Transport and metabolism of flavonoids from Chinese herbal remedy Xiaochaihu-tang across human intestinal Caco-2 cell monolayers1
Introduction
Xiaochaihu-tang is a combination of seven herbs, including Radix Bupleuri (chaihu in Chinese), Radix Scutellariae (huangqin), Rhizoma Pinelliae (banxia), Radix Ginseng (renshen), Radix Glycyrrhizae (gancao), Rhizoma Zingiberis Recens (shengjiang), and Fructus Jujubae (dazao). This herbal remedy was first described by Zhong-jing ZHANG (150 to 219 A.D in Chinese Eastern Han Dynasty) in his Shang Han Lun, a treatise on febrile diseases. Several controlled clinical trials have shown that Xiaochaihu-tang (also called Shosaiko-to in Japan) can be useful in the treatment of chronic hepatitis[1,2], and may decrease hepatic fibrosis and prevent hepatocellular carcinoma development in patients with chronic liver diseases[3]. However, data on absorption and disposition of the bioactive herbal constituents are scarce[4–7], which is very relevant to helping understand the link between the consumption of Xiaochaihu-tang and its medicinal effects.
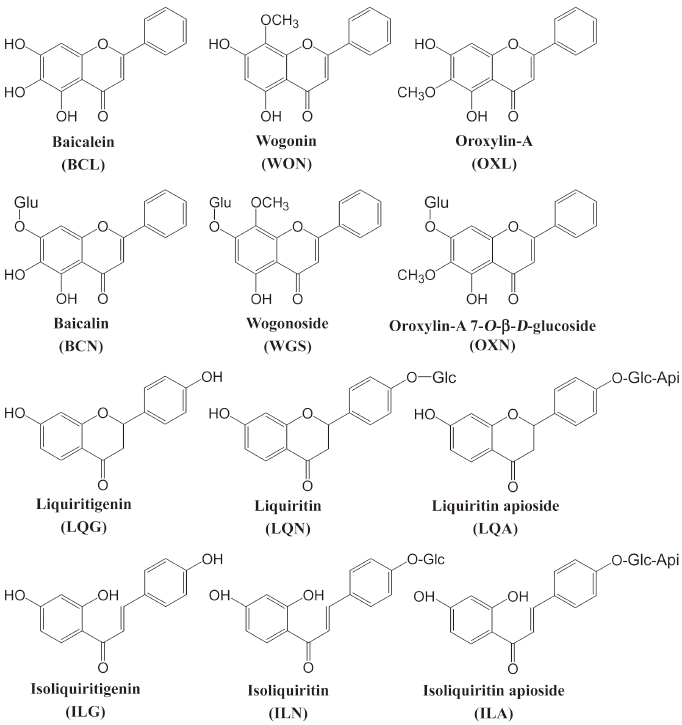
Flavonoids are one of the classes of putatively active constituents present in Xiaochaihu-tang, which have anti-inflammatory[8], anti-oxidant[9], anti-viral[10] and immunomodulating effects[11] and induce cancer cell apoptosis[12]. As part of our ongoing studies of Xiaochaihu-tang[13–15], we investigated intestinal permeability and metabolism of several important flavonoids and their aglycones using Caco-2 cell monolayers. In addition, a chemoinformatic approach was also used to assess the absorption properties of the investigational compounds. The present study aimed to understand some relevant mechanisms governing the oral bioavailability of these compounds. The major flavonoids present in Xiaochaihu-tang are baicalin, wogonoside, and oroxylin-A 7-O-β-D-glucopyranosiduronide from Radix Scutellariae and liquiritin, liquiritin apioside, isoliquiritin, and isoliquiritin apioside from Radix Glycyrrhizae, whereas their aglycones are present at lower levels[7]. Because flavonoids, reaching the colon either as unabsorbed flavonoids in the small intestine or as absorbed flavonoids secreted as conjugates into duodenum via biliary excretion[16], can be stripped of their sugar moieties by colonic microflora, the aglycones of the investigational Xiaochaihu-tang flavonoid glycosides were also included in the current study. These compounds were baicalein, wogonin, oroxylin-A, liquiritigenin, and isoliquiritigenin. The chemical structures of the investigational flavonoids are shown in Figure 1.
Materials and methods
Chemicals and reagents Purified baicalein (BCL; C15H10O5; MW: 270), baicalin (BCN; C21H18O11; 446), wogonin (WON; C16H12O5; 284), wogonoside (WGS; C22H20O11; 460), oroxylin-A (OXL; C16H12O5; 284), and oroxylin-A 7-O-β-D-glucopyranosiduronide (OXN; C22H20O11; 460), liquiritigenin (LQG; C15H12O4; 256), liquiritin (LQN; C21H22O9; 418), liquiritin apioside (LQA; C26H30O13; 550), isoliquiritin (ILN; C21H22O9; 418), and isoliquiritin apioside (ILA; C26H30O13; 550) were obtained either from the Phytochemistry Department of the Shanghai Institute of Materia Medica (Shanghai, China) or from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The purity of these compounds was ≥99.0%. Isoliquiritigenin (ILG; C15H12O4; 256; >98.0%) was purchased from Extrasynthèse (Genay, France). For transport study, the investigational compound was first dissolved in ethanol and then diluted with Hanks’ balanced salt solution (HBSS) to the desired concentration to yield the investigational compound solution. The final concentrations of ethanol in the solutions were not greater than 1%.
Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin, and MEM non-essential amino acids were obtained from Gibco Invitrogen Corporation (Grand Island, NY, USA). Fetal bovine serum (FBS) was supplied by Hyclone (Logan, UT, USA). HBSS, propranolol, atenolol, rhodamine123, verapamil, sulfasalazine, and indomethacin were obtained from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade water was prepared with an in-house Millipore Direct-Q 3 UV water purification system (Bedford, MA, USA).
Cell culture Caco-2 cells were purchased from American Type Culture Collection (passage no. 34; Manassas, VA, USA). Cell cultures were maintained at 37 °C in a humidified, 5% CO2 incubator. The cells were grown in 75-cm2 TC flasks in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 1% MEM non-essential amino acids. After harvesting at 90% confluence, the cells were seeded on 0.4-µm Millicell-PCF filter inserts (12-mm diameter; Bedford, MA, USA) at a density of 1×105 cells/cm2. To feed the seeded cells, the growth media were changed the day after seeding and every other day thereafter, ie, 400 and 600 µL in the apical and basolateral compartments, respectively. The integrity of cell monolayers was evaluated by measuring transepithelial electrical resistance (TEER) values with a Millicell electrical resistance system (Bedford, MA, USA). The cell monolayers were used for transport study on day 21 postseeding with TEER >400 Ω⋅cm2.
Transport experiments Before use, the Caco-2 cell monolayers were washed twice with pre-warmed HBSS (37 °C). Bidirectional transport experiments, ie, apical→basolateral and basolateral→apical, were conducted in triplicate at the concentration 5 µg/mL for OXL or BCL, 10 µg/mL for BCN, 20 µg/mL for WON, WGS, OXN, LQG, LQN, LQA, ILG, ILN, or ILA in HBSS (the investigational compound solutions). In the apical→basolateral experiment, the investigational compound solution (0.4 mL) was added into the apical chamber and 0.6 mL of HBSS to the basolateral side. Meanwhile, for the basolateral→apical study, 0.6 mL of the investigational compound solution and 0.4 mL of HBSS were added into the basolateral and the apical compartments, respectively. Samples (100 µL) were collected from the receiver compartment at 0, 30, 60, 90, and 120 min after the initiation of incubation. Sampling from the donor side was also carried out at 120 min to determine the recovery of the investigational compound. Propranolol (control compound of high permeability), atenolol (control compound of low permeability), rhodamine 123 (selective P-glycoprotein [P-gp] probe substrate), verapamil (P-gp inhibitor), sulfasalazine (multidrug resistance-associated protein [MRP] probe substrate), and indomethacin (MRP inhibitor) were also tested to evaluate the applicability of the cell monolayers. The apparent permeability coefficient, Papp, expressed in cm/s was calculated according to the following equation:
Papp = (ΔQ/Δt) / (A×C0)
where ΔQ/Δt is the linear appearance rate of the investigational compound on the receiver side in µmol/s, A is the surface area of the cell monolayer in cm2, and C0 is the initial concentration of the investigational compound on the donor side in µmol/L.
In silico assessment of permeability and solubility Chemoinformatic assessment of the physicochemical properties governing intestinal absorption was carried out for the investigational compounds. Number of hydrogen bond donors (HBD), number of hydrogen bond acceptors (HBA), number of rotatable bond (NROTB), and topological polar surface area (TPSA) were calculated using Molispiration Property Calculator (Molinspiration Cheminformatics, Bratislava, Slovak Republic). Aqueous solubility (LogS) and the partition coefficient (LogP) were determined using ALOGPS program[17] (http://www.vcclab.org). The permeability was predicted according to Lipinski’s Rule of 5[18] and the molecular surface properties.
Liquid chromatography/tandem mass spectrometry analysis Biological samples taken from the transepithelial permeability study were directly applied to liquid chromatography/tandem mass spectrometry (LC/MS/MS) analysis. The LC/MS/MS system consisted of a Thermo Finnigan TSQ Quantum triple stage quadrupole mass spectrometer (San Jose, CA, USA) interfaced with an Agilent 1100 series liquid chromatograph (Waldbronn, German). Finnigan Xcalibur and Agilent Chemstation software packages were applied to control the analytical system, as well as for data acquisition and processing. Samples were separated on a 5 µm Kromasil C18 column (50×2.1 mm i.d.; Chadds Ford, PA, USA) with a 0.2 µm pre-column filter (Upchurch Scientific, Oak Harbor, WA, USA). The LC mobile phase consisted of acetonitrile and water (except for BCL using mobile phase modified with 0.01% formic acid), which was delivered at a flow rate of 0.2 mL/min. A binary pulse gradient elution was carried out, which consisted of an initial isocratic elution at 0% acetonitrile from 0 to 0.5 min, followed by a 0.1-min increase of acetonitrile from 0 to 100% and then maintained acetonitrile at 100% for 2.7 min. At 3.3 min, acetonitrile was quickly returned to 0% and maintained until 7 min. The retention times of the investigational flavonoids ranged from 1.9 to 2.5 min. The MS/MS parameters in the negative-ion ESI mode were tuned to maximize generation of deprotonated molecule for the analyte, except for BCL in the positive-ion mode. The precursor-to-product ion pair used for selected reaction monitoring of BCL, BCN, WON, WGS, OXL, OXN, LQG, LQN, LQA, ILG, ILN, or ILA was m/z 271→123, 445→269, 283→268, 459→283, 283→268, 459→283, 255→119, 417→255, 549→255, 255→119, 417→255, or 549→255, respectively. Only LC eluent flow over a period of 1.5–3.0 min was introduced to the mass spectrometer for data acquisition. The linear dynamic ranges for the investigational compounds were 200–5000 ng/mL for BCL, WON, LQG, and ILG, and 10–2500 ng/mL for BCN, WGS, OXL, OXN, LQN, LQA, ILN, and ILA. Assay validation was conducted to demonstrate that the performance of the developed method was suitable and reliable for the intended application[19].
Glucuronide/sulfate profiling of the investigational flavonoids was conducted using the method described by Li et al[20]. In brief, initial detection of the possible glucuronides and sulfates was conducted by using full-scan LC/MS (m/z 200–900) in the negative-ion ESI mode (except for those of BCL in the positive-ion mode) according to predicted gains, ie, 176 Da for the glucuronides and 80 Da for the sulfates, in molecular mass of the metabolites compared with those of the parent compounds. In the second step, the glucuronide and sulfate candidates were selectively characterized by using LC/MS/MS and neutral loss scan of 176 and 80, respectively.
Results
Bidirectional transport of flavonoids across Caco-2 cell monolayers Before use, the Caco-2 cell monolayers were assessed with respect to barrier properties using model compounds known for passive diffusion, as well as the selective probe substrates and inhibitors of the efflux transporters. Propranolol and atenolol demonstrated Papp values of 31×10-6 and 0.21×10-6 cm/s, respectively, which are comparable to the reported data[21]. The results indicated that the monolayers could discriminate highly and lowly permeable compounds. Without the P-gp inhibitor verapamil, rhodamine 123 exhibited substantial directional preference with the efflux ratio (Papp(basolateral→apical)/Papp(apical→basolateral)) of 5.9, which was significantly reduced to 0.5 in the presence of verapamil. For sulfasalazine, the presence of the MRP inhibitor indomethacin caused reduction of efflux ratio from 6.2 to 0.9. These results confirmed that P-gp and MRP were present in the Caco-2 cell monolayers.
The Papp values of the investigational flavonoids are summarized in Table 1. The measured Papp data suggested that the membrane permeability of the aglycones were significantly better (3.51×10-6–22.7×10-6 cm/s) than that of their corresponding glycosides (0.04×10-6–0.45×10-6 cm/s). Among the aglycones, LQG demonstrated the highest membrane permeability (apical→basolateral), followed by WON, OXL, ILG, and BCL. For the glycosides, the magnitude of permeability varied to some extent as the number and type of sugar moiety changed, and the Papp values of basolateral→apical tended to be greater than the corresponding data of apical→basolateral. Notably, the glycosides OXN, ILN, and ILA exhibited substantially different bidirectional Papp values with efflux ratio between 5 and 10, suggesting the involvement of carrier-mediated transport of these compounds across the Caco-2 cell monolayers.
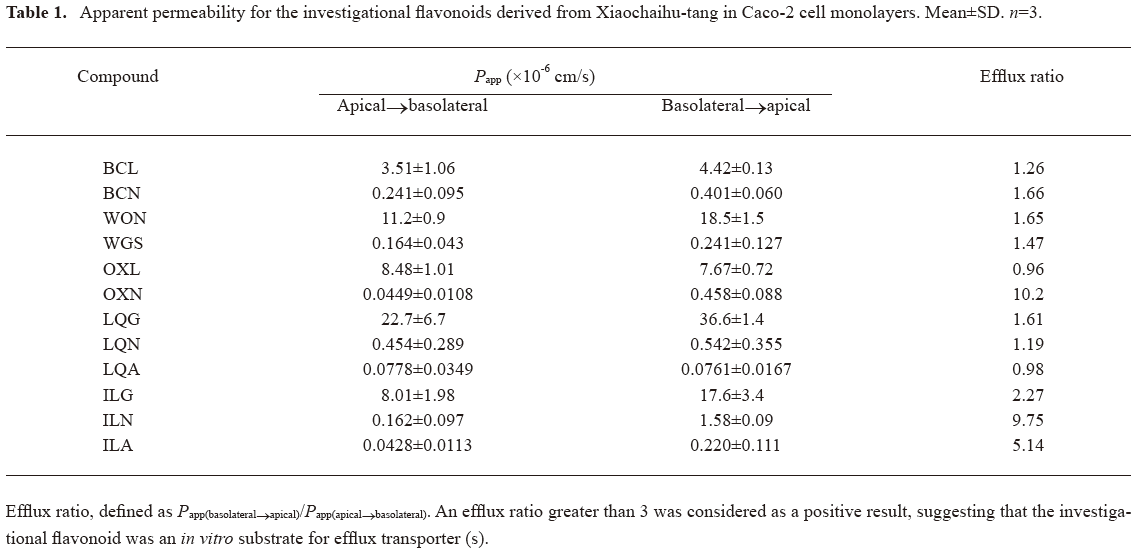
Full table
To identify the efflux transporters involved, bidirectional transport of OXN or ILN across Caco-2 monolayers was examined in the presence and absence of the transporter inhibitor. ILA, in contrast had very low membrane permeability and was not considered in further study. In the presence of indomethacin (50 µmol/L), the efflux ratios of OXN and ILN were significantly reduced (0.8 and 3.0, respectively) compared with the data (10.2 and 9.75, respectively) without the MRP inhibitor. The presence of the P-gp inhibitor verapamil (100 µmol/L) caused the efflux ratio of ILN reduction from 9.7 to 3.8, but did not influence the ratio of OXN. These results suggested that both MRP and P-gp were involved in the efflux of ILN. Meanwhile, only MRP was found to mediate the efflux of OXN.
Chemoinformatics assessment of physiochemical descriptors influencing flavonoids absorption To better understand the mechanism governing membrane permeability, chemoinformatics was carried out for the investigational flavonoids. Table 2 summarizes the calculated molecular and structural descriptors of the flavonoids. The in silico assessment was mainly focused on aqueous solubility and membrane permeability, two key factors affecting the drug property of oral absorption. All of the investigational flavonoids could be defined as soluble, demonstrating S values ranging from 55 to 1720 µg/mL, which were greater than the corresponding initial concentrations (C0) in the Caco-2 study (5–20 µg/mL). Important properties for determining membrane permeability are the molecule size, lipophilicity, ionization, and capacity to make hydrogen bonds[22,23]. The investigational aglycones had favorable properties for supporting good permeability, including molecular weight (MW: 256–284 Da; the favorable value <500 Da), lipophilicity (logP: 2.79–3.47; <5), hydrogen-bonding capacity (HBA+HBD: 6–8; <12 and TPSA: 67–91; <140 Å2), and molecular flexibility (NROTB: 1–3; <10). In contrast, poor membrane permeability of the flavonoid glycosides across Caco-2 cell monolayers might be attributed to their high hydrogen-bonding potential, demonstrating HBA+HBD ranging from 14 to 21 and TPSA from 146 to 216 Å2.
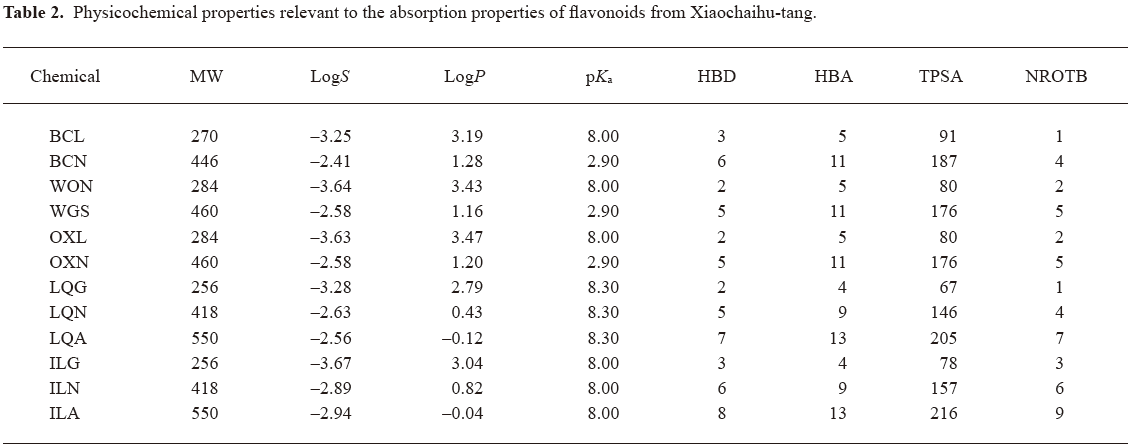
Full table
Glucuronidation and sulfation of flavonoids in Caco-2 cell monolayers The Caco-2 cells exhibit many morphological and biochemical features of adult human enterocytes including the expression of the phase II enzymes UDP-glucuronosyltransferases (UGT; including UGT1A3, UGT1A6, and UGT2B7)[24] and sulfotransferases (SULT; such as SULT1A1, SULT1A2, SULT1A3, SULT1B1, SULT1C1, SULT1C2, and SULT2A1)[25]. Formation of glucuronides or sulfates was not observed for the glycosides with the transport recoveries ranging from 85% to 122%, except for ILN (recovery: 82%). In contrast, the investigational flavonoid aglycones were metabolized by Caco-2 cells, including glucuronidation and/or sulfation (Table 3). The recoveries of the aglycones were between 72% and 79%. Monoglucuronides of BCL, WON, and OXL were detected both on the apical and basolateral sides as early as 30 min after apical loading, which were BCN, WGS, and OXN, respectively. The peak abundance of the BCL glucuronide was markedly greater than that of WON or OXL glucuronides. Meanwhile, glucuronide was also measured for LQG, but not for ILG. In addition, monosulfates were found on both sides for LQG, ILG, and ILN. The excretion of metabolites out of the cells seemed to be polarized. More glucuronidated BCL, WON, and OXL and sulfated LQG, ILG, and ILN were excreted into the apical media (1.8–4.6 fold) than into the basolateral ones, whereas glucuronidated LQG were preferentially excreted into the basolateral medium (1.4 fold more than the apical datum).
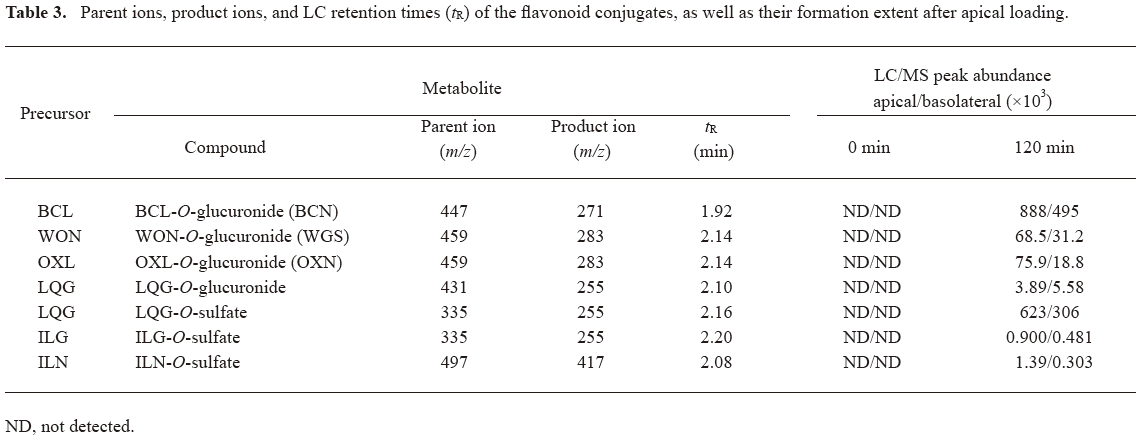
Full table
Discussion
Oral bioavailability is one of the most important pharmacokinetic properties for drugs, which has been traditionally defined as the fraction of the dose that reaches the systemic circulation. Various intestinal disposition processes such as permeation, efflux, and intestinal metabolism may affect the oral bioavailability of flavonoids, and microflora metabolism in the colon may introduce additional complexity to the intestinal disposition[16,26]. Due to their high hydrogen-bonding potential, the investigational flavonoid glycosides (BCN, WGS, OXN, LQN, LQA, ILN, and ILA) were poorly transported across the Caco-2 cell monolayers. It is assumed that flavonoids in general are absorbed as their aglycones after prior hydrolysis of the glycosides along the gastrointestinal tract[27,28]. For these glycosides, their aglycones (BCL, WON, OXL, LQG, and ILG) exhibited favorable in vitro membrane permeability, which was supported by their relevant physicochemical properties acquired via in silico calculation. Our transport and metabolism data of BCL are consistent with those reported by Zhang et al[29] and Akao et al[30]. Meanwhile, Asano et al[31] reported bidirectional Papp values for LQG comparable with our data, but their efflux ratios for LQN and LQA were significantly higher than ours. The reason for discrepancy might be associated in part with poor membrane permeability of these two glycosides, as well as the different experimental conditions used in the studies.
Transport of drugs across the intestinal epithelial is often accompanied by phase II conjugation reactions, which may profoundly affect the drug absorption. The conjugated metabolites measured in our Caco-2 study suggested that the intestinal absorption of the aglycones might be limited by efficient glucuronidation and/or sulfation by the enterocytes. The observed differences in the conjugation pattern among these investigational flavonoids might be due to non-uniform affinities of UGT and SULT for the compounds. Meanwhile, except for ILN sulfation, the conjugative metabolism of both types generally did not occur for the flavonoid glycosides, the reason for which might be the steric hindrance caused by the bulky sugar moiety attached. Wen and Walle reported some methylated flavone aglycones having improved metabolic stability compared with unmethylated flavone aglycones[32]. Our current study added more evidence with additional methylated (WON and OXL) and unmethylated flavones (BCL) to support these researchers’ conclusions.
Membrane transporters, especially the efflux transporters P-gp and MRP2, have been known to affect the extent of absorption and oral bioavailability of drugs. For flavonoids, P-gp does not seem to be involved in their transport[26], but recently Wang et al[33] reported this transporter mediating possible efflux of Ginkgo flavonoids. In the current study, we found that the in vitro transport of OXN, ILN, and ILA was mediated by the efflux transporter. Notably, besides MRP we also observed that P-gp was efficiently involved in the carrier-mediated transport of ILN. For licorice flavonoids, the absorptive transport (apical→basolateral) of the chalcones (ILG, ILN, and ILA) was uniformly slower than that of the corresponding flavonones (LQG, LQN, and LQA, respectively). Considering that the two classes of the flavonoids possess very close physicochemical properties, the efflux-transporter mediation was speculated to contribute to the difference in the in vitro absorptive transport.
In silico calculation of the detected glucuronic acid and sulfate conjugates of the flavonoids (data not shown) suggested that their poor membrane permeability was due to the elevated hydrogen-bonding capacity and increased polarity, especially for the glucuronides exceeding the favorable values. Occurrence of these conjugated metabolites on both sides of Caco-2 cell monolayers suggested possible involvement of both the apical and basolateral transport proteins for the metabolite efflux. Efflux proteins of the MRP family present in Caco-2 cells were found to eliminate glucuronides and sulfates of some xenobiotics[34]. Apical MRP2 has been demonstrated to be an important efflux transporter for glucuronic acid and sulfate conjugates of flavonoids[35]. Which transporters mediate the metabolite efflux on both sides requires further investigation. Conjugated flavonoids may function as inactive pools for their aglycones, prevent the aglycones from enzymatic oxidation, and extend the half-lives. The potential importance of hydrolysis of flavonoid glucuronides and re-formation of the aglycones in the liver and the serum/plasma has been addressed by some researchers[36,37]. The detection of the conjugated metabolites on the basolateral side of Caco-2 cell monolayers suggested that the traditional definition of oral bioavailability may not necessarily be meaningful for the naturally occurring flavonoids. Conjugated flavonoids measured in the systemic circulation may need to be involved, along with the unchanged flavonoids, in the calculation of oral bioavailability.
In summary, our data demonstrate that the limiting factors of oral bioavailability for flavonoids derived from Xiaochaihu-tang appeared to involve poor transport of the glycosides across the enterocyte and efficient metabolism of the aglycones. In addition, the efflux transporters might also negatively contribute to the transport of some flavonoid glycosides.
References
- Hirayama C, Okumura M, Tanikawa K, Yano M, Mizuta M, Ogawa N. A multicenter randomized controlled clinical trial of Shosaiko-to in chronic active hepatitis. Gastroenterol Jpn 1989;24:715-9.
- Tajiri H, Kozaiwa K, Ozaki Y, Miki K, Shimizu K, Okada S. Effect of Sho-saiko-to (Xiao-chai-hu-tang) on HBeAg clearance in children with chronic hepatitis B virus infection and with sustained liver disease. Am J Chin Med 1991;19:121-9.
- Oka H, Yamamoto S, Kuroki T, Harihara S, Marumo T, Kim SR, et al. Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9). Cancer 1995;76:743-9.
- Uchida E, Fukasawa I, Matuszaki Y, Inagaki M, Uchida N, Takeda S, et al. Pharmacokinetics and pharmacodynamics of TJ-9 after single administration in Japanese healthy male volunteers. Jpn Pharmacol Ther 1995;23:659-70.
- Li C, Homma M, Oka K. Chromatographic identification of phenolic compounds in human urine following oral administration of the herbal medicines Daisaiko-to and Shosaiko-to. J Chromatogr B 1997;693:191-8.
- Li C, Homma M, Ohkura N, Oka K. (S)-Dihydrooroxylin A in human urine following oral administration of the traditional Chinese medicines: Daisaiko-to and Shosaiko-to. Tetrahedron Asymmetry 1997;8:1145-7.
- Li C, Homma M, Oka K. Characteristics of delayed excretion of flavonoids in human urine after administration of Shosaiko-to, a herbal medicine. Biol Pharm Bull 1998;21:1251-7.
- Woo KJ, Lim JH, Suh SI, Kwon YK, Shin SW, Kim SC, et al. Differential inhibitory effects of baicalein and baicalin on LPS-induced cyclooxygenase-2 expression through inhibition of C/EBPbeta DNA-binding activity. Immunobiology 2006;211:359-68.
- Chang WT, Shao ZH, Yin JJ, Mehendale S, Wang CZ, Qin Y, et al. Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur J Pharmacol 2007;566:58-66.
- Guo O, Zhao L, You O, Yang Y, Gu H, Song G, et al. Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res 2007;74:16-24.
- Ohtake N, Nakai Y, Yamamoto M, Ishige A, Sasaki H, Fukuda K, et al. The herbal medicine Shosaiko-to exerts different modulating effects on lung local immune responses among mouse strains. Int Immunopharmacol 2002;2:357-66.
- Chao JI, Su WC, Liu HF. Baicalein induces cancer cell death and proliferation retardation by the inhibition of CDC2 kinase and surviving associated with opposite role of p38 mitogen-activated protein kinase and AKT. Mol Cancer Ther 2007;6:3039-48.
- Bao YW, Li C, Shen HW, Nan FJ. Determination of saikosaponin derivatives in Radix bupleuri and in pharmaceuticals of the Chinese multiherb remedy Xiaochaihu-tang using liquid chromatographic tandem mass spectrometry. Anal Chem 2004;76:4208-16.
- Chen P, Li C, Liang SP, Song GQ, Sun Y, Shi YH, et al. Characterization and quantification of eight water-soluble constituents in tubers of Pinellia ternata and in tea granules from the Chinese multiherb remedy Xiaochaihu-tang. J Chromatogr B 2006;843:183-93.
- Li L, Liang SP, Du FF, Li C. Simultaneous quantification of multiple licorice flavonoids in rat plasma. J Am Soc Mass Spectrom 2007;18:778-82.
- Hollman PCH, Katan MB. Absorption, metabolism, and bioavailability of flavonoids. In: Rice-Evans CA, Packer L, editors. Flavonoids in health and disease. New York, Basel, Hong Kong: Marcel Dekker, Inc; 1998. p 483–522.
- Tetko IV, Gasteiger J, Todeschini R, Mauri A, Livingstone D, Ertl P, et al. Virtual computational chemistry laboratory – design and description. J Comput Aid Mol Des 2005;19:453-63.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23:3-25.
- US FDA guidance for industry on bioanalytical method validation, 2001; http://www.fda.gov/cder/guidance/index.htm
- Li C, Meng XF, Winnik B, Lee MJ, Lu H, Sheng SQ, et al. Analysis of urinary metabolites of tea catechins by liquid chromatography/electrospray ionization mass spectrometry. Chem Res Toxicol 2001;14:702-7.
- Artursson P, Karlsson J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 1991;175:880-5.
- Van de Waterbeemd H, Gifford E. ADMET in silico modelling: towards prediction paradise? Nat Rev Drug Discov 2003;2:192-204.
- Hou TJ, Wang JM, Zheng W, Wang W, Xu XJ. Recent advances in computational prediction of drug absorption and permeability in drug discovery. Curr Med Chem 2006;13:2653-67.
- Sabolovic N, Magdalou J, Netter P, Abid A. Nonsteroidal anti-inflammatory drugs and phenols glucuronidation in Caco-2 cells: identification of the UDP-glucuronosyltransferases UGT1A6, 1A3 and 2B7. Life Sci 2000;67:185-96.
- Meinl W, Ebert B, Glatt H, Lampen A. Sulfotransferase forms expressed in human intestinal Caco-2 and TC7 cells at varying stages of differentiation and role in benzo[a]pyrene metabolism. Drug Metab Dispos 2008;36:276-83.
- Walle T. Absorption and metabolism of flavonoids. Free Radic Biol Med 2004;36:829-37.
- Akao T, Kawabata K, Yanagisawa E, Ishihara K, Mizuhara Y, Wakui Y, et al. Baicalin, the predominant flavone glucuronide of scutellariae radix, is absorbed from the rat gastrointestinal tract as the aglycone and restored to its original form. J Pharm Pharmacol 2000;52:1563-8.
- Liu Y, Hu M. Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perfused rat intestinal model. Drug Metab Dispos 2002;30:370-7.
- Zhang L, Lin G, Kovács B, Jani M, Krajcsi P, Zuo Z. Mechanistic study on the intestinal absorption and disposition of baicalein. Eur J Pharm Sci 2007;31:221-31.
- Akao T, Hanada M, Sakashita Y, Sato K, Morita M, Imanaka T. Efflux of baicalin, a flavone glucuronide of Scutellariae Radix, on Caco-2 cells through multidrug resistance-associated protein 2. J Pharm Pharmacol 2007;59:87-93.
- Asano T, Ishihara K, Morota T, Takeda S, Aburada M. Permeability of the flavonoids liquiritigenin and its glycosides in licorice roots and davidigenin, a hydrogeneted metabolite of liquiritigenin, using human intestinal cell line Caco-2. J Ethnopharmacol 2003;89:285-9.
- Wen X, Walle T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab Dispos 2006;34:1786-92.
- Wang Y, Cao J, Zeng S. Involvement of P-glycoprotein in regulating cellular levels of Ginkgo flavonols: quercetin, kaemferol, and isorhamnetin. J Pharm Pharmacol 2005;57:1297-303.
- König J, Nies AT, Cui Y, Leirer I, Keppler D. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2 mediated drug resistance. Biochem Biophys Acta 1999;1461:377-94.
- Walle UK, Galijatovic A, Walle T. Transport of the flavonoid Chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol 1999;58:431-8.
- O’Leary KA, Day AJ, Needs PW, Sly WS, O’Brien NM, Williamson G. Flavonoid glucuronides are substrates for human liver β-glucuronidase. FEBS Lett 2001;503:103-6.
- Shimoi K, Saka N, Nozawa R, Sato M, Amano I, Nakayama T, et al. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab Dispos 2001;29:1521-4.
