Identification of higenamine in Radix Aconiti Lateralis Preparata as a beta2-adrenergic receptor agonist1
Introduction
Beta2-adrenergic receptor (β2-AR) agonists have been used for the relief and prophylaxis of asthma symptoms for many years[1]. The β2-ARs are mainly located in the alveolar walls[2] and mediate the relaxation of human airway smooth muscle in vivo and in vitro[3]. β2-AR agonists activate adenylate cyclase, which increases the concentration of intracellular cAMP. It activates the cAMP-dependent protein kinase that phosphorylates myosin light chain kinase, leading to the relaxation of airway smooth muscle[4].
For a long time, the research of β2-AR agonists was focused on chemosynthetic drugs. However, traditional Chinese medicine (TCM) has provided a lot of valuable knowledge about the treatment of asthma for thousands of years. Its long history of use, traditions, faith, popularity, and anecdotes are widely taken as evidence of its efficacy[5]. Some effective traditional therapies have been studied to a certain extent by means of modern science. In modern western medicine, active compounds were isolated from plants and drugs were prepared in salt form. In China, several drugs isolated from plants have been prepared in salt form in recent years. However, herbal medications, developed in the ancient tradition, continue to be widely used in Chinese populations[6]. Nowadays the mechanism-centered approach to TCM is primarily concerned with the search for the molecular, cellular, and pharmacological bases of traditional medicines. It seeks to identify the active substances of herbal treatments and investigate their mechanism of action. TCM extracts prepared from medicinal plants and other natural sources contain a variety of molecules with potent biological activities. However, it is often difficult to analyze the biological activities of these extracts because of their complex nature and the possible synergistic effects of different components.
Radix Aconiti Lateralis Preparata (RALP), which is prepared with the lateral roots of Aconitum carmichaeli Debx, is prescribed fairly often in TCM formulas. This herb was recorded in the earliest extant TCM book “Shennong’s Chinese Material Medicine”. RALP exhibits numerous and distinct pharmacological activities, including cardiotonic, anti-inflammatory, anticancer, sedative, and analgesic effects. Its chemistry is composed mainly of alkaloids and hypaconitine. Hypaconitine, aconitine, mesacontine, talatisamine, and karakoline have been isolated from RALP[7]. Among them, hypaconitine, aconitine, and mesaconitine are toxic alkaloids that may cause arrhythmia or cardiac depression[8]. In the hydrosoluble component part of RALP, Kosuge et al 1976 found traces of dl-higenamine, which had cardiotonic effects[9]. Coryneine chloride[10] and salsolinol were also isolated from RALP and proved to be cardiotonic agents.
In TCM, RALP is often mixed with other herbs for the treatment of asthma, but the molecular mechanism is unknown. For hunting the mystery, a sensitive β2-AR agonist assay for assessing intracellular cAMP changes was established through stimulated cAMP response element (CRE)-regulated reporter gene activity[11,12], and the key compound from the RALP was isolated for ligand affinity and efficacy determination, and its potential molecular mechanism for curing asthma was partly clarified in this paper.
Materials and methods
Materials The CHOCRE-EGFP cells (CHO cells with CRE-enhanced green fluorescent protein reporter gene) were constructed by our laboratory[13]. The enzymes used for DNA manipulations were purchased from Takara Biotechnology Co Ltd (Dalian, China). Escherichia coli DH5α and pcDNA3.1 were purchased from Invitrogen Biotechnology Co Ltd (New York, NY, USA). Salbutamol, isoprenaline, BRL37344, and dobutamine were obtained from Sigma (St Louis, MO, USA). Anti-β2-AR specific antibody was purchased from Boster Biological Technology Ltd (Wuhan, China). Goat anti-rabbit IgG-TRITC was purchased from Jackson ImmunoResearch Laboratories (Jackson, West Grove, PA, USA). Cell culture media and reagents were obtained from GibcoBRL Life Technologies (New York, NY, USA). Standard higenamine was purchased from the Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). HP20 was supplied by the Chemical Plant of Nankai University (Tianjin, China). Other reagents used in this study were of analytical grade and commercially available.
Cloning of the β2-AR gene Rat genomic DNA was obtained from rat venous blood using the SiMax Genomic DNA Extraction kit from SBS Genetech Co Ltd (Beijing, China). The β2-AR gene was amplified by polymerase chain reaction (PCR using a sense primer, 5′-ATAAAGCTTATGGAGCCACACGGGAATGACAGC-3′, and an anti-sense primer, 5′-GCGGAATTCCTACAGTGGCGAGTCGTTTGTGTT-3′. The amplification was carried out for 30 cycles as follows: 94 °C for 50 s, 55 °C for 50 s, and 72 °C for 1.5 min. The PCR product was digested with Hind III and EcoR I, and the resulting fragment was gel-purified and then ligated into pcDNA3.1, which had been digested with the same restriction endonucleases. The β2-AR/pcDNA3.1 construct was sequenced.
Cell culture and transfection CHO-EGFP cells were maintained in RPMI-1640 medium (GibcoBRL) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin, incubated at 37 °C in 5% CO2, and grown to 80% confluency before transfection. Transfection was carried out using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. β2-AR/pcDNA3.1 was transfected into CHO-EGFP cells, and stable clones were selected in culture medium containing 200 mg/L Zeocin. Single-cell-derived clones were isolated using a limiting dilution approach in 96-well plates. Cells were subsequently tested by ligand binding. One sensitive and stable cell line was obtained and designated as CHOβ2-AR-CRE-EGFP. The control cell line transfected with pcDNA3.1 was designated as CHOCRE-EGFP.
Immunocytochemistry of β2-AR Cells were seeded onto 96-well plates. After washing 3 times with ice-cold phosphate-buffered saline (PBS), cells were fixed in PBS containing 4% formaldehyde for 1 h at room temperature (RT). Cells were washed 5 times in PBS and blocked in PBS containing 2% bovine serum albumin (BSA) for 1 h at RT. Subsequently, anti-β2-AR polyclonal antibody (1/100 in PBS) was added to the cells for 24 h at 4 °C. Cells were washed 3 times in PBS containing 1% BSA and incubated with goat anti-rabbit IgG-TRITC for 1 h at 37 °C. Cells were observed by inverted fluorescence microscope (Olympus BX51, Japan).
Flow cytometry assay CHOβ2-AR-CRE-EGFP cells were seeded in 12 well plates at 105 cells/well and incubated at 37 °C in 5% CO2 for 12 h in culture medium. Herb extracts or positive β2-AR agonist samples diluted with RPMI-1640 medium containing FBS (10%) were added to the wells (1 mL/well). After incubating for 10 h, the medium was discarded, and the drug-treated cells were trypsinized for 2 min and rinsed with RPMI-1640 medium containing FBS (10%). Single cell suspensions were centrifuged at 300×g for 3 min, and the supernatant was discarded. Pellets were washed and dissolved with PBS containing 2% FBS, protected from light, filtered twice, and then analyzed by flow cytometry. Ten thousand cells were collected on the FACS Calibur flow cytometer (Becton Dickinson, UT, USA) equipped with CellQuest software (Becton Dickinson). All data were carried out in triplicate, expressed as mean±SEM, and analyzed with the GraphPad Prism 4.0 program (GraphPad Software, California, USA).
Isolation and preparation of RALP extracts RALP (100 g) was powdered and soaked in 1 L 50% ethanol overnight. The supernatant was concentrated to 200 mL in vacuo, and passed through a column (2×20 cm) of HP20 macroporous resin (washed with water, eluted with 40% aqueous ethanol) to purify the aqueous phase. The eluted ethanol solution was concentrated to dryness in vacuo and then lyophilized. A yield of 1.3 g from the extract was obtained, and 100 mg dissolved in water was filtered through a 0.45 µm membrane, and separated by the 10A VP-HPLC system (Shimadzu Co., Kyoto, Japan), using a stainless-steel column filled with Kromasil C18 [150×4.6 mm, 5 μm particle size, (Eka Chemicals, Bohus, Sweden)] at 25 °C. The mobile phase was PBS (0.05 mol/L, pH 3.0): acetonitrile (90:10) (v/v) at a flow rate of 1.0 mL/min with UV detection at 270 nm. Each fraction was collected and vaporized to remove the organic solvent. The fractions were dissolved in dimethyl sulfoxide (10 µL) and then diluted 1000-fold with RPMI-1640 medium containing FBS (10%). The activity of the fractions was evaluated using CHOβ2-AR-CRE-EGFP cells. In the other hand, the CHOβ2-AR-CRE-GFP cells were pretreated with β2 adrenoceptor antagonist alprenolol (10–6 mol/L), and then higenaming was added into the cell assay system at the concentration of 10–2–10–7 mol/L. The competitive effects of alprenolol on higenamine were then evaluated by the same method.
Purification and identification of hit compounds The fraction that possessed activity (hit sample) was extracted on a larger scale and purified by semi-preparative reversed-phase HPLC, using a Kromasil C18 (250×10 mm, id, 10 µm) column with the same mobile phase. The active fractions were further isolated until single peaks were achieved in an effort to obtain single compounds. The electrospray mass spectrometer (ESI-MS) was operated under positive ion mode and an optimized collision energy level of 100% scanned from m/z 100 to 1 000. ESI was conducted using a spray voltage of 0.02 kV. High-purity nitrogen (99.999%) was used as dry gas at a flow rate of 15 L/min, with a capillary temperature of 230 °C. The ESI interface and mass spectrometer parameters were optimized to obtain maximum sensitivity.
The relaxant effect of higenamine on guinea pig tracheal muscle Hartley strain guinea pigs (250–350 g) were killed with an overdose of pentobarbital (75 mg/kg, ip). The tracheas were removed and cut spirally into 2 strips 15 mm long and 3 mm wide[14]. The tracheal strips were mounted vertically in a 20 mL water-jacketed organ bath filled with Krebs-bicarbonate buffer (124 mmol/L NaCl, 5 mmol/L KCl, 1.3 mmol/L MgSO4, 2.5 mmol/L CaCl2, 25 mmol/L NaHCO3, 0.6 mmol/L NaH2PO4, and 10 mmol/L glucose) aerated with a mixture of 95% O2 and 5% CO2 at 37 °C. The bottom end of the strip was fixed to an L-shaped hook, and the top end was tied to a stainless steel wire connected to a force displacement transducer. Changes in isometric tension were recorded. Before each experiment, each strip was subjected to a load of 2 g for at least 2 h, with frequent changes of Krebs-bicarbonate buffer, until a stable baseline tension was obtained. The strips were washed thoroughly with Krebs-bicarbonate buffer immediately after the peak tension developed, and were kept unstimulated until a stable baseline tension was obtained again. In all experiments, 200 µL of each drug was added into the organ bath. All drug concentrations indicated were expressed as the final concentration in the organ bath. The higenamine sample was added to the organ bath 10 min before the addition of acetylcholine (ACh) and propranolol was added 10 min before higenamine. The peak contractile response was taken as 100%. Tensions were expressed as percentages of the peak contractile response.
Asthma model in guinea pig induced by atomized histamine Thirty-five male Hartley strain guinea pigs (250–350 g) were divided into 5 groups. The experimental protocols were approved by the Committee of Animal Care of Nankai University (Tianjin, China). In the experiment, the animals were kept in an inhalation cage consisting of 3 boxes. An animal was placed into box A to which the test drug was applied using an ultra-sound nebulizer, which provided atomized test drug solutions. The drug was atomized at the rate of 0.5 mL/min and all pumped into box A. The animal was kept in box A for 1 min under spontaneous breathing for the administration of atomized test drugs or normal saline. Box B served as a sluice through which the animal was passed into box C. In box C the animal was exposed to atomized 0.1% histamine solutions for 20 s. The histamine was atomized at the rate of 0.5 mL/min and all pumped into box C. The animal was then withdrawn from the inhalation cage. The time until the appearance of asphyxial convulsion was considered the latent period of asthma. At first, the animals were stimulated only by histamine in box C and the latent periods were recorded. Two days later the animals were exposed to histamine again after their exposure to atomized drugs or normal saline in box A. The increasing rate of the latent period was calculated by Vogel’s method[15].
Statistical analysis Data are presented as mean±SEM. Statistical analysis of the data for multiple comparisons were carried out by ANOVA followed by the Bonferroni test[16]. For single comparison, the significance of differences between means was determined by t-test. A value of P<0.05 was considered statistically significant.
Results
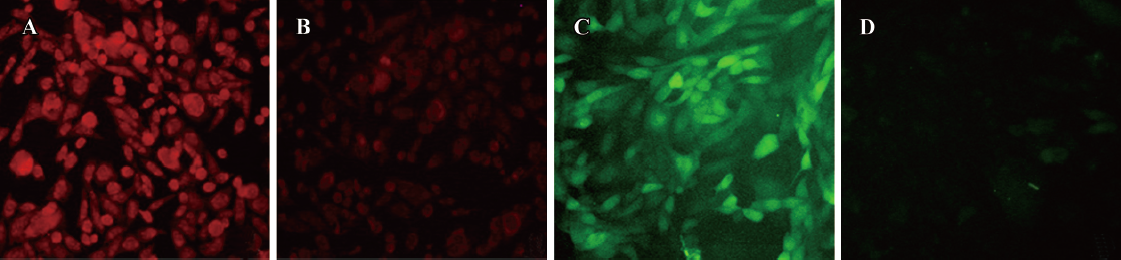
Properties of the CHOβ2-AR-CRE-EGFP cells assay system β2-AR protein expressed in CHOCRE-EGFP cells was detected by immunocytochemistry. CHOβ2-AR-CRE-EGFP cells (Figure 1A) showed much stronger red fluorescence than control cells transfected with pcDNA3.1 alone (Figure 1B). On the other hand, when CHOβ2-AR-CRE-EGFP cells were incubated with 1 mmol/L salbutamol, strong green fluorescence was emitted (Figure 1C), while no fluorescence was detected for the negative control cell line (Figure 1D).
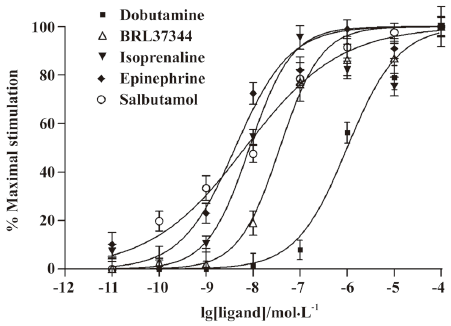
Next, CHOβ2-AR-CRE-EGFP cells were stimulated by different agonists at different concentrations to evaluate the function of the system. The fluorescence of the cells was measured by flow cytometry and the EC50 was determined by Scatchard analysis. As shown in Figure 2, salbutamol, isoprenaline, epinephrine, BRL37344, and dobutamine were used to assess the specificities of the reporter system. Among the agonists tested, epinephrine had the highest affinity for CHOβ2-AR-CRE-EGFP cells. As predicted, salbutamol, as a β2-AR-specific agonist, displayed a high activity. The β1-AR agonists, dobutamine and β3-AR agonists, BRL37344, respectively, displayed a much lower ability to stimulate β2-AR in this cell-based assay system. The order of the EC50 values may, therefore, be defined as follows: epinephrine (4.15 nmol/L), salbutamol (6.73 nmol/L), isoprenaline (8.35 nmol/L), BRL37344 (36.91 nmol/L), and dobutamine (894.5 nmol/L). The EC50 values were comparable with the Kact values for the accumulation of cAMP in CHO cells[17].
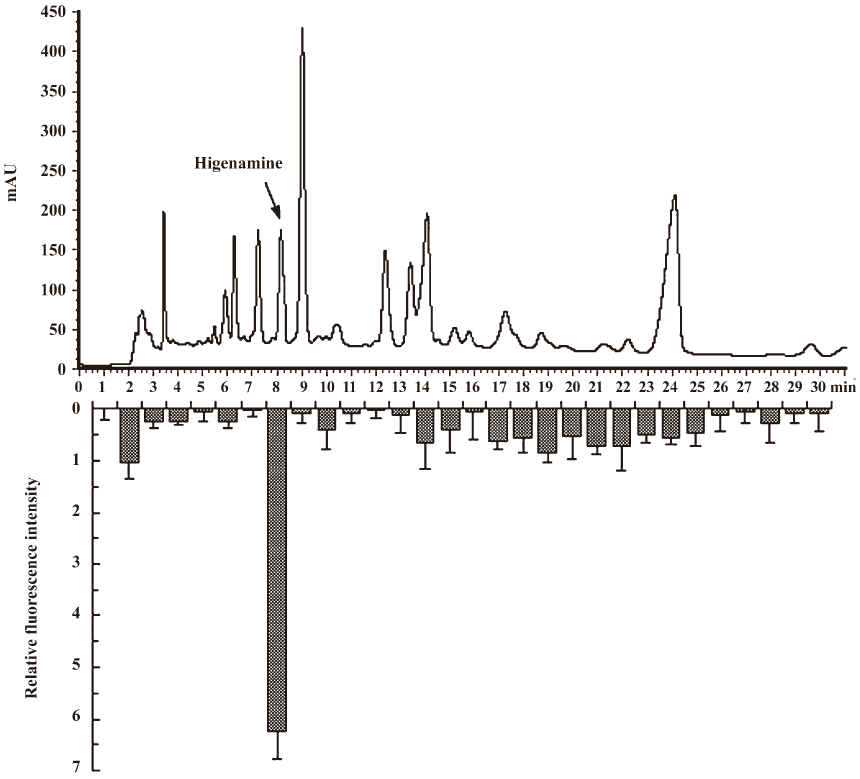
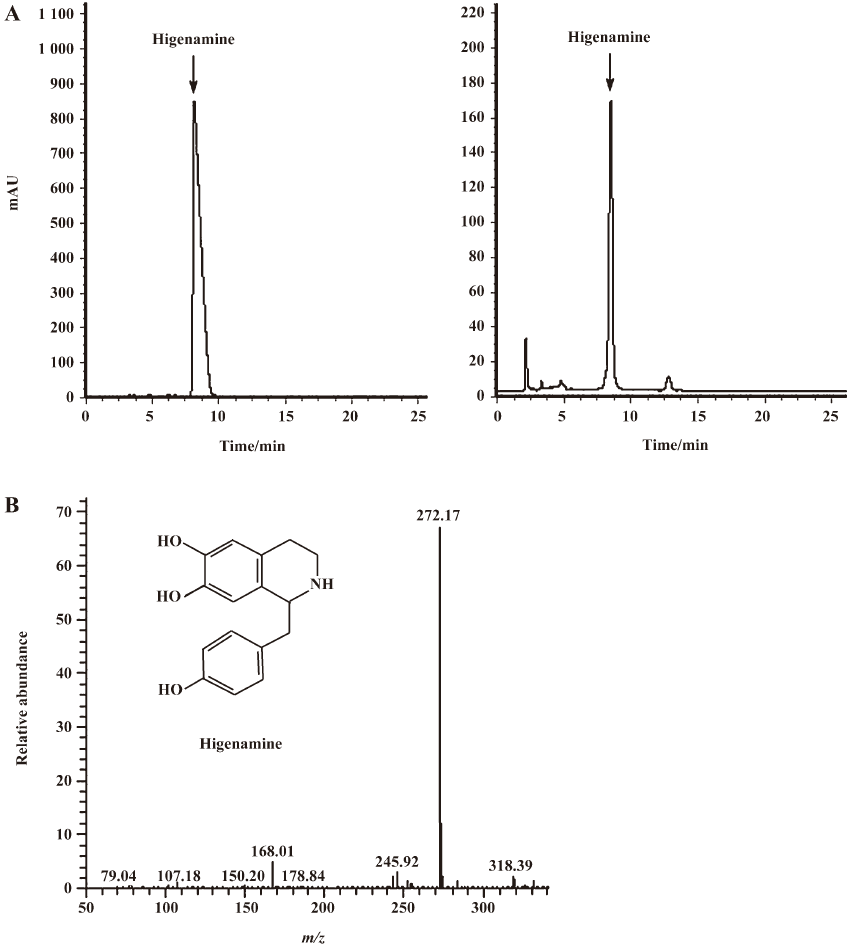
Identification of hit compounds To isolate the effective components from RALP, the active crude extract of RALP was further purified by HPLC. Thirty fractions were isolated and collected, and each fraction was tested for activity using CHOβ2-AR-CRE-EGFP cells. As shown in Figure 3, the 8th fraction showed the highest activity. Using semi-preparative reversed-phase HPLC, the bioactive fraction was finally obtained, and its mass spectra and structure are shown in Figure 4. The purified compound showed an intense molecular ion [M+H]+ at m/z 272, which coincided with the molecular weight of higenamine. Its retention time was also the same as standard higenamine, confirming that the effective compound of the active fraction was higenamine. Compared with the standard, the purity of the purified higenamine was about 90%. The dose-response curves for higenamine activity are shown in Figure 5. The EC50s of the crude extract and purified higenamine were 1.04 µg/mL and 0.127 µg/mL, respectively.
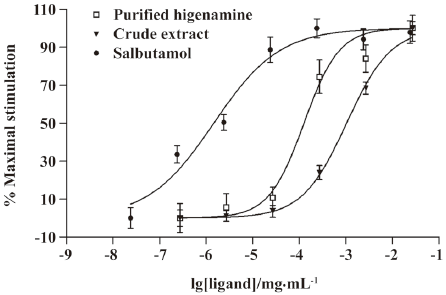
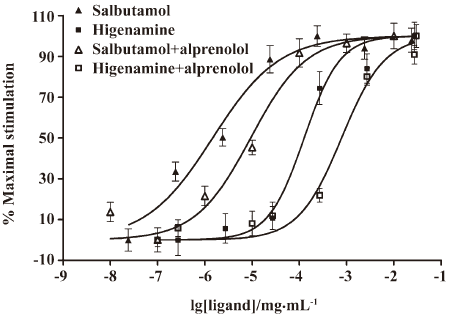
Competitive effect of alprenolol on higenamine Radiolabeled anopindolol, a β2-adrenoceptor antagonist, was often applied in ligand binding assay and cAMP radioimmunoassay G-protein-coupled receptors research[18,19]. In the present study, we used alprenolol to evaluate its competitive effect on higenamine by measuring the CRE-directed reporter gene expression. The dose-dependent curves of the activity of higenamine and salbutamol are shown in Figure 6. Alprenolol was shown as a competitive antagonist to salbutamol, which is a selective β2 agonist, by shifting right the dose-response curve with the EC50 value from 1.463 to 9.331 ng/mL. Alprenolol had the same effect on higenamine, the EC50 of higenamine was shifted from 126.5 ng/mL to 782.8 ng/mL. This result indicated that higenamine was a potential β2-adrenoceptor agonist.
The relaxant effect of higenamine on guinea pig tracheal muscle At concentrations of 10–8 and 10–9 mol/L, higenamine significantly inhibited the tracheal contractions induced by acetylcholine (ACh) (Figure 7). Higenamine relaxed tracheal muscle in a dose-dependent manner, because 10–8 mol/L higenamine was significantly more effective than 10–9 mol/L higenamine.
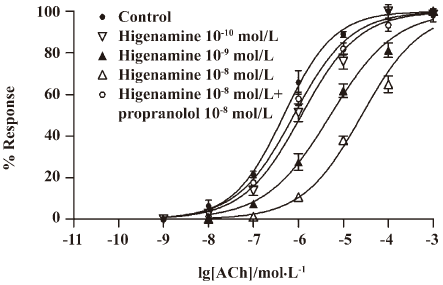
The blockade of β-ARs by 10–8 mol/L propranolol shifted the dose-response curve for ACh to the left. This concentration of propranolol had a significant effect on the inhibitory effect of 10–8 mol/L higenamine on tracheal contractions induced by ACh. Their EC50 values were significantly different, with the EC50 value for ACh in strips treated with 10–8 mol/L higenamine being (2.60±0.36)×10–5 mol/L, and that for ACh in propranolol-treated strips treated with 10–8 mol/L higenamine being (7.90±0.72)×10–7 mol/L. Higenamine activated β-ARs to inhibit tracheal contractions based on the observation that the propranolol block of β-ARs significantly altered the relaxant effect of higenamine.
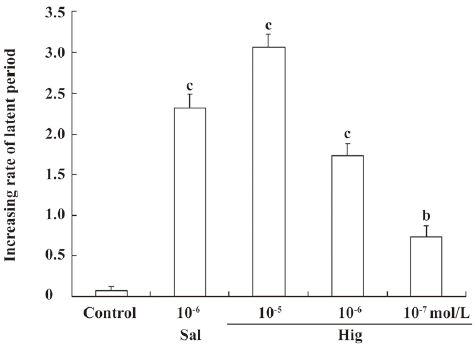
The effect of higenamine in a guinea pig experimental asthma model The guinea pig asthma model was established to certify the bronchiectasic effect of purified higenamine prepared from RALP. As shown in Figure 8, 10–6 mol/L salbutamol was taken as a positive control and prolonged the latent period 2.3-fold. Higenamine inhibited asphyxial convulsion in a concentration-dependent manner. The increasing rate of 10–5, 10–6, and 10–7mol/L higenamine was about 3.1-, 1.7-, and 0.74-fold, respectively. Therefore, higenamine was markedly effective in reducing the severity of bronchoconstriction following the exposure of guinea pigs to atomized histamine solutions.
Discussion
β2-ARs are found in vascular, bronchial, and uterine muscle, and their stimulation causes muscular relaxation. β2-ARs are major pharmaceutical targets, and the physiological and pharmacological manipulation of these receptors by agonists and antagonists have clinical relevance, particularly in airway smooth-muscle disorders. β2-AR agonists, as bronchodilators, have been used for the relief and prophylaxis of asthma for more than 30 years[1]. On the other hand, there are hundreds of different kinds of herbs used for the treatment of asthma in TCM. The empirical observations accumulated for over 2000 years by the clinical practices of generations of TCM physicians are valuable. Many herbal medicines are still used to treat patients’ illnesses and improve their quality of life. There are probably some potential β2-AR agonists in TCM, but empirical records and explanation are not enough. TCM needs to be evaluated by the general methods of basic biomedical sciences and by drug development models in conventional medicine. Modern medicine requires that the mechanisms of the herbs be explained at the molecular or cellular level, which can direct the clinical use and development of natural compounds.
However, each herb may contain hundreds of different compounds, complicating the understanding of their molecular mechanisms. To test the β2-agonist activity of hundreds of samples with animal models is tedious work. In vitro tissue models, such as tracheal strips or tracheal rings, can narrow the targets down to one tissue. However, their convenience and sensitivity cannot compare with cell-based assays. The screening assay in this paper indirectly detected the intracellular cAMP level by measuring CRE-directed reporter gene expression. It used no radioactive materials and evaluated the efficacy of β2-AR agonists. This assay is suitable for screening the β2-AR agonists from the complex natural compound library of herbs. Using this assay, the potent β2-AR agonist higenamine was identified from the 30 HPLC fractions of RALP. The purified higenamine was also tested with this cell line for its dose-response curve and EC50. We then used guinea pig tracheal strips as an in vitro tissue model to prove the inhibitory effect of higenamine on tracheal contractions. Finally, the bronchiectasic effect of purified higenamine was further studied in an in vivo model. From cell to tissue, and then to animal, we have built a systematic assay of β2-AR agonist to screen novel compounds and evaluate their efficacy at the level of cellular signaling pathways, in vitro in tissue, and in vivo in an animal model.
Radix Aconiti Lateralis Preparata as TCM is often combined with other herbs, such as Ephedra, Asarun, Ramulus Cinnamomi, and Radix Ginseng, at different ratios according to the patients’ conditions. They have been used clinically for hundreds of years for asthma, but their molecular mechanism has only been studied for a few years[20]. Most of the research on RALP has centered on the cardiotonic agents, which are aconitine alkaloids[21]. Higenamine, as a potent cardiotonic and vasodilator, is an α1 antagonist and a weak α2 agonist[22,23]. The β2-agonist activity of higenamine was first verified at the molecular and cellular level in the present study. Also, its relaxant effects on tracheal muscle to reduce the severity of bronchoconstriction were certified in vitro and in vivo. The β2-agonist-like effect resulted in the relaxation of airway smooth muscle and improved patient condition. Our results indicated that RALP had an active compound higenamine with the ability to produce relaxation in tracheal muscle. The bronchodilatory effect might explain in part their traditional use as an anti-asthmatic remedy. These findings may provide a new theoretical basis for guiding modern biochemical science to study the basis for TCM.
References
- Rabe KF, Schmidt DT. Pharmacological treatment of asthma today. Eur Respir J 2001;18:34s-40s.
- Carstairs JR, Nimmo AJ, Barnes PJ. Autoradiographic visualization of beta-adrenoceptor subtypes in human lung. Am Rev Respir Dis 1985;132:541-7.
- Emilien C, Maloteaux JM. Current therapeutic uses and potential of ß-adrenoceptor agonists and antagonists. Eur J Clin Pharmacol 1998;53:3890-4.
- Barany M. Protein phosphorylation in cardiac and vascular smooth muscle. Am J Physiol Heart Circ Physiol 1981;241:H1172-8.
- Tang JL. Research priorities in traditional Chinese medicine. JMB 2006;333:3919-4.
- Cheng JT. Review: Drug Therapy in Chinese Traditional Medicine. J Clin Pharmacol 2000;40:4455-0.
- Chen DH. The study of Chinese Radix Aconiti Lateralis Preparata II-the chemical composition of Baifupian. Chin Tradit Herbal Drugs 1965;13:4818-16.
- Huang TK. A handbook of the composition and pharmacology of common Chinese drugs. Beijing: Chinese Medicine Technology Press; 1994.
- Kosuge T, Yokota M. Cardioactive principle of Aconitum japonicum Thunb. Chem Pharm Bull (Tokyo) 1976;24:1768-2.
- Konno C, Shiruasaka M, Hikion H. Cardiac active principle of Aconitum Carmichaeli roots. Planta Med 1979;35:1505-7.
- Durocher Y, Perret S, Thibaudeau E, Gaumond MH, Kamen A, Stocco R, et al. A reporter gene assay for high-throughput screening of G-protein-coupled receptors stably or transiently expressed in HEK293 EBNA cells grown in suspension culture. Anal Biochem 2000;284:316-26.
- Kunapuli P, Ransom R, Murphy KL, Pettibone D, Kerby J, Grimwood S, et al. Development of an intact cell reporter gene beta-lactamase assay for G protein-coupled receptors for high-throughput screening. Anal Biochem 2003;314:16-29.
- Chen J, Bai G, Yang Y, Geng P, Cao Y, Zhu Y. Identifying glucagon-like peptide-1 mimetics using a novel functional reporter gene high-throughput screening assay. Peptides 2007;28:9283-4.
- Hashiba E, Sato T, Hirota K, Hashimoto Y, Matsuki A. The relaxant effect of propofol on guinea pig tracheal muscle is independent of airway epithelial function and ß-adrenoceptor activity. Anesth Analg 1999;89:1911-6.
- Vogel HG, Vogel WH. Drug discovery and evaluation: pharmacological assays (2 edn). Berlin Heidelberg: Springer-Verlag; 1997.
- Kaur M, Chivers JE, Giembycz MA, Newton R. Long-acting β2-adernoceptor agonist synergistically enhance glucocorticoid-dependent transcription in human airway epithelial and smooth muscle cells. Mol Pharmacol 2008;73:2031-4.
- Tate KM, Briend-Sutren MM, Emorine LJ, Delavier-Klutchko C, Marullo S, Strosberg AD. Expression of three human beta-adrenergic-receptor subtypes in transfected Chinese hamster ovary cells. Eur J Biochem 1991;196:357-61.
- Yanagisawa T, Sato T, Yamada H, Sukegawa J, Nunoki K. Selectivity and potency of agonists for the three subtypes of cloned human beta-adrenoceptors expressed in Chinese hamster ovary cells. Tohoku J Exp Med 2000;192:181-93.
- Gan LL, Wang MW, Cheng MS, Pan L. Trachea relaxing effects and beta2-selectivity of SPFF, a newly developed bronchodilating agent, in guinea pigs and rabbits. Biol Pharm Bull 2003;26:323-8.
- Kang YJ, Lee YS, Lee GW, Lee DH, Ryu JC, Yun-Choi HS, et al. Inhibition of activation of nuclear factor kappaB is responsible for inhibition of inducible nitric oxide synthase expression by higenamine, an active component of aconite root. J Pharmacol Exp Ther 1999;291:314-20.
- Lee YS, Kang YJ, Kim HJ, Park MK, Seo HG, Lee JH, et al. Higenamine reduces apoptotic cell death by induction of heme oxygenase-1 in rat myocardial ischemia-reperfusion injury. Apoptosis 2006;11:1091-100.
- Liu XJ, Henry NW, Shouchi T. Measurement of effects of the Chinese herbal medicine higenamine on left ventricular function using a cardiac probe. Eur J Nucl Med 1983;8:233-6.
- Feng YP, Gao H, Zeng GY. Effect of higenamine on α-adrenergic receptor. Acta Pharm Sin 1986;7:208-11.
