltration into the lungs in rats, mice, and ferrets. J Pharmacol Exp Ther 2000;295:1149-55.
- Barnes PJ, Adcock IM. NF-κB: a pivotal role in asthma and a new target for therapy. Trends Pharmacol Sci 1997;18:46-51.
- Bielekova B, Lincoln A, McFarland H, Martin R. Therapeutic potential of phosphodiesterase-4 and -3 inhibitors in Th1-mediated autoimmune diseases. J Immunol 2000;164:1117-24.
- Dinter H, Tse J, Halks-Miller M, Asarnow D, Onuffer J, Faulds D, et al. The type IV phosphodiesterase specific inhibitor mesopram inhibits experimental autoimmune encephalomyelitis in rodents. J Neuroimmunol 2000;108:136-46.
- Van Wauwe J, Aerts F, Walter H, de Boer M. Cytokine production by phytohemagglutinin-stimulated human blood cells: effects of corticosteroids, T cell immunosuppressants and phosphodiesterase IV inhibitors. Inflamm Res 1995;44:400-5.
- Kanda N, Watanabe S. Regulatory roles of adenylate cyclase and cyclic nucleotide phosphodiesterases 1 and 4 in interleukin-13 production by activated human T cells. Biochem Pharmacol 2001;2:495-507.
- Kumar RK, Herbert C, Thomas PS, Wollin L, Beume R, Yang M, et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. J Pharmacol Exp Ther 2003;307:349-55.