Possible arrhythmiogenic mechanism produced by ibuprofen1
Introduction
Ibuprofen (Fenbid) is a non-steroid anti-inflammatory drug (NSAID) often used to relieve fever and the symptoms of arthritis. Low doses of ibuprofen (200–1200 mg/d) are available over the counter in many countries as an analgesic. Its mechanism of action is believed to non-selectively inhibit both variants of cyclooxygenase (COX-1 and COX-2) and thus block prostaglandin synthesis. However, unlike aspirin, which also blocks both COX isoforms and prevents blood clotting, ibuprofen does not have antiplatelet effects and thus is not routinely used to prevent cardiac infarction.
The wide use of ibuprofen attributes to its lowest incidence in causing gastrointestinal dysfunction, a side-effect common to all non-selective COX inhibitors. Despite this advantage, the recent withdrawal of selective COX-2 inhibitor Vioxx (Merck & Co, Inc, Whitehouse Station, New Jersey, USA). from the US market due to its cardiac toxicity brought all COX inhibitors under the spotlight and raised the question as to whether they could lead to elevated cardiovascular risk.
The initial study of potential cardiac toxicity in an ibuprofen cohort identified significantly higher rates of life-threatening ventricular arrhythmias and cardiac arrest than those in the control naproxen cohort[1]. To date, with the extensive use of ibuprofen, the number of frequent ventricular premature beats, atrial fibrillation[2,3], and other untoward cardiac reactions have been reported as increasing steadily[4–6].
In spite of these clinical studies and case reports, very little work has been done to characterize the electrophysiological effects of ibuprofen on the animal heart. In the present study, we used isolated guinea pig hearts to identify the arrhythmiogenic effects of ibuprofen on the electrocardiogram (ECG); we examined its effects on the fast- and slow-response cardiac action potentials (AP), and analyzed its dynamic process of combination and dissociation with the Na+ channels. In conclusion, our results demonstrate for the first time that ibuprofen, a non-selective COX inhibitor, may induce cardiac arrhythmias by shortening the effective refractory period (ERP) and decreasing the excitation propagation.
Materials and methods
Solution preparation In total, 300 mg ibuprofen was dissolved in 5 mL DMSO to make a stock solution of 60 mg/mL. The stock solution would be further diluted to the designed concentrations in the Tyrode’s solution. The normal Tyrode’s solution for perfusing the cardiac ventricular cells of guinea pigs consisted of the following (in mmol/L): 137 NaCl, 23 NaHCO3, 0.5 MgCl2, 5.4 KCl, 1.8 CaCl2, 0.4 NaH2PO4, and 10 glucose, pH 7.4±0.05. The Tyrode’s solution for the rabbit sinus node preparations consisted of the following (mmol/L): 140 NaCl, 1.0 MgCl2, 5.4 KCl, 1.8 CaCl2, 5 HEPES, and 10 glucose, pH 7.4±0.05. Both solutions were gas-saturated by 95% O2+ 5% CO2 and 100% O2, respectively.
ECG recording and measurement in vivo Guinea pigs of either sex (weighing approximately 500 g) were anesthetized by an intraperitoneal injection (ip) of urethane (20%, 5 mL/kg). Needle electrodes, inserted subcutaneously, were used to record the ECG of standard limb lead II. The RR interval, QRS duration, QT interval, and heart rate variation (HRV) were measured, respectively. The corrected QT interval (QTc) was calculated with the Bazett formula (QTc=QT×RR-1/2) to reduce the influence of the fluctuation of the heart rate on the measurement of the QT interval. Different concentrations of ibuprofen were injected into the external jugular vein to evaluate its effects on the heart.
ECG recording in isolated heart The hearts of the anesthetized guinea pigs were quickly taken out of the chest and perfused with Langendorff perfusion set-up at a recorded pressure of 80 cmH2O. The temperature of the Tyrode’s solution was maintained at 37±0.5 °C. Three platinum electrodes placed on the cardiac apex, right atrium, and aortic root were used to record the ECG. The signals were documented on computer using the PowerLab system (PowerLab ML135, ML 785, ADInstruments Castle Hill, NSW, Australia). The RR interval, QRS duration, QT interval, and QTc were analyzed.
Preparation of guinea pig papillary muscles Guinea pigs of either sex (weighing approximately 250~300 g) were sacrificed by venesection under deep anesthesia with an injection of sodium pentobarbital (35 mg/kg, ip). The hearts were rapidly removed into the dissection chamber filled with the Tyrode’s solution. The right ventricular papillary muscles were excised and pinned to the bottom of a recording chamber. The recording chamber was perfused with the Tyrode’s solution at a constant rate of 3 mL/min and maintained at a temperature of 37±0.5 °C.
Recording of fast-response AP Bipolar platinum electrodes were used to drive the preparations with rectangular current pulses at a stimulation frequency of 1 Hz. Each pulse lasted for 0.1 ms, with the amplitude approximately 1.5 times the threshold value. After 30 min stimulation, transmembrane AP were recorded by a conventional glass microelectrode, which was filled with 3 mol/L KCl and had a tip resistances of 15–20 MΩ. The records were sampled and stored in the computer through the amplifier (MEZ8201, Nihon Kohden, Tokyo, Japan) and PowerLab interface (PowerLab ML785, ADInstruments, Australia)[7].
The calculated parameters for the fast-response AP included the resting potential (RP), AP amplitude (APA), maximum upstroke velocity of phase 0 (Vmax), AP duration at 50% and 90% repolarization (APD50 and APD90), and the ERP.
The preparations were driven by a series of 8 stimuli pulses at a frequency of 1 Hz. Then an additional pulse was added following the eighth one. Adjusting the time interval between the eighth and the extra stimulation pulse, the minimum interval for the evoked AP by the extra stimulus would be calculated as the ERP.
Measurements of time constants for ibuprofen combined with and dissociated from fast Na+ channels The preparations were stimulated at a frequency of 1, 2, and 3 Hz, respectively. For each frequency, the stimulation would last until the Vmax decreased gradually to a steady state, which could indicate the dynamics of the combination of ibuprofen with the fast Na+ channels after repetitive activation of the channels. The first-order exponential decay equation was employed to fit the relationship between the decreased Vmax values and the duration of the stimuli. The time constant (τon) thus obtained is the index of the combination dynamics of ibuprofen with the fast Na+ channels. Origin 6 software (Microcal Software, Northampton, MA, USA) was used to analyze the data.
With a similar protocol, the preparations were activated for 30 s at a frequency of 1, 2, or 3 Hz, respectively. After the Vmax for each frequency decreased to a steady state, a various delayed supra-threshold stimulus was added to invoke an additional AP, and the Vmax of the first evoked AP with various delays were measured. As the drug needs time to dissociate from the channels, it was found that the longer the retardation time for evocation, the greater the Vmax of the evoked AP. The relationship of the Vmax value and the retardation time was fitted by the first-order exponential decay equation. The time constant (τoff) would represent the dissociation index of ibuprofen from the Na+ channels.
Recording of slow-response AP of guinea pig papillary muscles When the preparations were perfused with the Tyrode’s solution containing 27 mmol/L KCl, the resting potential would gradually change to approximately -45 mV. The Na+ channels would be inactivated at this membrane level. After 0.4 mg/L isoprenaline was added into the Tyrode’s solution and a stronger stimulus was employed to the preparation, the slow-response AP, which were evoked by the slow inward current of the Ca2+ rather than the Na+ channels, could be initiated[8].
Spontaneous AP of rabbit sinus node cells Adult rabbits of either sex were anesthetized by injection of sodium pentobarbital (1%, 3 mL/kg). The hearts were removed to a chamber containing the Tyrode’s solution. The sinus nodes were excised from the heart and pinned to the bottom of the recording chamber, which was perfused with the special Tyrode’s solution for sinus nodes. An intracellular microelectrode (containing 3 mol/L KCl with a tip resistance of 25 MΩ) was employed to record the spontaneous AP of the sinus node cells. The parameters of AP were sampled with the same method as described in fast-response AP. The spontaneous beating rate, APA, APD, Vmax and spontaneous depolarization rate of phase 4 (SDR) were calculated, respectively.
Statistical analysis All of the data were sampled by Chart 5 software (ADInstruments, Australia). Origin 6 was used for the statistical analysis. The results were expressed as mean±SD. Statistical significance was determined by Student’s t-test for paired data. P<0.05 was considered statistically significant.
Results
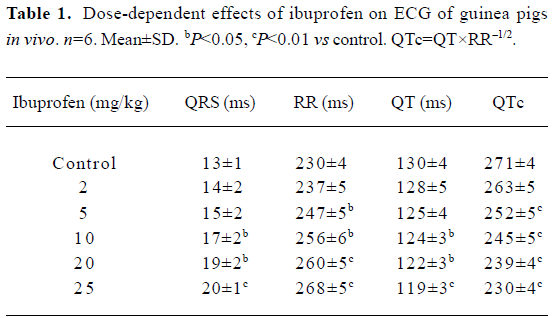
ECG from anesthetized guinea pigs ECG of standard limb lead II from urethane-anesthetized guinea pigs were recorded. Every 10 min, ibuprofen was consecutively injected into the external jugular vein to reach a concentration of 2, 5, 10, 20, 25 mg/kg, respectively (Table 1; Figure 1A). After 25 mg/kg ibuprofen, ECG exhibited a marked increase of the QRS duration (from 13±1 ms to 20±1 ms vs control) and RR intervals (from 230±4 ms to 268±5 ms) with percentage changes of 53.8% and 16.5%, respectively. However, the QTc decreased from 271±4 ms to 230±4 ms by a significant change of 15.1%. A HRV analysis showed that the main parameters of HRV (including the standard deviation of the RR intervals (SDNN), the low frequency power density component (LF), the high frequency power density component (HF), LF/HF ratio (LF/HF)] were not changed significantly after an intravenous injection of ibuprofen. In order to exclude the possible effect from DMSO, we performed the 5 control experiments, with an intravenous injection of DMSO (417 μL/kg) alone, 50 min after the injection of DMSO, QRS (ms), RR (ms), QT (ms), and QTc were measured. These did not change significantly on the ECG of the guinea pigs: 13.7±0.7, 213±7, 119±11, and 259±14 to 13.8±0.6, 206±9, 123±13, and 272±18, respectively. The results indicate that DMSO has no significant effect on the ECG of guinea pigs in vivo (P>0.05).
Full table
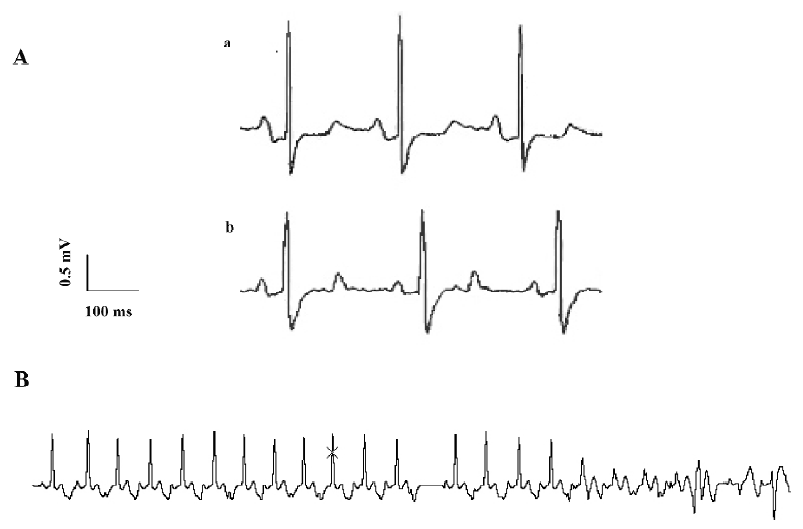
Premature contractions and ventricular fibrillations occurred in some animal experiments. Ventricular fibrillations occurred in 2 of 6 guinea pigs, which later recovered with an injection of a low dosage of ibuprofen (5 or 10 mg/kg); 25 mg/kg ibuprofen induced fatal ventricular fibrillations in another two guinea pigs (Figure 1B).
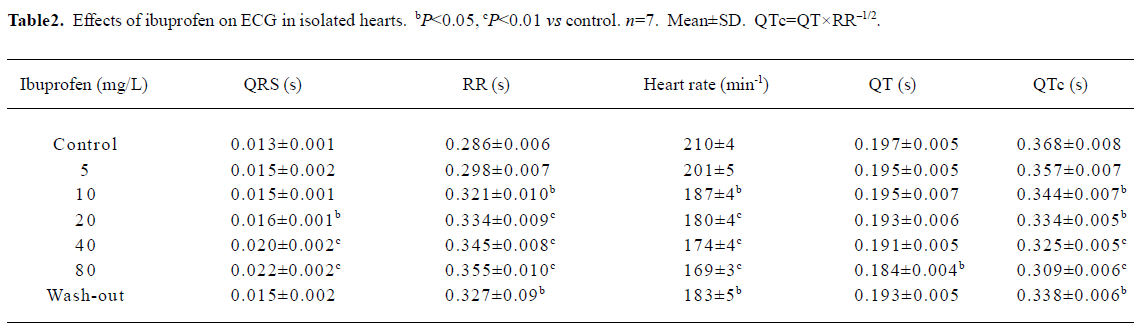
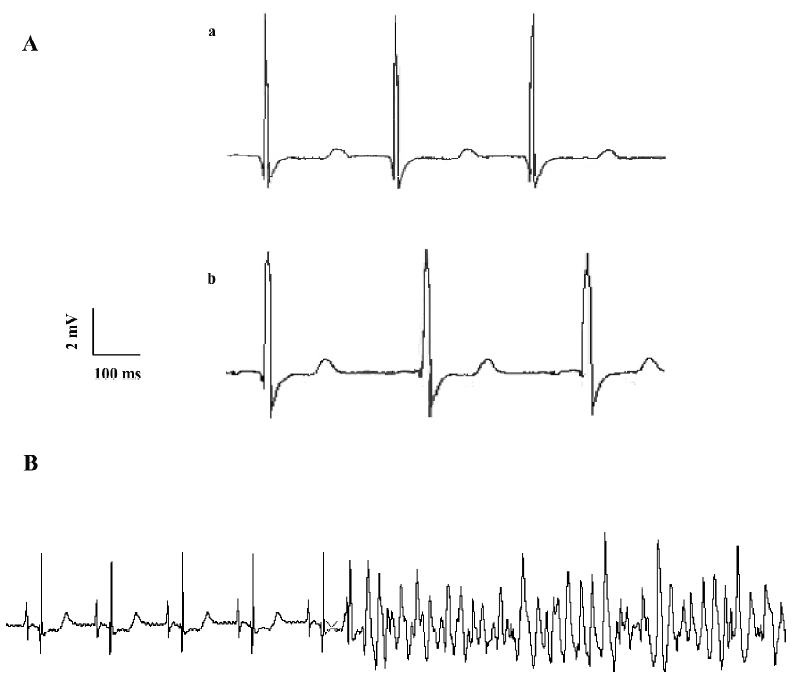
Effects of ibuprofen on ECG in isolated hearts The isolated guinea pig hearts were perfused with the Tyrode’s solution. Every 10 min, the heart was consecutively perfused with ibuprofen at concentrations of 5, 10, 20, 40, and 80 mg/L respectively, while the ECG was recorded. The results in Table 2 show that like the in vivo experiments, ibuprofen could prolong the duration of the QRS wave and the RR interval (Figure 2A). Although ibuprofen decreased the heart rate, it shortened the QT interval, especially the QTc. When the hearts were exposed to 20 mg/L ibuprofen, some ECG showed arrhythmias (especially ventricular premature contractions), but most disappeared within 30 min. With 40 mg/L ibuprofen, ventricular fibrillations occurred in 3 of 7 experiments and recovered later after washing out with the control Tyrode’s solution. In another experiment with a higher concentration of ibuprofen (80 mg/L), 2 hearts stopped beating after sustained ventricular fibrillations (Figure 2B).
Full table
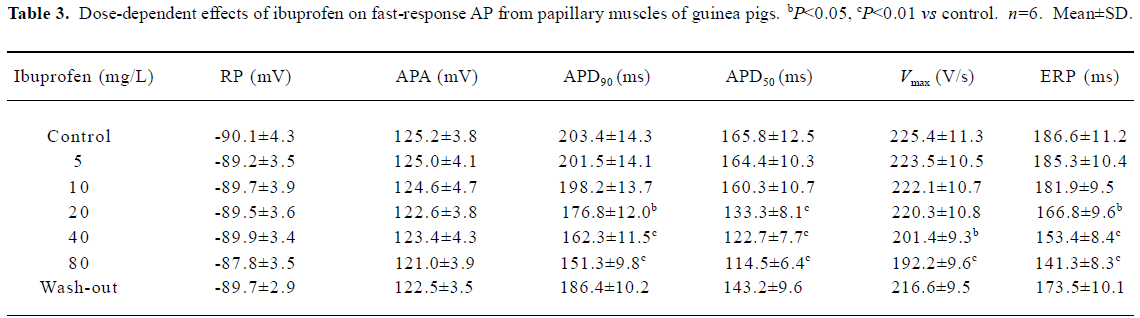
Effects of ibuprofen on fast-response AP of guinea pig papillary muscles In order to get a stable recording of fast-response AP, the papillary muscles of the guinea pigs were stimulated at a frequency of 1 Hz for approximately 30 min. Then the preparations were perfused consecutively with 10, 20, 40, and 80 mg/L Tyrode’s solution containing ibuprofen. Each performance lasted for 10 min. The results are shown in Figure 3 and Table 3. Ibuprofen can dose dependently shorten the APD50 by 30.9% (from 165.8±12.5 ms to 114.5±6.4 ms), APD90 by 25.6% (from 203.4±14.3 ms to 151.3±9.8 ms), Vmax by 14.7% (from 225.4±11.3 V/s to 192.2±9.6 V/s), and ERP by 24.3% (from 186.6±11.2 ms to 141.3±8.3 ms), respectively. No obvious influence of ibuprofen on RP and APA were observed. When the preparations were washed out with the control Tyrode’s solution for 30 min, all the changes could obviously be reversed.
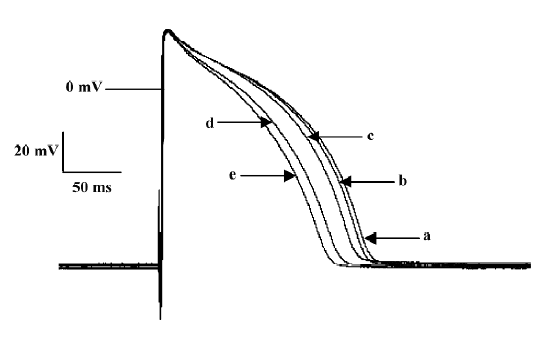
Full table
Dynamics of ibuprofen combined with and dissociated from the fast Na+ channels Ibuprofen can inhibit the Na+ channels; the Vmax of the fast-response AP is dramatically depressed by ibuprofen (Table 3). In a series of stimuli with frequencies lasting approximately 30 s, the value of the Vmax will decrease from the first evoked response, then gradually diminish into a steady state (Figure 4). With the different concentrations of ibuprofen (20 and 40 mg/L for 15 min, shown in the lower two rows of the Figure 4), the stimulation rate was increased from 1 to 2 or 3 Hz for more than 30 s for each frequency. The combination time constant (τon) of ibuprofen with the Na+ channels was calculated and shown in Table 4. Our results show that the higher the concentration of ibuprofen used, the shorter the time constant of τon; the higher the stimulus frequency, the shorter the time constant of τon (Figure 4; Table 4). These results indicate that ibuprofen is much easier to combine with the Na+ channels at a high stimulation frequency than that at a lower one.
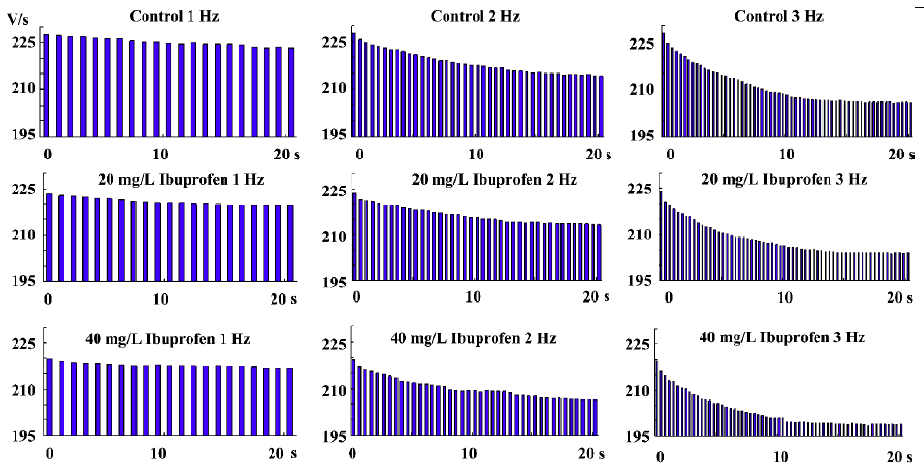
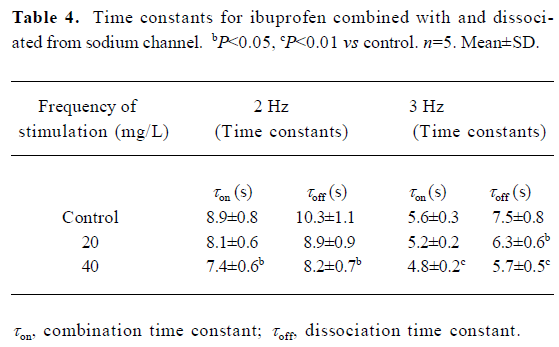
Full table
The dissociation time constant is longer than that of the combination ones for each different stimulation frequency (Table 4). As the ibuprofen concentration or the stimulation frequency increased, the dissociation course became much faster.
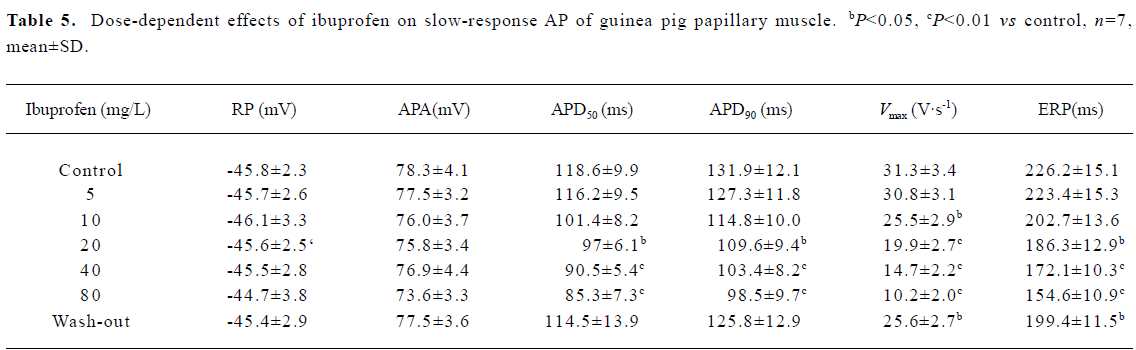
Effects of ibuprofen on slow-response AP After the slow-response AP of papillary muscles were recorded, the preparation were consecutively perfused with 5, 10, 20, 40, and 80 mg/L ibuprofen contained in Tyrode’s solution at an interval of 10 min for each concentration, as shown in Figure 5. The results in Table 5 show that ibuprofen (80 mg/L) could attenuate APD50 (from 118.6 ms to 85.3 ms), APD90 (from 131.9 ms to 98.5 ms), Vmax (from 31.3 V/s to 10.2 V/s), and ERP (from 226.2 ms to 154.6 ms), with percentage changes of 28.1%, 25.3%, 67.4%, and 31.7%, respectively. No substantial alterations were observed regarding RP and APA.
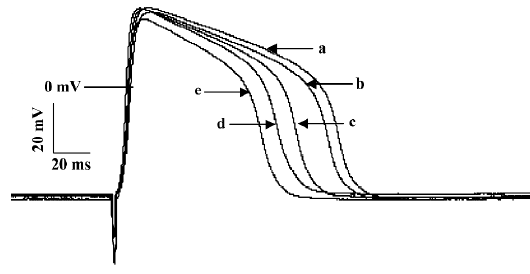
Full table
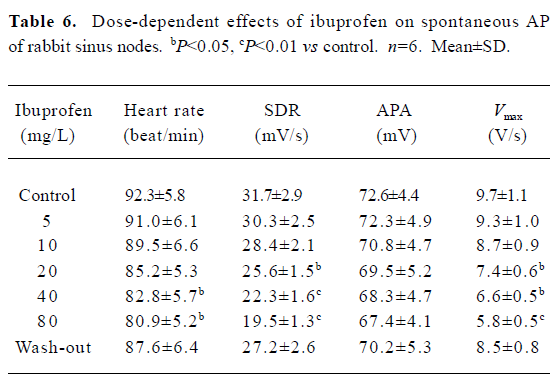
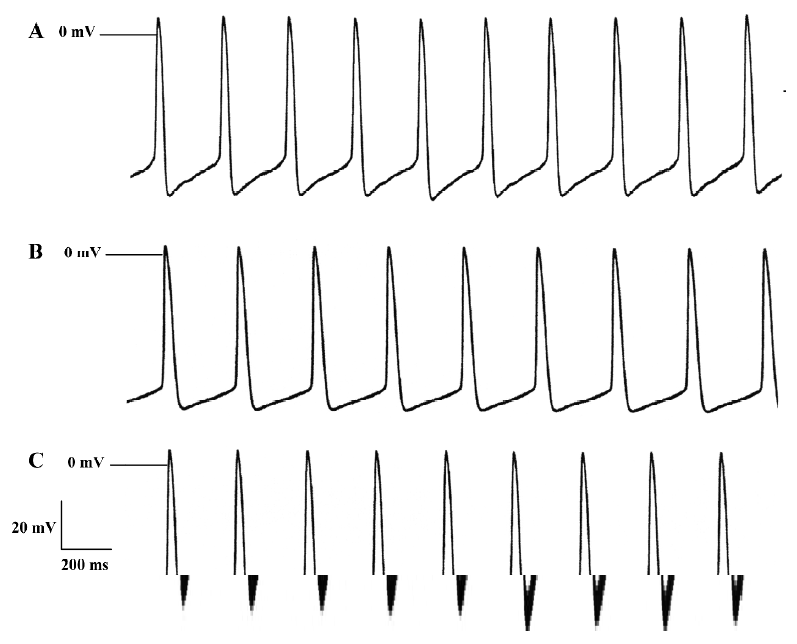
Effects of ibuprofen on spontaneous AP The spontaneous AP were recorded in rabbit sinus node preparations. The sinus node were exposed to 5, 10, 20, 40, and 80 mg/L ibuprofen consecutively. The results in Table 6 demonstrate that 80 mg/L ibuprofen decreases the Vmax by 40.2%, the beating rate by 12.4%, and the SDR by 38.5%, respectively. These effects of ibuprofen are dose dependent and reversible after washing out with control Tyrode’s solution. Figure 6 shows the effects of ibuprofen on the spontaneous AP of sinus nodes.
Full table
Discussion
NSAIDs are chemicals that present analgesic and anti-inflammatory effects, and most of them, including aspirin and ibuprofen, act as non-selective inhibitors of prostaglandin synthesis enzymes COX-1 and COX-2. By contrast, selective COX-2 inhibitors specifically block the production of the type of prostaglandins responsible for pain and inflammation. Hence, it was believed that selective COX-2 antagonists had more curative and fewer side-effects.
Ever since Merck withdrew its COX-2 antagonist Vioxx, a $2.5 billion/year sales blockbuster, because of high cardiovascular risk, the safety concerns over all COX inhibitors have received wide attention across the entire pharmaceutical industry. The cardiotoxicity of COX-2 inhibitors has been considered to result from the suppression of prostaglandin synthesis in endothelial cells[9,10]. Reduced prostaglandin in blood vessels would prevent vasorelaxation and facilitate clot formation, and thus predispose patients with existing heart diseases higher chances of myocardial infarction[11,12]. Although a similar association was obtained in other large population studies, in which high doses of traditional non-selective COX inhibitors were used to compare with placebo controls[13–15], the underlying mechanisms remain unknown.
In the present research, we studied the effects of ibuprofen on fast- and slow-response AP in guinea pig papillary muscles, on the spontaneous AP of rabbit sinus nodes, and the ECG of guinea pigs in order to elucidate its cardiac side-effects. During both the in vitro and in vivo ECG recording, ibuprofen prolonged the duration of the QRS complex wave and inversely shortened the QTc. The duration of QRS represents the propagating velocity of excitation in the heart. We also found that at a low dosage of ibuprofen (10–20 mg/L), certain types of arrhythmias, such as premature ventricular contraction and short duration ventricular fibrillation, could occur, and these events were reversible after ibuprofen was washed out with control solution. At a high dosage of ibuprofen (40–80 mg/L), 2 of 8 hearts stopped beating because of long-lasting ventricular fibrillations. These observations suggest that ibuprofen exhibit detrimental effects on heart function.
Next, we investigated the effect of ibuprofen at the cellular level, that is, its influence on cardiac AP. Figures 3 and 5 demonstrate that ibuprofen could dose dependently suppress Vmax in fast-response AP by 14.7%, and decrease the Vmax of slow-response AP by 67.4%, suggesting that ibuprofen is able to block the fast Na+ channel and the slow Ca2+ channel. Tables 3 and 5 further show that ibuprofen could not only decrease the fast and slow AP duration (APD50) by 30.9% and 28.1%, but also shorten the effective refractory periods by 24.3% and 31.7%, respectively. In sinus node cells, we found that ibuprofen decreased the SDR, slowing the heart rate in 6 experiments.
Interestingly, our data also imply that ibuprofen may directly interact with the cardiac Na+ channel. The changes of the association and dissociation time constants (τon and τoff) in Figure 4 provide evidence that the drug molecule may interact and inhibit the Na+ channel quicker as its concentration increase or during fast heart rates. The results presented in Tables 2, 3, 5, and 6 indicate that these alterations could not be reversed completely to the baseline after wash out. We believe that this effect may be induced by the prolonged exposure to high concentrations of ibuprofen during recording, lack of solvent DMSO in the wash solution, or a higher affinity of ibuprofen to the Na+ channel (τon<τoff), as illustrated in Table 4.
It might be speculated that ibuprofen could dose dependently suppress the Na+ and Ca2+ channels, and decrease the excitation spreading within the heart. The slower conduction is displayed on the ECG by the prolongation of the QRS wave. The decrease of the APD in AP recording could explain the shortening of the QTc produced by ibuprofen on ECG.
It is well known that slow conduction, shortening of the refractory period, and unidirectional blocking are the 3 important preconditions for re-entry that results in fibrillations in the heart. Since ibuprofen could slow conduction and decrease the refractory period, it might therefore lead to arrhythmias in clinical practice.
On the contrary, some reports indicate that NSAIDs, such as salicylic acid (SA), can reduce ventricular dysfunction and arrhythmias[16–18]. The investigators proposed that ibuprofen could prevent the atrial fibrillation mediated by inflammation, and SA could trap the hydroxyl radicals to reduce postischemic ventricular arrhythmias. Yet, those experiments were conducted on patients with heart diseases or on isolated ischemic hearts. By contrast, our study was performed on healthy animals and heart preparations. Nevertheless, they found that only low concentration of SA (0.5 and 1.0 mmol/L) prevented the risk of arrhythmias; at higher concentrations, the drugs lost their protective functions in the trials. It is known that anti-arrhythmia drugs are 2-edged swords; they can be used to treat one type of arrhythmia, but at the same time become pro-arrhythmic to another. It is also known that their arrhythmiogenic effects depend on, to some extent, the pathophysiological conditions of the diseased heart. Taken together, the results presented in this paper suggest that patients with existing heart diseases should cautiously take NSAIDs, unless they are under close observation of the side-effects.
References
- Pratt CM, Hertz RP, Ellis BE, Crowell SP, Louv W, Moyé L. Risk of developing life-threatening ventricular arrhythmia associated with tefenadine in comparison with over-the-counter antihistamines, ibuprofen and clemastine. Am J Cardiol 1994;15:346-52.
- Wu ZG, Zhou XL, Shu CM. Arrhythmia induced by the use of ibuprofen. Chin Foreign Med J 2005;3:88-9.
- Shi YL, Wang YZ, Zhao XL. One case of atrial fibrillation induced by ibuprofen. J Med Theor Prac 2004;17:447.
- Huerta C, Varas-Lorenzo C, Castellsague J, Garcia Rodriguez LA. Non-steroidal anti-inflammatory drugs and risk of first hospital admission for heart failure in the general population. Heart 2006;92:1610-5.
- Hudson M, Richard H, Pilote L. Differences in outcomes of patients with congestive heart failure prescribed celecoxib, rofecoxib, or non-steroidal anti-inflammatory drugs: population based study. Br Med J 2005;300:1370-3.
- Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemop prevention trial. N Engl J Med 2005;352:1092-102.
- Yang ZF, Shi GZ, Li CZ, Wang HW, Liu K, Liu YM. Electrophysiologic effects of nicorandil on the guinea pig long QT1 syndrome model. J Cardiovasc Electr 2004;15:815-20.
- Lu HL, Li CZ, Liu YM, Weng EQ, Liu B. The effects of Guanxinling on coronary blood flow–contraction force of cardiac muscle and electrical activity of the heart. Chin Heart J 2004;16:323-6.
- Buchanan FG, Holla V, Katkuri S, Matta P, Dubois RN. Targeting cyclooxygenase-2 and the epidermal growth factor receptor for prevention and treatment of intestinal cancer. Cancer Res 2007;67:9380-8.
- Martinez-Gonzalez J, Badimon L. Mechanisms underlying the cardiovascular effects of Cox-inhibition: benefits and risks. Curr Pharm Res 2007;13:2215-27.
- Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet 2005;365:475-81.
- Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA 2001;286:954-9.
- Leroy S, Mosca A, Landre-Peigne C, Cosson MA, Pons G. Ibuprofen in childhood: evidence-based review of efficacy and safety. Arch Pediatr Adolesc Med 2007;3:51-8.
- Huang WF, Hsiao FY, Wen YW, Tsai YW. Cardiovascular events associated with the use of four nonselective NSAIDs (etodolac, nabumetone, ibuprofen, or naproxen) versus a cyclooxygenase-2 inhibitor (celecoxib): a population-based analysis in Taiwanese adults. Clin Ther 2006;28:1827-36.
- Johnsen SP, Larsson H, Tarone RE, McLaughlin JK, Norgard B, Friis S, et al. Risk of hospitalization for myocardial infarction among users of rofecoxib, celecoxib, and other NSAIDs: a population-based case-control study. Arch Intern Med 2005;165:978-84.
- Liu XK, Tosaki A, Engleman RM, Das DK. Salicylate reduces ventricular dysfunction and arrhythmias during reperfusion in isolated rat hearts. J Cardiovasc Pharm 1992;19:209-15.
- Liu XK, Tosaki A, Engleman RM, Das DK. Reduction of postischaemic ventricular dysfunction and arrhythmias by trapping hydroxyl radicals with salicylic acid. Int J Tissue React 1993;15:25-30.
- Cheruku KK, Ghani A, Ahmad F, Pappas P, Silverman PR, Zelinger A, et al. Efficacy of nonsteroidal anti-inflammatory medications for prevention of atrial fibrillation following coronary artery bypass graft surgery. Prev Cardiol 2004;7:13-8.
