Protein tyrosine kinase, JNK, and ERK involvement in pseudolaric acid B-induced apoptosis of human breast cancer MCF-7 cells1
Introduction
Pseudolaric acid B (PAB; Figure 1) is a diterpene acid isolated from the root and trunk bark of Pseudolarix kaempferi Gordon (Pinaceae), known as “Tu-Jin-Pi” in Chinese, which has been used to treat dermatological fungi infections. PAB exerts potent antifungal, antimicrobial[1], antifertility[2,3], and cytotoxic activity in vitro[4]. We previously reported that PAB induced apoptosis, senescence, and mitotic arrest in human breast cancer MCF-7 cells; the apoptotic pathway was not through the Fas death receptor[5]. However, the apoptotic mechanism of PAB in MCF-7 cells was not entirely clear. Therefore, in this study the protein tyrosine kinases (PTK) and the mitogen-activated protein kinases (MAPK) on the apoptotic pathways were investigated to explore the apoptotic mechanism of PAB on MCF-7 cells.
Apoptosis is a highly regulated process of cell death and plays a fundamental role in the maintenance of tissue homeostasis. In recent years many studies have revealed that apoptosis is a constitutive suicide program triggered by a variety of extrinsic and intrinsic signals[6,7]. Breast cancer is the second most common cause of cancer death in women, and human breast cancer has a poor prognosis on the basis of present therapeutic measures[8].
PTK plays key roles in the regulation of cell proliferation, differentiation, metabolism, migration, and survival[9–13]. They are classified as receptor PTK and non-receptor PTK[14]. Due to their involvement in various forms of cancers, PTK have become prominent targets for therapeutic intervention. The focus of PTK inhibitors has been as a promising class of antitumoral agents, and these agents have been shown to inhibit multiple features of cancer cells, including proliferation, survival, invasion, and angiogenesis. Genistein, a soybean-derived isoflavone, is a PTK inhibitor that attenuates growth factor-stimulated proliferation of cancer cells[15]. Genistein can inhibit the activity of certain PTK, such as Src, the epidermal growth factor receptor, and topoisomerase II[16–19], therefore it has potent effects on both receptor PTK and non-receptor PTK. AG1024 is also effective in blocking insulin-like growth factor receptor 1[20], but in this study, genistein or AG1024 together with PAB showed unexpected growth stimulant effect.
In mammalian cells there are 3 major pathways of MAPK: stress-activated protein kinase/c-Jun-N-terminal kinase (JNK), p38 kinase, and extracellular signal-regulated kinase (ERK)[21,22]. Generally, the JNK and p38 protein promotes inflammation, apoptosis, growth, differentiation, and oncogenic transformation, while ERK are implicated in growth, differentiation, and development[23–26]. MAPK are induced by a PTK cascade mechanism[27,28]. The pathway of MAPK includes sequence MAPK kinases (MAPKK) kinases (MAPKKK), MAPKK, and MAPK. The MAPKKK is a serine–threonine kinase, which is a contact molecule that passes active signals from the membrane receptors to its downstream protein, MAPKK. MAPKK is a kinase with dual specificity, which phosphorylates threonine and tyrosine residues on its substrate, MAPK. MAPK, that transmit the signal from cytoplasm to the nucleus, are also a serine–threonine kinase, which phosphorylate and activate cytoplasmic proteins[28]. In these ways by regulating the expression of different genes, MAPK are implicated directly in distinct cellular processes. Many PTK, including receptor PTK and non-receptor PTK phosphorylate MAPKKK to active or inactive MAPK in the apoptotic process[29,30]. Therefore, the mutual relationship between PTK and MAPK was examined in the PAB-induced apoptotic process.
Materials and methods
Materials PAB, which was purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China), was dissolved in DMSO to make a stock solution. The DMSO concentration was kept below 0.01% in all the cell cultures and did not exert any detectable effect on cell growth or cell death. 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), AG1024, and genistein were purchased from Sigma (St Louis, MO, USA). PD98059 and SP600125 were from Calbiochem (La Jolla, CA, USA). Rabbit polyclonal antibodies against p38, phospho-p38 (p-p38), JNK, phospho-JNK(p-JNK), ERK1, ERK2, phospho-ERK(p-ERK), β-actin, and horseradish peroxidase-conjugated secondary antibodies (goat antirabbit) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture Human breast cancer cells MCF-7 were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640 medium (Hyclone, Logan, UT, USA) supplemented with 10% heat inactivated (56 ℃, 30 min) fetal calf serum (Beijing Yuanheng Shengma Research Institution of Biotechnology, Beijing, China), 2 mmol/L glutamine (Gibco, Grand Island, NY, USA), penicillin (100 U/mL), and streptomycin (100 μg/mL), and maintained at 37 ℃ with 5% CO2 in a humidified atmosphere.
Cell growth inhibition test The inhibition of cell growth was determined by MTT test. In a previous study at 36 h, the half-maximal inhibitory concentration IC50 value in MCF-7 cells was 3.4 µmol/L; therefore, we adopted 4 µmol/L at 36 h as the best concentration[5]. MCF-7 cells (1.2×104 cells/well) were seeded into 96-well culture plates (Nunc, Roskilde, Denmark). After overnight incubation, various concentrations of PAB (4 µmol/L), SB203580 (20 µmol/L), PD98059 (10 µmol/L), SP600125 (20 µmol/L), AG1024 (4 µmol/L), and /or genistein (50 µmol/L) were added to the plates. Following incubation, cell growth was measured at different time points by the addition of MTT at 37 ℃ for 3 h. and DMSO (150 µL) was added to dissolve the formazan crystals. Absorbance was measured at 492 nm with an ELISA plate reader (Bio-Rad, Hercules, CA, USA). The percentage of inhibition was calculated as follows:
Cell death (%)=(A492[control]–A492[sample])/(A492[control]–A492[blank])×100%.
Observation of morphological changes by light microscopy MCF-7 cells (6×105 cells/well) were seeded into 6-well culture plates (Nunc, Denmark). After overnight incubation, various concentrations of PAB, SP600125, AG1024, and/or genistein were added to the plates for 36 h. Morphological changes were observed by phase contrast microscopy (Leica, Nusslich, Germany).
Lactate dehydrogenase activity-based cytotoxicity assays[31] It was reported that the rate of lactate dehydrogenase (LDH) released from viable cells, floating dead cells, and the culture medium was used to distinguish the proportion of apoptotic and necrotic cells. The cells were cultured with PAB, genistein, SP600125, PD98059 or/and SB203580 for 36 h. Floating dead cells were collected from the culture medium by centrifugation (240×g for 10 min at 4 ℃), and the LDH content from the pellets lysed in 0.1% NP-40 for 15 min was used as an index of apoptotic cell death (LDHp). The LDH released in the culture medium (extracellular LDH or LDHe) was used as an index of necrotic cell death. The adherent and viable cells were lysed in 0.1% NP-40 for 15 min to release LDH (intracellular LDH or LDHi). The substrate reaction buffer of LDH (0.5 mmol/L L (+)-lactic acid, 0.66 mmol/L indonitrotetrazolium, 0.28 mmol/L phenazine methosulfate, and 1.3 mmol/L nicotinamide adenine dinucleotide in pH 8.2 Tris-HCl) was added. The A value at 490 nm of reaction for 1 and 5 min was assayed.
LDH activity=(A5min–A1min)/4.
The percentage of apoptotic and necrotic cell death was calculated as follows:
Apoptosis (%)=LDHp/(LDHp+LDHe+LDHi)×100 and Necrosis (%)=LDHe/(LDHp+LDHe+LDHi)×100.
Western blot analysis of protein expression[32] MCF-7 cells (1.2×106 cells/well) were seeded into a 25 mL culture bottle. After overnight culture, PAB and/or genistein was treated for the indicated time. Both adherent and floating cells were collected and frozen at –80 ℃. Western blot analysis was performed for total proteins as follows. Briefly, the cell pellets were resuspended in lysis buffer, including 50 mmol/L HEPES, pH 7.4, 1% Triton-X 100, 2 mmol/L sodium orthovanadate, 100 mmol/L sodium fluoride, 1 mmol/L edetic acid, 1 mmol/L EGTA, 1 mmol/L phenylmethylsulfonyl fluoride, 0.1 g·L–1aprotinin, and 0.01 g·L–1 leupeptin, then lysed at 4 ℃ for 1 h. After centrifugation at 13 000×g for 10 min, the protein content of the supernatant was determined using Bio-Rad protein assay reagent (Bio-Rad, USA). The protein was loaded in each lane, then separated by 12% SDS–PAGE, and blotted onto a nitrocellulose membrane. The protein expression was detected using a primary polyclonal antibody (1:200-1:1000) and secondary polyclonal antibody (1:500) conjugated with peroxidase.
Statistical analysis All data represent at least 3 independent experiments and are expressed as mean±SD. Statistical comparisons were made using Student’s t-test. P-values of less than 0.05 were considered to represent statistical significance.
Results
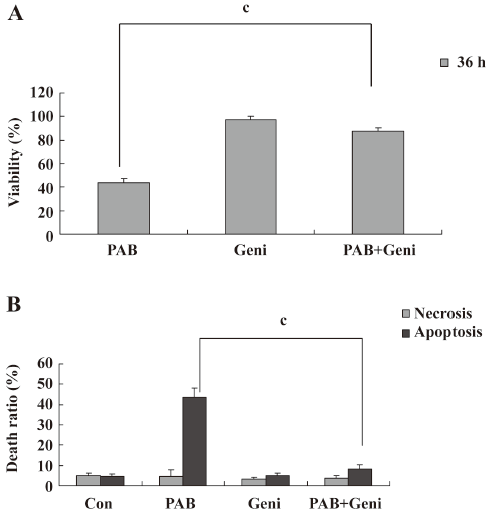
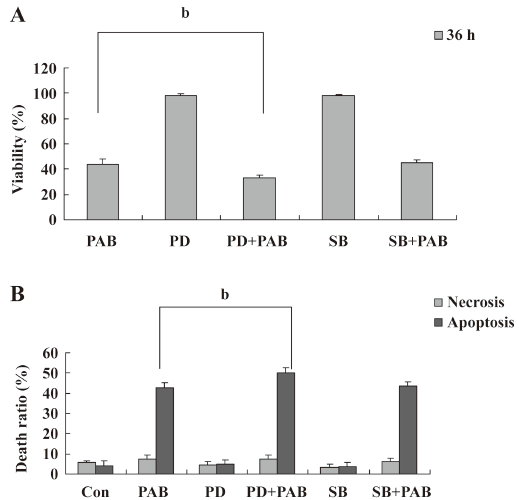
PAB induced MCF-7 cell apoptosis, but genistein and AG1024 inverted the effect of PAB The MTT analysis showed that after 4 µmol/L PAB treatment, the survival ratio of MCF-7 cell was 43.76%±3.22%, but 50 µmol/L genistein increased the viability induced by PAB to 87.79%±2.40% (Figure 2A). The LDH analysis further proved that PAB mainly induced apoptosis, but not necrosis. Genistein mainly decreased the apoptotic ratio from 43.56%±4.36% to 8.11%±2.33% and had no obvious effect on the necrotic ratio induced by PAB (Figure 2B). After 4 µmol/L PAB treatment, the number of apoptotic bodies increased, and the number of surviving cells decreased. However, after genistein treatment, PAB did not decrease the number of surviving cells and did not promote the appearance of apoptotic bodies. It was also observed that the volume of MCF-7 cells became larger in contrast to the control cells, whereas genistein decreased the apoptotic ratio induced by PAB (Figure 3).
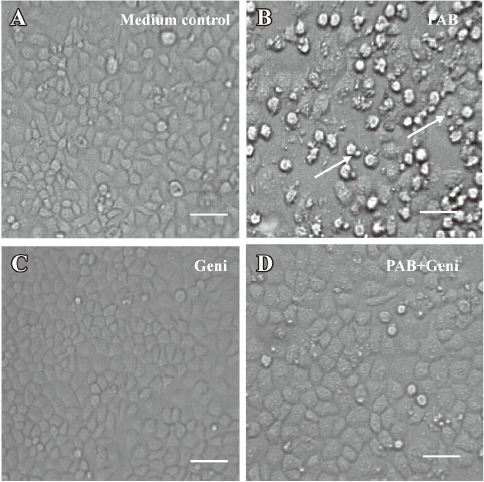
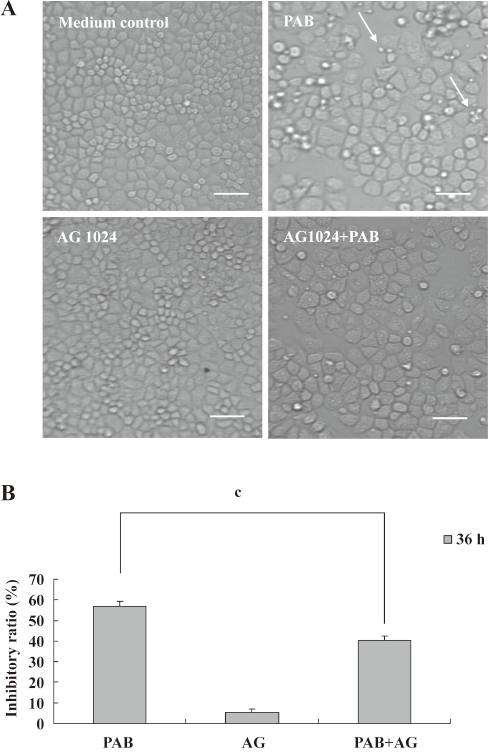
To further detect the effect of PTK on PAB-treated L929 cells, AG1024, another inhibitor of PTK, was applied in the MTT analysis and morphological observation. Like genistein, 4 µmol/L AG1024 inhibited the appearance of apoptotic bodies induced by PAB. Meanwhile, in the presence of AG1024, MCF-7 cells displayed the shape of giant cells after PAB treatment. From the MTT analysis, AG1024 decreased the inhibitory ratio induced by PAB from 56.78%±2.52% to 40.47%±1.76% (Figure 4). Therefore, in a following study, genistein was applied as an inhibitor of PTK.
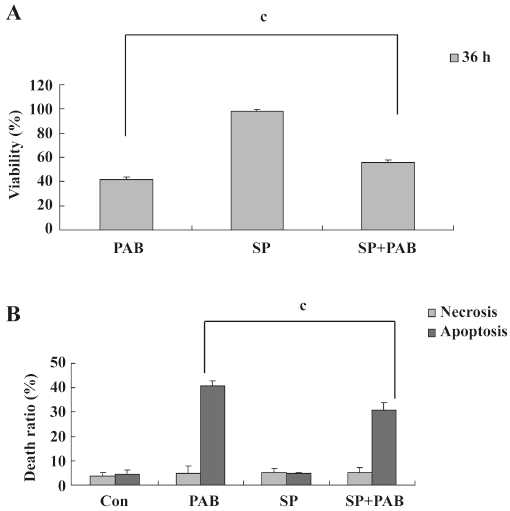
SP600125 decreased the apoptotic ratio induced by PAB SP600125 is an inhibitor of JNK[33]. SP600125 (20 µmol/L) in the presence of PAB had a strong protective effect on MCF-7 cells, and the viability of PAB-treated cells was increased from 41.66%±2.33% to 55.73%±2.30% (Figure 5A). The LDH analysis showed that PAB and SP600125 had no obvious effect on necrosis, but was effective on only apoptosis in MCF-7 cells from 40.78%±2.06% to 30.96%±2.83% (Figure 5B). In the morphological observation, SP600125 obviously increased MCF-7 cell survival and decreased the appearance of the apoptotic body when it was used together with PAB (Figure 6).
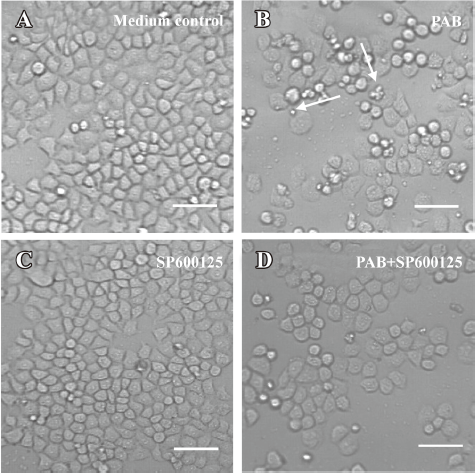
Effect of PD98059 and SB203580 on PAB-induced apoptosis PD98059 is a selective and cell-permeable inhibitor of MAP kinase kinase that acts by inhibiting ERK activation. In the MTT analysis, it was observed that PD98059 in addition of PAB decreased the survival ratio induced by PAB from 44.11%±3.97% to 33.36%±1.74%, while p38 inhibitor SB203580[33] in the presence of PAB had no obvious effect on apoptosis induced by PAB (Figure 7A). The LDH analysis showed that SB203580 or PD98059 did not affect necrosis in MCF-7 cells treated with PAB (Figure 7B).
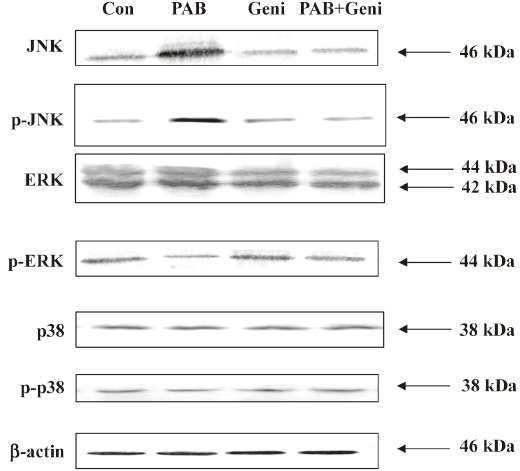
PAB upregulated the expressions of JNK and p-JNK, downregulated the expression of p-ERK, and had no obvious effect on the expressions of ERK, p38, and p-p38 It was observed that 4 µmol/L PAB had no obvious effect on the expression of ERK at 12, 24, 36, and 48 h. However PAB began to reduce the expression of p-ERK at 24 h, and sustained this decrease to 48 h. PAB upregulated the expressions of JNK and p-JNK at 24 h, and this effect continued to 48 h. On p38 and p-p38 expressions, PAB exerted no obvious changes (Figure 8).

Genistein downregulated the expressions of JNK and p-JNK, and upregulated p-ERK in PAB-treated MCF-7 cells PAB upregulated the expressions of JNK and p-JNK at 36 h, but together with genistein, PAB downregulated the expressions of JNK and p-JNK. Genistein in addition to PAB promoted the expression of p-ERK, which was downregulated by PAB. Genistein in the presence of PAB had no obvious effect on the expressions of p38, p-p38, and ERK compared with PAB alone (Figure 9).
Discussion
As a mechanism of cell death, apoptosis is an important process for the normal development and suppression of oncogenesis. Apoptosis is characterized by a series of typical morphological events, such as cell shrinkage, DNA fragmentation, formation of apoptotic bodies, and rapid phagocytosis by neighboring cells[6]. Therefore, unlikely necrosis, apoptosis does not induce inflammation, and is appreciated in clinical research and treatment. In the LDH analysis, genistein, PD98059, SB203580, and SP600125 were not involved in necrosis when PAB was used to elucidate the mechanism of apoptosis in MCF-7 cells. Therefore, PTK and MAPK were implicated to exert their functions mainly in the process of PAB-induced apoptosis. Genistein was first isolated from Pseudomonas spp, targeting the ATP-binding site of PTK[16,18]. PTK were highly expressed in various types of cancers. Therefore, genistein was researched as an anticancer reagent[34,35]. However, in this study, when PAB exerted obvious inhibitory effects on MCF-7, genistein unexpectedly inhibited the apoptotic effect of PAB and promoted MCF-7 cell survival. To further ascertain the effect of PTK on PAB-treated MCF-7 cells, AG1024, another inhibitor of PTK, was applied to explore the function of PTK. Like genistein, AG1024 inverted the effect of PAB to inhibit apoptosis. Therefore, it was highly possible that PTK promoted apoptosis in MCF-7 cells after PAB treatment. The use of genistein and AG1024 in clinical cancer treatment should be emphasized carefully, especially during combined use of drugs. Although genistein and AG1024 retrieved most MCF-7 cells treated with PAB at 36 h, the shape of MCF-7 cells became larger than the untreated control cells. Because PAB induced mitotic arrest in MCF-7 cells[5] and microtubule disruption[36,37], both mitotic arrest and microtubule disruption might be reasons for the cells becoming larger. It was probable that PTK were involved only in growth and death, not in mitotic arrest and the inhibition of microtubule formation in the PAB-treated MCF-7 cells. A more detailed mechanism would be elucidated in future research. MAPK, especially JNK, ERK, and p38, had various functions on the apoptotic process[22–26]. In this study, PAB upregulated the expressions of JNK and p-JNK, and the inhibitor of JNK reduced the PAB-induced apoptosis, indicating that JNK promoted apoptosis in PAB-treated MCF-7 cells. The expression of p-ERK was downregulated by PAB, and the inhibitor of ERK increased the apoptotic ratio of MCF-7 cells, indicating that ERK promoted the survival of PAB-treated MCF-7 cells. Because the inhibitor of p38 had no obvious effect on cell growth, and the expressions of p38 and p-p38 were not altered by PAB, p38 was not involved in PAB-induced apoptosis in MCF-7 cells. It was concluded that through activating JNK and inhibiting ERK, PAB induced apoptosis. MAPK proteins could be activated when it was phosphorylated serially by PTK[27,28]. Genistein together with PAB inhibited the increase of PAB-induced JNK, therefore JNK was downstream of PTK. SP600125 decreased the suppressive effect of PAB on MCF-7 cells, but it was not as potent as genistein. Therefore, besides JNK, there might be other kinases downstream of PTK. Genistein upregulated the expression of p-ERK, which was downregulated by PAB, indicating that besides JNK, ERK was also downstream of PTK and promoted survival of the PAB-treated MCF-7 cells after PAB treatment.
Therefore, it was concluded that PTK were upstream of ERK and JNK, and PTK induced apoptosis through activating JNK and inactivating ERK in PAB-treated MCF-7 cells.
References
- Li E, Clark AM, Hufford CD. Antifungal evaluation of PAB, a major constituent of pseudolarix kaempfeAri. J Nat Prod 1995;58:57-67.
- Wang WC, Lu RF, Zhao SX, Gu ZP. Comparison of early pregnancy-terminating effect and toxicity between pseudolaric acids A and B. Acta Pharmacol Sin 1988;9:445-8.
- Wang WC, Lu RF, Zhao SX, Zhu YZ. Antifertility effect of PAB. Acta Pharmacol Sin 1982;3:188-92.
- Pan DJ, Li ZL, Hu CQ, Chen K, Chang JJ, Lee KH. The cytotoxic principles of Pseudolarix kaempferi: pseudolaric acid-A and B and related derivatives. Planta Med 1990;56:383-5.
- Yu JH, Cui Q, Jiang YY, Yang W, Tashiro SI, Onodera S, et al. Pseudolaric acid B induces apoptosis, senescence, and mitotic arrest in human breast cancer MCF-7. Acta Pharmacol Sin 2007;28:1975-83.
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972;26:239-57.
- Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Int Med 2005;258:479-517.
- Kaufmann SH. Induction of endonucleolytic DNA cleavage in human acute myelogenous breast cancer cells by etoposide, camptothecin, and other cytotoxicanticancer drugs: a cautionary note. Cancer Res 1989;49:5870-8.
- Rucci N, Recchia I, Angelucci A, Alamanou M, Fattore AD, Fortunati D. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther 2006;318:161-72.
- Salvi M, Brunati AM, Toninello A. Tyrosine phosphorylation in mitochondria: A new frontier in mitochondrial signaling. Free Radical Biol Med 2005;38:1267-77.
- Linassier C, Pierre M, Le PJB., Pierre J. Mechanisms of action in NIH-3T3 cells of genistein, an inhibitor of EGF receptor tyrosine kinase activity. Biochem Pharmacol 1990;39:187-93.
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990;61:203-12.
- Noble MEM, Endicott JA, Johnson LN. Protein kinase inhibitors: insights into drug design from structure. Science 2004;303:1800-5.
- Kim Hk, Jeong MJ, Kong MY, Han MY, Son KH, Kim HM, et al. Inhibition of Shc/Grb2 protein-protein interaction suppresses growth of B104-1-1 tumors xenografted in nude mice. Life Sci 2005;78:321-8.
- Conklin CM, Bechberger JF, MacFabe D, Guthrie N, Kurowska EM, Naus CC. Genistein and quercetin increase connexin43 and suppress growth of breast cancer cells. Carcinogenesis 2007;28:93-100.
- Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science 1995;267:1782-8.
- Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein tyrosine kinases. Methods Enzymol 1991;201:362-70.
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein-Induced Changes in Lipid Metabolism of Ovariectomized Rats. J Biol Chem 1987;226:5592-5.
- Schmidt F, Knobbe CB, Frank B, Wolburg H, Weller M. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol Rep 2008;19:1061-6.
- Michael H, Alexander H, Andreas PS, Viola B, Detlef S, Hans S. Blockade of IGF-1 receptor tyrosine kinase has antineoplastic effects in hepatocellular carcinoma cells. Biochem Pharmacol 2006;71:1435-48.
- Klekotka PA, Santoro SA, Wang H, Zutter MM. Specific residues within the alpha 2 integrin subunit cytoplasmic domain regulate migration and cell cycle progression via distinct MAPK pathways. J Biol Chem 2001; 276: 32 353–61.
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol 1999;19:2435-44.
- Xia ZG, Dikens M, Raingesud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995;270:1336-41.
- Hagemann C, Blank JL. The ups and downs of MEK kinase interactions. Cell Signal 2001;13:863-75.
- Bradham CA, McClay DR. p38 MAPK is essential for secondary axis specification and patterning in sea urchin embryos. Development 2006;133:21-32.
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006;24:21-44.
- Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004;23:2838-49.
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther 2005;4:139-63.
- Matsuda S, Gotoh Y, Nishida E. Signaling pathways mediated by the mitogen-activated protein (MAP) kinase/MAP kinase cascade. J Leukoc Biol 1994;56:548-53.
- Kharbanda S, Ren R, Pandey P, Shafman TD, Feller SM, Weichselbaum RR, et al. Activation of the c-Abl tyrosine kinase in the stress response to DNA damaging agents. Nature 1995;376:785-8.
- Kim YM, Talanian RV, Billiar TR. Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J Biol Chem 1997; 272: 31 138–48.
- Nalini K, Rayudu G, Vsha G, Jinah C, Henry JF. Role of protein kinase C in basal and hydrogen peroxide stimulated NF-κB activation in the murine macrophage. Arch Biochem Biochem Biophys 1998;350:79-86.
- Cui Q, Tashiro S, Onodera S, Minami M, Ikejima T. Oridonin induced autophagy in human cervical carcinoma HeLa cells through Ras, JNK, and p38 regulation. J Pharmacol Sci 2007;105:317-25.
- Yang C, Han R. Genistein suppresses adhesion induced protein tyrosine phosphorylation and invasion of BI6 BL6 melanoma cells. Cancer Lett 1998;129:117-25.
- Akimoto T, Nonaka T, Ishikawa H, Sakurai H, Saitoh JI, Takahashi T, et al. Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: possible involvement of inhibition of survival signaltransduction pathways. Int J Radiat Oncol Biol Phys 2001;50:195-201.
- Tong YG, Zhang XW, Geng MY, Yue JM, Xin XL, Tian F, et al. Pseudolarix acid B, a new tubulin-binding agent, inhibits angiogenesis by interacting with a novel binding site on tubulin. Mol Pharmacol 2006;69:1226-33.
- Wong VK, Chiu P, Chung SS, Chow LM, Zhao YZ, Yang BB, et al. Pseudolaric acid B, a novel microtubule-destabilizing agent that circumvents multidrug resistance phenotype and exhibits antitumor activity in vivo. Clin Cancer Res 2005;11:6002-11.

