Development and evaluation of lipid nanoparticles for camptothecin delivery: a comparison of solid lipid nanoparticles, nanostructured lipid carriers, and lipid emulsion
Introduction
Camptothecin is a potent antitumoral agent, first isolated from extracts of Camptotheca acuminate (“Xi Shu” in Chinese or “tree of joy”), and is a deciduous tree native to China[1]. Its insolubility in most biocompatible solvents has made camptothecin very difficult to deliver into the body through conventional routes, including orally or by an intravenous or intramuscular injection[2]. A number of camptothecin analogs are currently used for clinical treatment. However, none of these analogs surpasses camptothecin in potency of action[3]. The synthesis of these semisynthetic derivatives is also time consuming and expensive. Camptothecin thus appears to be a promising drug if the barriers against its clinical use can be resolved.
In the pharmaceutical field, several advantages of drug delivery systems in a nanosize range have been shown, including increasing solubility, reducing side-effects, prolonging pharmacological effects, and improving bioavailability[4,5]. Solid lipid nanoparticles (SLN) are a new generation of nanoparticulate active-substance vehicles, and are attracting major attention as novel colloidal drug carriers for parenteral use. SLN are particles made from crystalline solid lipids with a mean diameter of 50~1000 nm[6]. The distinct advantages of SLN are the solid state of their particle matrix, the ability to protect chemically-labile ingredients against chemical decomposition, and the possibility of modulating drug release. Nanostructured lipid carriers (NLC), composed of a solid lipid matrix with a certain content of a liquid lipid phase, are another generation of SLN[7]. SLN and NLC may overcome the problems of membrane stability and drug leaching associated with liposomes and conventional emulsions[8].
The development of lipid nanoparticles has received considerable attention in the field of cancer therapy[2]. Due to the lipophilic nature of their matrices, lipid nanoparticles are considered particularly useful for administering lipophilic drugs, such as camptothecin. They may protect camptothecin from rapid hydrolysis of its lactone ring in plasma to the essentially inactive carboxylate form[9]. Moreover, camptothecin requires a prolonged schedule of administration and drug targeting to expand its efficacy[10]. Some investigations have focused on using certain nanoparticles as carriers for camptothecin[2,11]. However, relatively little work has completely and comprehensively compared various types of nanoparticles with each other. The aim of this study was to compare physicochemical properties and cytotoxicity against cancer cells of SLN, NLC, and a lipid emulsion (LE). The feasibility of using camptothecin-loaded nanoparticle systems as a parenteral formulation was demonstrated through extensive characterization of the size, charge, molecular environment, drug release, cytotoxicity, safety, and stability.
Materials and methods
Materials Camptothecin, squalene, pluronic F68 (PF68), and Nile red were purchased from Sigma (St Louis, MO, USA). Precirol ATO5 and Compritol 888 ATO were obtained from Gattefossé (Gennevilliers, France). Myverol 18-04K was supplied by Quest (Naarden, the Netherlands). The cellulose membranes (Cellu-Sep T3, with a molecular weight cut-off of 3500) were supplied by Membrane Filtration Products (Seguin, TX, USA). The melanoma cell line (B16-F0) was purchased from American Type Culture Collection (Rockville, MD, USA).
Preparation of lipid nanoparticles The lipid and aqueous phases were separately prepared in glass vials. The lipid phase consisted of solid or liquid lipids, a lipophilic emulsifier (Myverol), and camptothecin, while the aqueous phase consisted of double-distilled water and a hydrophilic emulsifier (PF68). The 2 phases were separately heated to 85 °C for 15 min. The aqueous phase was added to the lipid phase and then mixed using a high-shear homogenizer (Pro 250; Pro Scientific, Monroe, CT, USA) for 5 min. The mixture was further treated using a probe-type sonicator (VCX600; Sonics and Materials, Newtown, CT, USA) for 10 min at 25-35 W. The whole systems consisted of the water phase, the lipid phase, and the lipid/water interphase. The compositions of the SLN, NLC, and LE are given in Table 1.
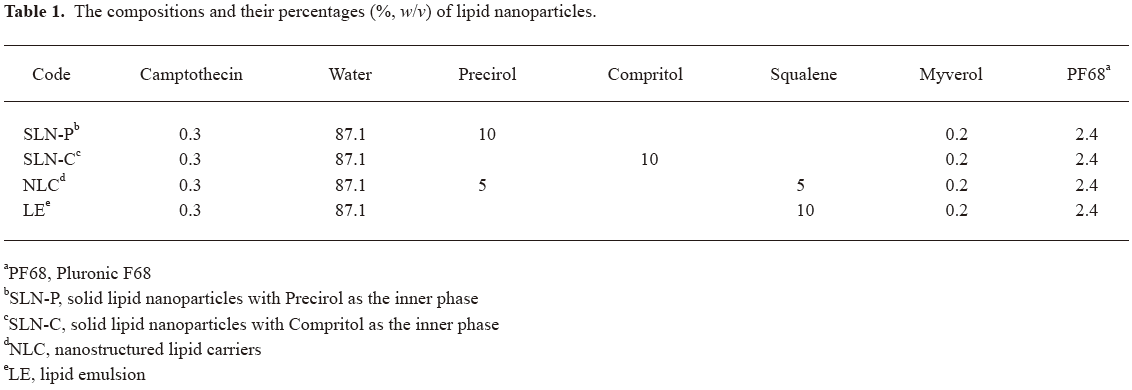
Full table
Determination of the mean diameter and surface charge The mean particle size (z-average) and zeta potential of the SLN, NLC, and LE were measured by photon correlation spectroscopy (Nano ZS90; Malvern, Worcestershire, UK) using a helium-neon laser with a wavelength of 633 nm. Photon correlations of spectroscopic measurements were carried out at a scattering angle of 90°. A 1:100 dilution of the formulations was made using double-distilled water before the measurement. The stability of the drug delivery systems was determined by monitoring the size and zeta potential at 37 °C for 28 d.
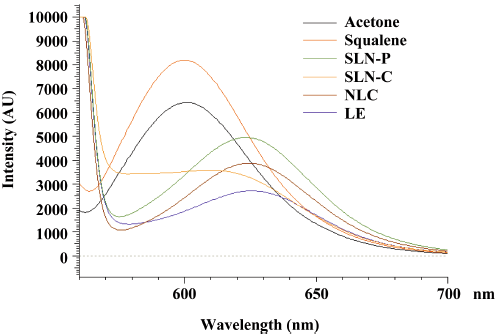
Molecular environment of lipid nanoparticles The lipophilic fluorescent marker, Nile red, was used as the model solute, and the molecular environment (polarity) was elucidated by fluorometric spectroscopy based on the solvatochromism of Nile red. The lipid nanoparticles with 1 ppm Nile red were prepared as described earlier. Emission fluorescence spectra were determined with a Hitachi F-2500 fluorescence spectrophotometer (Tokyo, Japan). The spectra of the drug carrier systems with Nile red were recorded at room temperature with both slit widths set to 10 nm. The excitation wavelength was fixed at 546 nm, and the emission spectra were recorded from 550 to 700 nm at a scanning speed of 300 nm/min.
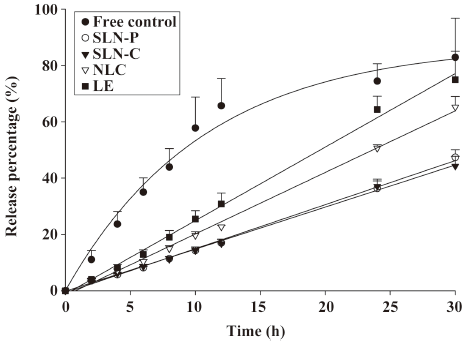
Camptothecin release from lipid nanoparticles Camptothecin release from the drug carrier systems was measured using a Franz diffusion cell. A cellulose membrane was mounted between the donor and receptor compartments. The donor medium consisted of 1 mL vehicle containing camptothecin. The receptor medium consisted of 10 mL of 30% ethanol in pH 7.4 buffer in order to maintain sink conditions during the experiments. The available diffusion area between cells was 1.767 cm2. The stirring rate and temperature were kept at 600 r/min and 37 °C, respectively. At appropriate intervals, 300 μL aliquots of the receptor medium were withdrawn and immediately replaced with an equal volume of fresh buffer. The amount of drug released was determined by HPLC. Camptothecin in solution was used as the control by dissolving camptothecin (3 mg) in a 10 mL mixture of polyethylene glycol 400, propylene glycol, and Tween 80 (40:58:2)[11].
HPLC analysis of camptothecin The HPLC system for camptothecin included a Hitachi L-7110 pump (Tokyo, Japan), a Hitachi L-7200 sample processor, and a Hitachi L-7485 fluorescence detector. A 25 cm-long, 4 mm inner diameter stainless steel RP-18 column (Merck, Darmstadt, Germany) was used. The mobile phase was an acetonitrile, a pH 4 aqueous solution adjusted by acetic acid (35: 65) at a flow rate of 1 mL/min. The wavelengths of excitation and emission were set to 360 and 440 nm, respectively.
Cytotoxicity assay Melanoma cells were seeded at an initial concentration of 1×104 cells/well in a 24-well plate, and incubated in 1 mL medium (10% fetal bovine serum, 89% Dulbecco’s modified Eagle’s medium, and 1% penicillin–streptomycin). Ten microliters of lipid nanoparticles with or without camptothecin diluted with medium was added at 24 h postinoculation, and the plates were incubated in a 5% CO2 atmosphere at 37 ºC for 12 and 24 h. The final concentration of camptothecin in the medium was 8.6 µmol/L. After washing with phosphate-buffered saline, the cells were incubated with 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in RPMI-1640 for 2 h at 37 °C. Formazan crystals resulting from MTT reduction were dissolved by adding 200 μL DMSO with gentle agitation for 30 min. The absorbance of the supernatant was then measured spectrophotometrically in an ELISA reader at 550 nm. Cell viability was calculated as the percentage of the control.
Erythrocyte hemolysis Blood samples were obtained from a healthy donor by venipuncture and collected into test tubes containing 124 mmol/L sodium citrate (1 volume of sodium citrate solution+9 volumes of blood). The erythrocytes were immediately separated by centrifugation at 2000×g for 5 min and washed 3 times with 4 volumes of a normal saline solution. Erythrocytes collected from 1 mL blood were resuspended in 10 mL normal saline. Immediately thereafter, 2.5 mL of 2% (w/v) dispersions of the formulations and mixtures in saline were incubated with 0.1 mL of the erythrocyte suspension. Incubations were carried out at 37 °C with gentle shaking of the test tubes. After 1 h of incubation, the samples were centrifuged for 5 min at 2000×g. The absorbance of the supernatant was measured at 415 nm to determine the percentage of cells undergoing hemolysis. Hemolysis induced with double-distilled water was taken as 100%.
Statistical analysis Statistical analysis of differences between different treatments was performed using unpaired Student’s t-test. A 0.05 level of probability was taken as the level of significance. An ANOVA test was also used.
Results
Mean diameter and zeta potential of lipid nanoparticles SLN made of Precirol (SLN-P) or Compritol (SLN-C) as the core material were stabilized with PF68 and Myverol. Precirol is a glycerol palmitostearate with a melting point of 58 °C. Compritol is a glycerol behenate consisting of mono-, di-, and triglycerides[12]. Squalene was used as the liquid matrix in the NLC and LE formulations. Squalene is an all-trans isoprenoid containing 6 isoprene units, which has been used without evidence of safety concerns according to the World Health Organization Weekly Epidemiological Record (14 July 2006). The lipid nanoparticle systems were developed by hot homogenization followed by ultrasonication. In the camptothecin-loaded mixtures, we observed no distinct, undissolved crystals. Even though the exact solubility of camptothecin in the inner phase could not be measured, it appeared that most of input drug was solubilized.
Values of the particle size and surface charge of the developed SLN, NLC, and LE are shown in Table 2. After production, the mean diameters of the particles were in the range of 190~310 nm, depending on the lipid loading. The characterization of the particle size revealed that the average diameter of the SLN-P was considerably smaller (P<0.05) than that of the SLN-C. The average diameter of the LE was comparable (P>0.05) to that of the SLN-P. NLC exhibited the smallest size (P<0.05) among the formulations tested. As depicted in Table 2, the zeta potentials of the lipid nanoparticles were negative. There was no significant difference (P>0.05) among the zeta potentials of the SLN and NLC formulations. The LE showed a surface charge of –12.6 mV, which was lower (P<0.05) than those of the systems with solid lipids (approximately -35 mV).
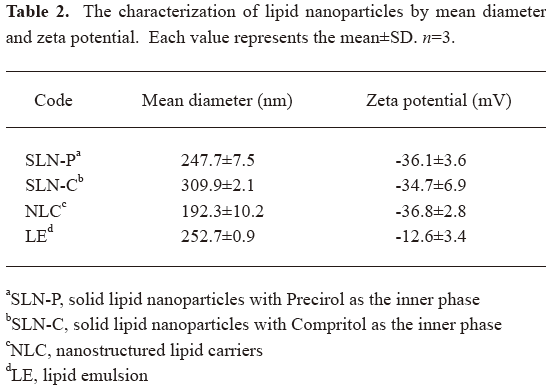
Full table
Molecular environment of lipid nanoparticles Nile red is a dye whose absorption bands vary in shape, position, and intensity with the nature of the environment. The emission spectra of Nile red in the nanoparticulate systems are shown in Figure 1. Nile red is very soluble in organic solvents, such as acetone, and strongly fluorescent in a lipophilic environment[13]. Corresponding to high lipophilicity, such as with acetone and squalene, the emission maxima were found to be near 600 nm. When incorporated into the nanoparticle systems, the increased re-orientation probability of the surrounding water molecules led to emission shifts to longer wavelengths. A reduction in the fluorescence intensity was observed for lipid nanoparticles as well. The results indicated a decreasing trend of intensity: LE<SLN-C<NLC<SLN-P.
Camptothecin release from lipid nanoparticles A key issue investigated in this study was the feasibility of using lipid nanoparticles to deliver camptothecin. The ability of nanoparticles to deliver camptothecin was examined by determining the drug release, as shown in Figure 2. The amount of camptothecin released from each formulation was plotted as a function of time. The free control showed a quick release of camptothecin. The inclusion of the drug in lipid nanoparticles significantly reduced the release. The release kinetics from nanoparticles could be fitted with a zero-order model. It was found that the release rate of the drug greatly depended on the inner phases in the lipid nanoparticles. Both SLN systems showed the most sustained release (P<0.05), with ~45% of total drug amount released within 30 h. A more rapid release of camptothecin from the NLC and LE was observed, with ~65% and ~75% of camptothecin being released within 30 h, respectively.
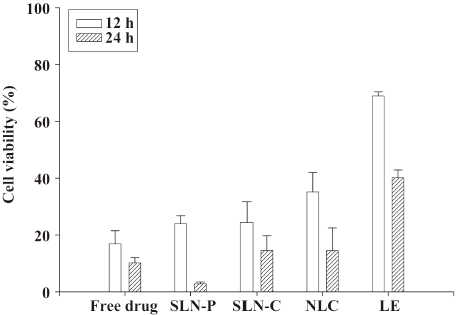
Cytotoxicity assay To assess the cytotoxicity of camptothecin-loaded formulations, their tumor-killing activity was determined against a melanoma cell line by MTT assay. The effects of lipid nanoparticles on the viability (%) of melanomas studied after incubation periods of 12 and 24 h are presented in Figure 3. A stronger inhibition of cell proliferation was observed with 24 h rather than 12 h of incubation. No statistically significant difference (P>0.05) in cell viability was found between free camptothecin and the drug-loaded SLN systems in the 12 h incubation experiment. Camptothecin loaded in the NLC and LE showed lower in vitro cytotoxic activities (P<0.05) than the free drug at 12 h. Camptothecin showed different cytotoxicity behaviors between the 12 and 24 h incubations. At 24 h, the SLN-C and NLC showed similar activities as the free control. It is surprising that camptothecin loaded in the SLN-P could almost completely inhibit the proliferation of melanoma cells. Empty nanoparticles without camptothecin were also tested, and it was found that the particles themselves had no effect on cytotoxicity. This indicates that the cytotoxicity toward the melanomas was mainly a consequence of the camptothecin molecules.
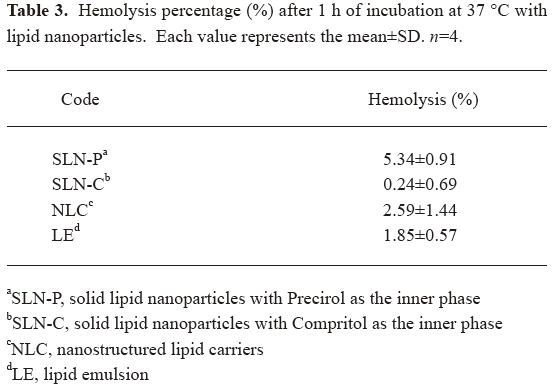
Erythrocyte hemolysis The use of lipid nanoparticles for parenteral administration is subject to rigorous demands to determine the non-toxicity of the formulation. To evaluate the safety of the nanoparticles themselves, the hemolytic activity was determined. As shown in Table 3, the hemolysis percentages of the SLN-P, SLN-C, NLC, and LE were 5.34%, 0.24%, 2.59%, and 1.85%, respectively. Thus all systems showed tolerable hemolysis of erythrocytes.
Full table
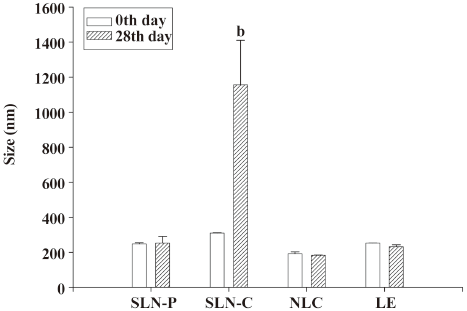
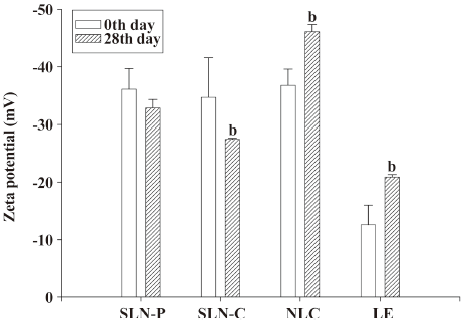
Storage stability After 28 d of storage at 37 °C, the mean diameter of the SLN-C had increased from 310 to 1157 nm, as observed in Figure 4. This suggests the instability of this system. The particle sizes of the SLN-P, NLC, and LE were maintained during the 28 d period (P>0.05). Concerning the zeta potentials of lipid nanoparticles in Figure 5, the surface charge of the SLN-P was almost unchanged (P>0.05) over 28 d. The zeta potential of the SLN-C decreased (P<0.05) during storage at 37 °C. Contrary to the case of the SLN-C, the addition of squalene to the lipid phase (with the NLC and LE) resulted in a slight but significant (P<0.05) increase in the zeta potential after storage.
Discussion
We are attempting to develop an approach which will permit the utilization of camptothecin, which is not currently used in clinical practice due to the lack of a suitable drug carrier system. Thus the use of drug delivery technologies, such as SLN and NLC, should permit effective formulations and utilization of this potent agent in cancer therapy. In this report we demonstrate that camptothecin can be effectively delivered using lipid nanoparticles, and that camptothecin-loaded nanoparticles displayed activity against melanomas, especially with SLN. Nano- or submicron-sized particles were obtained with the prepared formulations. The drug-containing nanoparticles remained within the injectable range for intravenous administration and were similar in size or smaller than fat emulsions presently being used for parenteral nutrition (200-400 nm)[14]. The difference of lipid types in the inner phase greatly influenced the physicochemical characteristics of the lipid nanoparticles.
It was observed that glycerides with longer alkyl chains (Compritol) produced larger particles compared to those with shorter chains (Precirol). The crystalline lipid core (of the SLN) produced larger diameter particles compared to that with the amorphous core (of the NLC). Below 70 °C, the viscosities of Precirol and Compritol increased rapidly[15]. In those case, the addition of a low viscosity oil might reduce the size distribution[16]. However, that was not the case for the LE, since squalene was the sole lipid present in the inner phase. The composition of the nanoparticle shell significantly affects the physicochemical properties and drug release profiles[17]. The particulate systems were stabilized with the surfactants PF68 [with a hydrophile–lipophile balance (HLB) of 29] and Myverol (with an HLB of 7). We found that the polarity of these systems particularly differed according to the molecular environment test. The surfactant system with a determined HLB may well stabilize NLC, which had a moderate polarity, but not the LE, which showed the highest polarity among all systems.
Surface potentials should play an important role in nanoparticle stability due to electrostatic repulsion. The LE exhibited a lower charge than either the SLN or NLC. Since PF68 is a non-ionic species, Myverol (palmitinic acid monoglycerides) and lipid cores may be responsible for the negative surface charges. The negative charge was likely caused by the slightly ionized fatty acids from glycerides including Precirol, Compritol, and Myverol[18]. This may explain the additional negative charges introduced by the SLN and NLC.
The lipophilic fluorescent marker, Nile red, was used as a model molecule, and the molecular environment was elucidated with a fluorescence spectrophotometer by utilizing the solvatochromism of Nile red. The emission spectrum of Nile red shifts to longer wavelengths with increasing environment polarity. The fluorescence is also quenched in a more-hydrophilic environment[19]. As shown in Figure 1, the SLN-P exhibited a more lipophilic environment than the others. The pronounced lipophilic character of Precirol expressed by a low HLB of 2 may have contributed to this result. The lipophilicity decreased following an increase in the squalene content in the formulations (LE>NLC>SLN-P). It should be noted that the SLN-C showed a lower intensity compared to the SLN-P and NLC. The nanoparticles exhibited high fluorescence intensity when the majority of the Nile red molecules were dissolved in the inner phase. A possible cause of the reduced intensity is the aggregation of Nile red and its relocation to a more polar environment[19]. Crystallization of lipids in the inner phase reduces the capacity to accommodate foreign molecules and caused the expulsion and aggregation of Nile red. Lipids which form highly crystalline particles with a perfect lattice, such as Compritol, may lead to stronger drug expulsion than Precirol. The incorporation of liquid lipids to solid lipids can lead to massive crystal order disturbances, and the resulting matrix of lipid particles exhibits great imperfections in the crystal lattice, thus leaving enough space to accommodate other molecules[7,20].
A zero-order release was suitable to fit the curve of all lipid nanoparticles during 30 h of administration (Figure 2), indicating the sustained release of the drug. Camptothecin shows a high octanol/water partitioning (logP=1.74)[21]. The water solubility of camptothecin is also low (1.34 μg/mL)[22]. It can be expected that most camptothecin molecules were included in the inner phase of lipid nanoparticles. The drug is stably retained in the lipid cores for a determined duration, followed by its slow release into the external phase. The release from the inner phase supplements the depletion of the drug from the external phase, supplying sustained and controlled delivery of the drug[23]. The zero-order fashion may also imply a membrane-controlled mechanism. The composition of emulsifiers in the interface was the same for all systems. This may suggest that the lipid phase could alter the characteristics of the interface, thus modulating the drug delivery. As demonstrated in Figure 2, it is possible to modify the release profiles as a function of the lipid matrix. SLN showed the slowest release rate. Drug mobility is drastically reduced in solidified or crystallized nanoparticles, which is a prerequisite for slow drug release[24,25].
The incorporation of liquid lipids into the solid lipid matrix caused the NLC to become more imperfect and allowed easier release of the loaded drug[7], thus increasing the camptothecin release rate when liquid lipids were included in the NLC matrix. Another possibility is that the smaller size of the NLC particles should increase the total surface area. An increasing release rate would therefore be expected. According to the Kelvin and Ostwald–Freundlich equation, for small particles, especially in the nanometer range, the saturation solubility would significantly increase. Both the increase in the saturation solubility and the enlargement of the surface area contribute to the enhancement of dissolution velocity according to the Noyes–Whitney equation[26]. However, that was not the case for the LE with a larger mean diameter, but a higher camptothecin release rate. This can be explained by traditional oil-in-water emulsions not being suited for slow release because of the low viscosity and rigidity of the dispersed liquid phase which causes rapid drug diffusion out of the lipid phase[24]. Lundberg[27] prepared emulsions to deliver camptothecin derivatives. He indicated that most drugs intercalated in the shell of the emulsion droplets. Hence the easier release of camptothecin from the shell monolayer may have contributed to the rapid drug release from the LE, although there were some differences in ingredients and preparation procedures between the emulsions developed by Lundberg and our laboratory.
Camptothecin is considered to be a potent drug against melanomas[28,29]. Camptothecin possesses a mechanism of action involving the inhibition of DNA relaxation by DNA topoisomerase I, and more specifically, the stabilization of a covalent binary complex formed between topoisomerase I and DNA[3]. The result of the in vitro cytotoxicity assay against melanomas indicated that the camptothecin-loaded SLN-P showed stronger inhibition of cell proliferation, followed by the SLN-C, NLC, and LE. The SLN-P even exhibited higher cytotoxicity than the free drug after a 24 h incubation. The cytotoxicities of the SLN-C and NLC were comparable to that of the free control. Camptothecin was slowly released from lipid nanoparticles. Hence the activity against melanomas might not have predominantly been due to the direct penetration of free camptothecin into the cytoplasm. Nanoparticles need to enter cells and diffuse through the viscous cytosol to access the particular cytoplasmic targets where the sites of action are located[30]. Cellular uptake of the drug can possibly occur by an endocytotic pathway of particles or by fusion of the particle surfaces with the cell membrane, leading to increased internalization of the nanoparticles and drug release inside the tumor[2,14]. Previous studies also show that SLN can enhance DNA transfection into the cells by an endocytosis process[31,32]. The SLN-P may exhibit excellent endocytotic activity to induce high cytotoxicity. Additional work is required to confirm this hypothesis.
There may be other molecular mechanisms involved in the increased cell uptake of the drug by the nanoparticles besides the influence of endocytosis or fusion. In the case of camptothecin, the pH of the microenvironment around tumor cells was found to affect drug uptake[33]. The acidic extracellular environment leads to a pH gradient unique to tumor cells. This gradient favors the uptake and retention of camptothecin and its analogs[9]. The lipid nanoparticles tested showed a pH range of 4.3-5.1, as measured by a pH meter. Moreover, as the protons in the systems are attracted to the negatively-charged particles, the pH of the microenvironment around the particles will be lower than the bulk pH[34]. This may also explain the low cytotoxicity of the LE, since it showed a lower negative surface charge compared to the SLN and NLC. The high cytotoxicity of the SLN-P is especially important for melanomas, since this tumor is highly resistant to chemotherapy and radiation therapy, and therapies for melanomas have generally been ineffective at best and have rarely resulted in sustained responses[35].
The hemolytic potential of the injectable forms has generally been found to correlate with the severity of lesions[36]. Any hemolytic effect is expected to be mediated by direct contact between the lipid/water interface and erythrocytes[23,37]. The overall results of the hemolysis study indicated that treatment with the developed nanoparticle systems was less toxic, suggesting the potential for therapeutic applications.
Besides the issue of safety, the physical stability of a nanoparticle system is one of the most important desired product characteristics. Lipid nanoparticles are heterogeneous systems and thermodynamically unstable, and therefore, may have a significant tendency to lose physical stability during storage. Measuring the zeta potential indicates the storage stability of colloidal dispersions[6]. In general, the zeta potential decreased to -20 to -30 mV, which is not enough for sufficient electrostatic stabilization[38]. However, the LE, which possessed a surface charge of -12.6 mV, showed good stability over 28 d of storage according to the size determination. A great proportion of the SLN-C aggregated to larger particles, although its surface charge was more negative than -30 mV. This may have been due to the crystallinity of Compritol. Freitas and Müller[12] indicated that the particle diameter of SLN composed of Compritol increased from 0.77 μm to 23.34 μm during a 3 d storage. The continuous recrystallization in the inner phase may have caused particle collisions and partial destruction of the lipid/water interface. The destruction of the particle shell may also have resulted in a reduction of surface charges of the SLN-C during storage.
In this study, we attempted to evaluate the feasibility of using lipid nanoparticles for camptothecin delivery. Nano- to submicron-sized particles were achieved with the lipid nanoparticles developed in this investigation, and the NLCs showed the smallest mean diameter. The controlled adjustment of camptothecin release could be attained by modifying the lipid matrix, with SLN showing the most sustained delivery followed by the NLC and LE. Camptothecin incorporated into the SLN-P showed a higher in vitro cytotoxicity against melanomas compared to the free drug form. However, the cytotoxicity of the LE was relatively lower than that of the free drug. All nanoparticle systems were shown to be well tolerated according to the erythrocyte hemolysis test. The experimental results indicated that the SLN-P obtained in this study could potentially be exploited as a novel camptothecin carrier with respect to its sustained drug release, high cytotoxicity to melanomas, low hemolysis, and good stability. The lipid nanoparticle approach can thus enhance the utilization of camptothecin as a potential tool for cancer therapy.
References
- Lorence A, Nessler CL. Camptothecin, over four decades of surprising findings. Phytochemistry 2004;65:2735-49.
- Hatefi A, Amsden B. Camptothecin delivery methods. Pharm Res 2002;19:1389-99.
- Thomas CJ, Rahier NJ, Hecht SM. Camptothecin: current perspectives. Bioorg Med Chem 2004;12:1585-604.
- Hung CF, Fang CL, Liao MH, Fang JY. The effect of oil components on the physicochemical properties and drug delivery of emulsions: tocol emulsion versus lipid emulsion. Int J Pharm 2007;335:193-202.
- Teeranachaideekul V, Müller RH, Junyaprasert VB. Encapsulation of ascorbyl palmitate in nanostructured lipid carriers (NLC)––effects of formulation parameters on physicochemical stability. Int J Pharm 2007;340:198-206.
- Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery -- a review of the state of the art. Eur J Pharm Biopharm 2000;50:161-77.
- Hu FQ, Jiang SP, Du YZ, Yuan H, Ye YQ, Zeng S. Preparation and characteristics of monostearin nanostructured lipid carriers. Int J Pharm 2006;314:83-9.
- Ruckmani K, Sivakumar M, Ganeshkumar PA. Methotrexate loaded solid lipid nanoparticles (SLN) for effective treatment of carcinoma. J Nanosci Nanotechnol 2006;6:1991-5.
- Adams DJ. The impact of tumor physiology on camptothecin-based drug development. Curr Med Chem Anti-Cancer Agents 2005;5:1-13.
- Hatefi A, Knight D, Amsden B. A biodegradable injectable thermoplastic for localized camptothecin delivery. J Pharm Sci 2004;93:1195-204.
- Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J Control Release 1999;59:299-307.
- Freitas C, Müller RH. Correlation between long-term stability of solid lipid nanoparticles (SLN) and crystallinity of the lipid phase. Eur J Pharm Biopharm 1999;47:125-32.
- Lombardi Borgia S, Regehly M, Sivaramakrishnan R, Mehnert W, Korting HC, Danker K, et al. Lipid nanoparticles for skin penetration enhancement––correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopy. J Control Release 2005;110:151-63.
- Lee MK, Lim SJ, Kim CK. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials 2007;28:2137-46.
- Jenning V, Thünemann AF, Gohla SH. Characterization of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int J Pharm 2000;199:167-77.
- Mason TG. New fundamental concepts in emulsion rheology. Curr Opin Colloid Interface Sci 1999;4:231-8.
- Kim BD, Na K, Choi HK. Preparation and characterization of solid lipid nanoparticles (SLN) made of cacao butter and curdlan. Eur J Pharm Sci 2005;24:199-205.
- Wong HL, Bendayan R, Rauth AM, Wu XY. Development of solid lipid nanoparticles containing ionically complexed chemotherapeutic drugs and chemosensitizers. J Pharm Sci 2004;93:1993-2008.
- Jores K, Haberland A, Wartewig S, Mäder K, Mehnert W. Solid lipid nanoparticles (SLN) and oil-loaded SLN studied by spectrofluorometry and Raman spectroscopy. Pharm Res 2005;22:1887-97.
- Souto EB, Wissing SA, Barbosa CM, Müller RH. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm 2004;278:71-7.
- Hansch C, Telzer BR, Zhang L. Comparative QSAR in toxicology: examples from teratology and cancer chemotherapy of aniline mustards. Crit Rev Toxicol 1995;25:67-89.
- Kang J, Kumar V, Yang D, Chowdhury PR, Hohl RJ. Cyclodextrin complexation: influence on the solubility, stability, and cytotoxicity of camptothecin, an antineoplastic agent. Eur J Pharm Sci 2002;15:163-70.
- Wang JJ, Sung KC, Hu OYP, Yeh CH, Fang JY. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J Control Release 2006;115:140-9.
- Westesen K, Bunjes H, Koch MHJ. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J Control Release 1997;48:223-36.
- zur Mühlen A, Schwarz C, Mehnert W. Solid lipid nanoparticles (SLN) for controlled drug delivery -- drug release and release mechanism. Eur J Pharm Biopharm 1998;45:149-55.
- Hou DZ, Xie CS, Huang KJ, Zhu CH. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials 2003;24:1781-5.
- Lundberg BB. Biologically active camptothecin derivatives for incorporation into liposome bilayers and lipid emulsions. Anti-Cancer Drug Design 1998;13:453-61.
- Cheung KJ, Li G. The tumor suppressor p33ING1 does not enhance camptothecin-induced cell death in melanoma cells. Int J Oncol 2002;20:1319-22.
- Quotob SS, Ng CE. Comparison of apoptotic, necrotic and clonogenic cell death and inhibition of cell growth following camptothecin and X-radiation treatment in a human melanoma and a human fibroblast cell line. Cancer Chemother Pharmacol 2002;49:167-75.
- Vasir JK, Reddy MK, Labhasetwar VD. Nanosystems in drug targeting: opportunities and challenges. Curr Nanosci 2005;1:47-64.
- Tabatt K, Kneuer C, Sameti M, Olbrich C, Müller RH, Lehr C, et al. Transfection with different colloidal systems: comparison of solid lipid nanoparticles and liposomes. J Control Release 2004;97:321-32.
- Asasutjarit R, Lorenzen SI, Sirivichayakul S, Puxrungtham K, Ruktanonchai U, Ritthidej GC. Effect of solid lipid nanoparticles formulation compositions on their size, zeta potential and potential for in vitro pHIS-HIV-Hugag transfection. Pharm Res 2007;24:1098-107.
- Adams DJ, Dewhirst MW, Flowers JL, Gamcsik MP, Colvin OM, Manikumar G, et al. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother Pharmacol 2000;46:263-71.
- Pongcharoenkiat N, Narsimhan G, Lyons RT, Hem SL. The effect of surface charge and partition coefficient on the chemical stability of solutes in o/w emulsions. J Pharm Sci 2002;91:559-70.
- Tang CH, Grimm EA. Depletion of endogenous nitric oxide enhances cisplatin-induced apoptosis in a p53-dependent manner in melanoma cell lines. J Biol Chem 2004;279:288-98.
- Bjerregaard S, Wulf-Andersen L, Stephens RW, Lund LR, Vermehren C, Söderberg I, et al. Sustained elevated plasma aprotinin concentration in mice following intraperitoneal injections of w/o emulsions incorporating aprotinin. J Control Release 2001;71:87-98.
- Ahyayauch H, Goni FM, Bennouna M. Interaction of electrically neutral and cationic forms of imipramine with liposome and erythrocyte membranes. Int J Pharm 2004;279:51-8.
- Weyenberg W, Filev P, Van den Plas D, Vandervoort J, De Smet K, Sollie P, et al. Cytotoxicity of submicron emulsions and solid lipid nanoparticles for dermal application. Int J Pharm 2007;337:291-8.
