A novel anti-cancer effect of genistein: reversal of epithelial mesenchymal transition in prostate cancer cells1
Introduction
Prostate cancer (PCa) is the second most frequent cause of cancer death among males in the USA[1]. The invasion and metastasis of cancer is a major cause of death among PCa patients. Despite the widespread presence of clinically insignificant tumors in elderly men, PCa commonly has an aggressive phenotype that requires prompt intervention[2]. Therefore, PCa is a highly desirable target for effective and tolerable antimetastatic drugs.
Epithelial mesenchymal transition (EMT) is an important process during tumor progression by which epithelial cells acquire mesenchymal, fibroblast-like properties and show decreased intercellular adhesion and increased motility[3]. Increasing evidence emphasizes a critical role of EMT during PCa progression and malignant transformation, endowing the incipient cancer cell with invasive and metastatic properties[4–6]. So EMT could be a very promising therapeutic target, and the inhibition of EMT may prevent or restrain the invasion and metastasis of PCa. Ideally, it should inhibit the early steps of invasion and metastasis.
Genistein (4´,5,7-trihydroxyisoflavone) is a major isoflavone constituent of soybeans and soy products. The consumption of dietary genistein in the form of soy has been associated with a lower risk of PCa[7]. Genistein displays multiple biological activities in several preclinical model systems that relate to cancer prevention and is considered a cancer chemopreventive agent[8,9]. In addition, genistein was also suggested to have antimetastatic activity. Epidemiological studies indicate that genistein consumption is associated with a lower incidence of clinical PCa metastasis[10], and the potential effect of genistein on the metastatic activity of tumor cells has been confirmed in several experimental studies[11–13]. At pharmacologic doses, the main effect of genistein on PCa cells appears to be the induction of apoptosis[14,15]. Some investigators have also reported that genistein suppresses PCa cell proliferation[16–18]. However, the genistein concentrations used in the majority of these studies are much higher than that is physiologically achievable. Because chemopreventive agents must be administered over long periods without toxicity, and must be given at doses associated with effective concentrations, mechanistic studies need to take these parameters into consideration[19]. Therefore, studies of genistein that take into consideration mechanisms that operate at physiologically-relevant concentrations are needed and can shed light on a widely consumed agent that may have cancer chemopreventive activity.
In our previous research[20,21], we established a HIF-1a (hypoxia-inducible factor 1 alpha) overexpression PCa LNCaP cell line (LNCaP/HIF-1a), and successfully proved that HIF-1a could induce the LNCaP cells to undergo EMT. IA8-ARCaP, a highly invasive clone of the parental ARCaP cell line[4,22], was derived from the ascites fluid of a patient with late-stage or advanced metastatic disease, which have been identified to process EMT in a previous report[4]. The 2 PCa cell lines were used in this study as an EMT cell model.
Focusing specifically on the physiological concentrations of genistein, we found for the first time that low-dose genistein can reverse the cell phenotypes of EMT in PCa IA8-ARCaP and LNCaP/HIF-1a cells without significantly affecting in vitro cell growth. Furthermore, low-dose genistein can inhibit tumor cell invasion of IA8-ARCaP and LNCaP/HIF-1a in vitro.
Materials and methods
Genistein Genistein (G-6649; Sigma, St Louis, MO, USA) was dissolved in DMSO as a 100 mmol/L stock and stored at –20 °C in dark conditions for a maximum of 3 weeks. Cells in the genistein-free treatment were incubated using the vehicle (DMSO of a similar concentration).
Cell culture Experiments were performed on 3 PCa cell lines, LNCaP, LNCaP/HIF-1a, and IA8-ARCaP. LNCaP/HIF-1a, with the stable overexpression of HIF-1a, was established in our previous research[21], and was found to process EMT in our previous report[20,23]. IA8-ARCaP (a kind gift of Professor Leland WK CHUNG, Emory University, Atlanta, GA, USA), a highly invasive clone of the parental ARCaP cell line, was derived from the ascites fluid of a patient with late-stage or advanced metastatic disease and cultured in T-medium (Life Technologies, Gaithersburg, MD, USA) containing 5% fetal bovine serum[22]. IA8-ARCaP cells used in this study were from early passages, which have been identified to process EMT in previous report[4].
Cell viability assay The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine viable cell number[24]. Briefly, PCa cells were plated in 96-well microtiter plates (1000 cells/well) for 12 h. Experimental medium containing different concentrations (0, 0.1, 0.5, 1, 5, 10, 15, 20, 50, and 75 μmol/L) of genistein was added, and the cells were allowed to incubate for an additional 24, 48, and 72 h. MTT (5 mg/mL in phosphate-buffered saline [PBS]; Sigma, USA) was added, and the cells were incubated for 2 h. After careful removal of the medium, 0.1 mL buffered DMSO was added to each well, and the plates were shaken. Absorbance at 570 nm (proportional to viable cell number) was then read with a multiplate reader (Bio-Rad Laboratories, Hercules, CA, USA).
Immunofluorescence PCa cells were treated with genistein (48 h, 15 μmol/L) and fixed with 4% paraformaldehyde. When needed, the cells were permeabilized for 15 min with 0.5% Triton X-100 in PBS. After blocking in PBS buffer containing 10% bovine serum (BS), the cells were incubated (2 h at room temperature) with an anti-E-cadherin monoclonal antibody diluted at 1:150 or with an anti-vimentin polyclonal antibody diluted at 1:100 in PBS buffer containing 3% BS. The cells were washed and incubated with a fluorescein isothiocyanate-conjugated secondary antibody diluted at 1:100 in PBS containing 3% BS. Cells were analyzed with a fluorescence inverted microscope (IX 50; Olympus, Yokogawa Japan).
Western blot analysis Cells were harvested in RIPA lysis buffer (1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 150 mmol/L NaCl, 10 mmol/L Tris-HCl, and a protease inhibitor mixture) for 10 min on ice. The total protein concentration was determined using Bio-Rad protein assay reagent (Bio-Rad, USA). Equal amounts of total protein were separated by SDS–PAGE, transferred to nitrocellulose membranes, and probed with a human-specific anti-E-cadherin monoclonal antibody (Santa Cruz, CA, USA), an anti-vimentin polyclonal antibody (Santa Cruz, USA), or an anti-β-actin antibody (Sigma, USA). Horseradish peroxidase (HRP)-conjugated secondary antibodies were used (Santa Cruz, USA), and secondary antibody detection was performed using Super Signal HRP (Pierce, Rocford, IL, USA).
RT–PCR analysis Total RNA was isolated from the cultured cells in the log phase using TRIzol (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. One microgram of total RNA was reverse transcribed at 42 °C for 60 min using oligo(dT) as a primer and avian myeloblastosis virus RT (Promega, Madison, WI, USA). PCR was performed using Taq (TaKaRa, Shiga, Japan) polymerase. The sequences of primers used in this report were as follows: E-cadherin 5´-GTAACCGATCAGAATGAC-3´ (forward primer) and 5´-CGTGGTGGGATTGAAGAT-3´ (reverse primer); vimentin 5´-TGGCACGTCTTGACCTTGAA-3´ (forward primer) and 5´-GGTCATCGTGATGCTGAGAA-3´ (reverse primer); and β-actin 5´-GTGGGGCGCCCCAGGCACCA-3´ (forward primer) and 5´-CTTCCTTAATGTCACGCACGATTTC-3´ (reverse primer). PCR products were visualized by eletrophoresis through 1.2% agarose gels and quantified with Glyko Bandscan gel analyzing software (Glyko, Novato, CA, USA). Parallel reactions were run using human β-actin as a control for RT–PCR. All assays were performed at least 3 times.
Invasion assay The invasiveness of tumor cells was performed in vitro using a transwell chamber system with 8.0 μm pore polycarbonate filter inserts (Corning Coster, Cambridge, MA, USA). The lower side of the filter was coated with 10 μL gelatin (1 mg/mL), and the upper side was coated with 10 μL of the matrigel. PCa cells (5×103) were placed in the upper part of the filter. The chamber was then incubated at 37 °C for 24, 48, and 72 h. The cells were fixed with methanol and stained with Giemsa. The invasiveness of tumor cells was determined by counting the total number of cells on the lower side of the filter at 100× magnification. For each replicate, the tumor cells in 10 randomly selected fields were determined, and the counts were averaged.
Data analysis Results were expressed as mean±SEM. Statistical comparisons were made with the Student’s t-test. ANOVA for multiple comparisons was used as noted. In all cases, P<0.05 was considered significant. All statistical tests were performed with statistical analysis software (SPSS, Chicago, IL, USA).
Results
Identification of EMT in PCa cell lines Cells that have undergone EMT are characterized by a loss of epithelial cell adhesion and cytoskeleton components and acquisition of mesenchymal components. LNCaP/HIF-1a, with a stable overexpression of HIF-1a, was established in our previous research[21], and was found to process EMT in our previous report[20,23]. IA8-ARCaP cells have been identified to process EMT in a previous report[4]. In the present study, we first identified the characteristics of EMT in LNCaP, LNCaP/HIF-1a and IA8-ARCaP cells by examining the expression of EMT-relative markers, cell proliferation, and invasion.
As observed in Figure 1, LNCaP cells expressed higher E-cadherin, which is typically associated with epithelial cells; however, LNCaP/HIF-1a and IA8-ARCaP cells displayed a higher expression of the vimentin protein, which is associated with mesenchymal cells, and an undetectable expression of epithelium-associated E-cadherin genes. All the results were confirmed by Western blotting. Since EMT has been identified as being associated with increased cancer cell invasion and proliferation, we evaluated the possible correlation among these cell lines with different characteristics of EMT. Cell invasion detected using a transwell chamber coated with a matrigel barrier (Figure 2), and cell growth as assessed by MTT assay (Figure 3), were also analyzed. From the results, we can see that compared with the LNCaP cells, the invasive potency and growth rate for LNCaP/HIF-1a and IA8-ARCaP increased significantly (P<0.05).
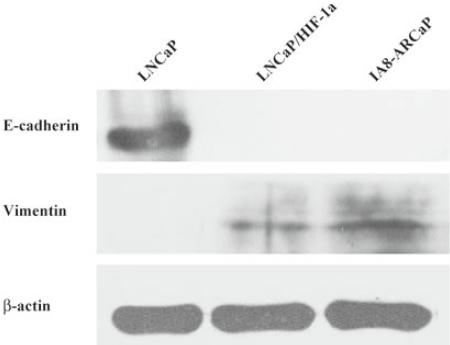
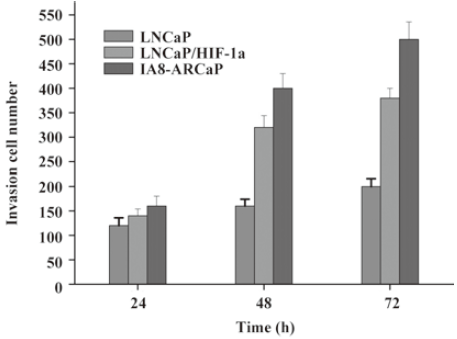
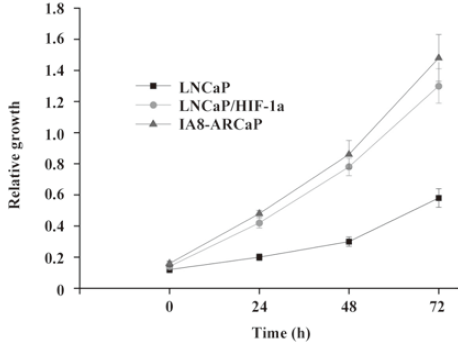
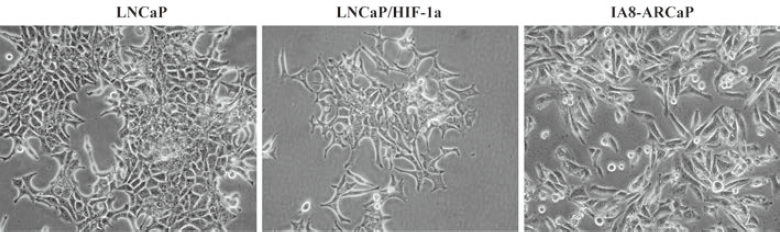
Together, these results suggest that there was an actual difference in the EMT phenotypes among these cell lines. According to our result, LNCaP was an EMT-negative cell line, whereas LNCaP/HIF-1a and IA8-ARCaP have undergone the EMT process. Moreover, we further observed the difference in cell morphology. As observed in Figure 4, IA8-ARCaP cells exhibited a fibroblast-like shape and LNCaP cells displayed an epithelial-like shape. However, LNCaP/HIF-1a cells still showed an epithelial-like phenotype, despite having undergone the EMT process.
Inhibitory effect of genistein on PCa cell proliferation Several in vitro studies have shown that genistein inhibits the proliferation of a wide range of cancers[25,26]. However, the inhibitory effect of genistein is often reported to be at concentrations exceeding its physiological level. Moreover, the estimation of the inhibition of cell growth by genistein is frequently, at least in part, affected by the induction of cell death by a high concentration of genistein. To investigate if genistein exerted any effect on the growth of PCa cells, MTT assay was performed on human PCa IA8-ARCaP and LNCaP/HIF-1a cells. As shown in Figure 5, genistein almost completely inhibited the growth of IA8-ARCaP and LNCaP/HIF-1a cells in the concentration range of 15–75 μmol/L. The addition of genistein to the medium reduced IA8-ARCaP and LNCaP/HIF-1a-viable cell numbers in a dose-dependent manner (with increasing concentrations from 15 to 75 μmol/L). However, low achievable genistein concentrations (<15 μmol/L) did not exert potent effects of growth inhibition (P>0.05).
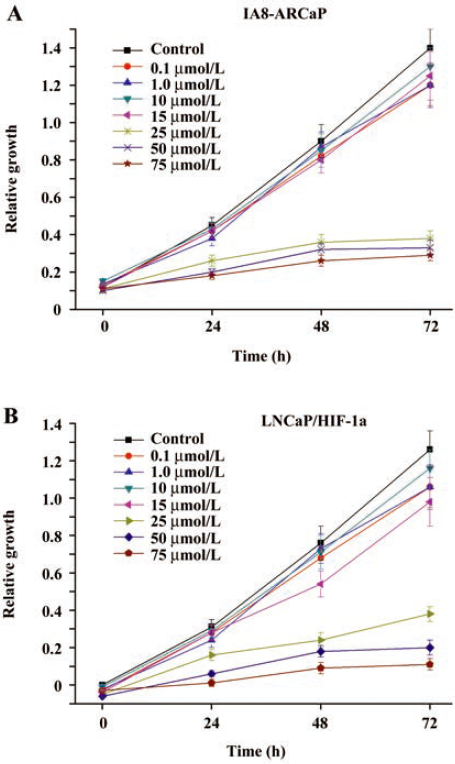
Since the aim of our study was to characterize the effect of genistein on EMT and cell invasion in physiologically-relevant and non-toxic concentrations, in subsequent experiments, genistein was used at concentrations of 0.1–15 μmol/L.
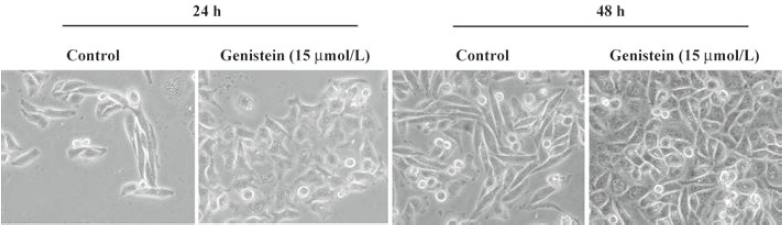
Reversal of EMT induced by low-dose genistein in IA8-ARCaP and LNCaP/HIF-1a cells Changes in the cell morphology of IA8-ARCaP were assessed under an inverted phase-contrast microscope (Figure 6). We found that culturing cells with low-dose genistein (15 μmol/L) for 24 h resulted in morphological changes of these cells from a fibroblast-like shape to an epithelial-like shape. After incubation for 48 h, the cells adopted a more epithelial-like morphology and enhanced their cell–cell contact.
In addition to the morphological changes, the phenotypic markers expressed in IA8-ARCaP cells after low-dose genistein treatment were also detected using immunofluorescence staining, RT–PCR, and Western blot assay. We observed that genistein induced the E-cadherin expression of IA8-ARCaP cells within 24 h (Figure 7; P<0.05). However, in parallel with the marked increase in the E-cadherin epithelial marker, genistein significantly decreased the expression of mesenchymal marker vimentin at a low-dose concentration (Figure 7; P<0.05).
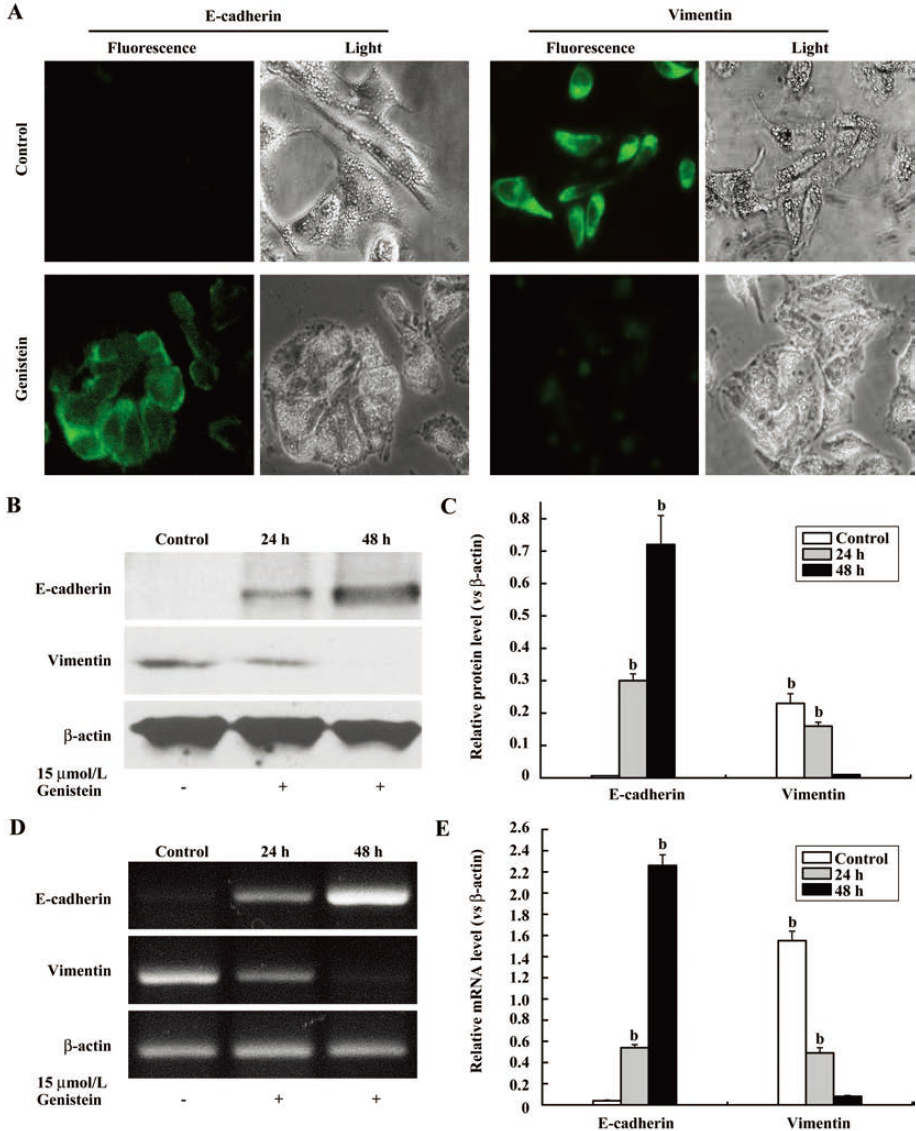
Immunostaining with antibodies to E-cadherin and vimentin showed the changes in the localization and expression level of these EMT-related proteins as an effect of the low-dose genistein (Figure 7A). Our data suggested that genistein can reverse the EMT phenotype of IA8-ARCaP cells at a low-dose concentration.
Although LNCaP/HIF-1a cells have undergone the EMT process, these cells still displayed an epithelial-like phenotype. So we detected the changes of the phenotypic markers expressed in LNCaP/HIF-1a cells after low-dose genistein (15 μmol/L) treatment using Western blot assay. The same results as IA8-ARCaP cells were found in LNCaP/HIF-1a cells, which showed that low-dose genistein induced the E-cadherin expression in parallel with the marked decrease in the mesenchymal marker vimentin (Figure 8C).
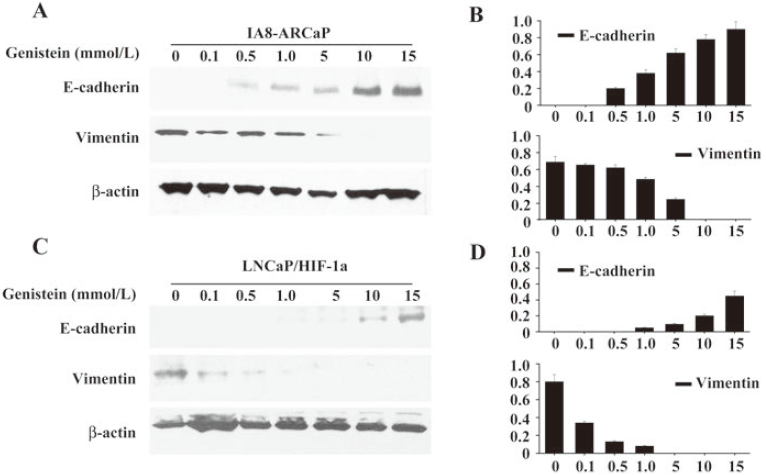
Concentration-dependent manner of EMT reversal in PCa cells by low dose genistein To further characterize the reversal of EMT induced by low-dose genistein, we analyzed the effect of genistein on EMT-related markers, E-cadherin and vimentin, in PCa cells separately treated with various doses less than 15 μmol/L for 48 h.
As shown in Figure 8, exposure to low doses of genistein (0.1, 0.5, 1.0, 5, 10, and 15 μmol/L) led to the upregulation of E-cadherin expression in a concentration-dependent manner. At the same time, the expression of vimentin was reduced in the same manner. We found that low-dose genistein was able to induce the reversal of EMT in PCa cells, IA8-ARCaP, and LNCaP/HIF-1a. These results indicate that this phenomenon was in a concentration-dependent manner
Low-dose genistein inhibits PCa cell invasion To investigate whether low-dose genistein could inhibit tumor invasion, the in vitro invasion assay with a matrigel model was performed. As shown in Figure 9, the invasive activity of LNCaP/HIF-1a and IA8-ARCaP cells was decreased by low-dose genistein.
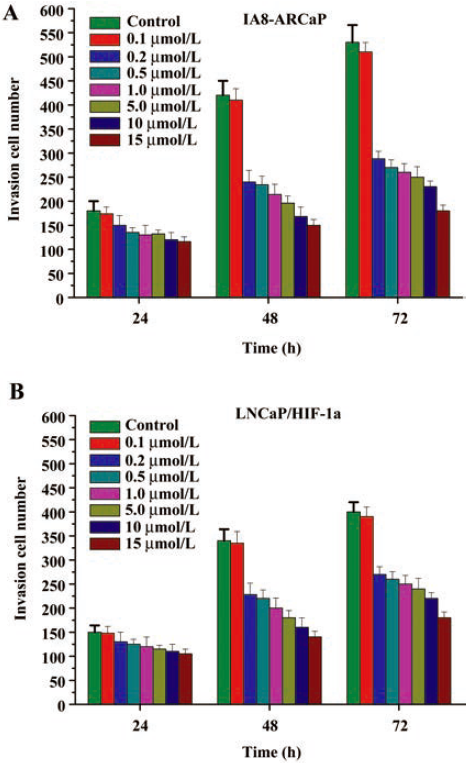
Cells were exposed to various doses (0.1, 0.2, 0.5, 1, 5, 10, and 15 μmol/L) genistein for 24, 48, and 72 h. The numbers of cells that digested matrigel and migrated through the 8 μm pores in the filter were counted. We found that genistein treatment (0.2–15 μmol/L doses) resulted in the significant inhibition of IA8-ARCaP cell invasion at every time point (Figure 9A; P<0.05). Similar results were obtained for LNCaP/HIF-1a cells (Figure 9B). However, treatment with 0.1 μmol/L genistein could not inhibit PCa cell invasion (P>0.05). These results suggest that genistein can inhibit the in vitro cell invasion of IA8-ARCaP and LNCaP/HIF-1a cells at a low-dose concentration, and the threshold of the concentration to inhibit cell invasion was 0.2–15 μmol/L.
Discussion
To explore the effect of genistein on EMT in PCa cells, we selected 2 human PCa cell lines, IA8-ARCaP and LNCaP/HIF-1a. IA8-ARCaP cells can uniquely represent the molecular basis of PCa metastases and EMT, which was identified in a previous study[4]. Following cellular interaction between human PCa ARCaP cells and the mouse host, several changes in morphology, gene expression, and behavior were observed in this cell clone. This lineage-derived IA8-ARCaP cells undergoing EMT changes showed a spindle-shape fibroblastic morphology, and exhibited reduced cell adhesion and enhanced metastasis to the bone and adrenal gland[4]. LNCaP/HIF-1a, with a stable overexpression of HIF-1a, was established in our previous research[21], and was found to process EMT in our previous report[20,23]. In the present study, we further confirmed the characteristics of EMT in IA8-ARCaP and LNCaP/HIF-1a cells by examining the expression of EMT relative markers, cell proliferation, and invasion.
In present study, we found that human PCa cells, IA8-ARCaP, and LNCaP/HIF-1a treated with genistein displayed a decreased viable cell number, which at low, physiologically-relevant genistein concentrations (<15 μmol/L), appears to have no inhibitory effect on cell proliferation, however, can significantly inhibit cell invasion in vitro. Our findings confirmed the results of previous reports that indicated that genistein suppresses the growth of human PCa cell lines. Peterson and Barnes[27] showed that pharmacological concentrations of genistein block the in vitro growth of LNCaP cells and DU145 cells. Onozawa et al[28] reported that the genistein concentration required to induce a 50% reduction in LNCaP cell growth was 40 μmol/L. Santibanez et al[17] also reported that high levels of genistein inhibit the proliferation and the in vitro invasion potential of LNCaP cells. Geller et al[29] reported that genistein decreased the growth of both BPH (50% reduction at 18.4 μmol/L genistein) and PCa tissue (37% reduction at 18.4 μmol/L genistein) in histoculture. Our finding of a genistein-induced growth inhibition in IA8-ARCaP and LNCaP/HIF-1a cells are consistent with these reports of the effects of genistein on PCa cell lines. At higher concentrations (>15 μmol/L), genistein also induced IA8-ARCaP and LNCaP/HIF-1a cell growth inhibition. However, at lower, more physiologically-relevant concentrations, the primary effect of genistein on these cells appeared to be cell invasion modulation, with minimal effects on proliferation. Although plasma genistein in heavy soy consumers can reach micromolar levels[30], concentrations above 15 μmol/L are considered supraphysiological. We therefore focused on evaluating the mechanisms underlying the anti-invasive effects of genistein at concentrations no higher than 15 μmol/L.
Recently, EMT has received much attention. EMT is actively involved in tumor invasion and metastasis. The reversal of EMT, that is MET, in cancer cells can decrease the invasive ability[3]. Therefore, the inhibition or reversal of EMT may prevent or restrain cancer invasion and metastasis. Ideally, it should inhibit the early steps of invasion and metastasis. In our study, we found that after treatment with low-dose genistein (15 μmol/L) for 48 h, IA8-ARCaP cells displayed morphological alterations from the mesenchymal phenotype to epithelial phenotype and possessed an epithelial-like morphology, accompanied by an upregulated expression of epithelial marker E-cadherin and the loss of expression of mesenchymal marker vimentin. The same results can be found in LNCaP/HIF-1a cells, which showed that low-dose genistein induced the E-cadherin expression parallel with the marked decrease in the mesenchymal marker vimentin. Our results indicated that low-dose genistein (<15 μmol/L) was able to induce the reversal of EMT in PCa cells, IA8-ARCaP, and LNCaP/HIF-1a. This phenomenon was in a concentration-dependent manner. Moreover, our data also indicated that the inhibitory effect of PCa cell invasion by low-dose genistein was in a time-dependent manner, and the threshold of concentration to inhibit cell invasion of IA8-ARCaP and LNCaP/HIF-1a was 0.2 to 15 μmol/L.
Up until now, no data have been published about the effect of genistein on EMT of cancer cells. Here, we first demonstrated a novel anticancer effect of genistein: the reversal of EMT. Our results suggested that treatment with low-dose genistein may be a potential strategy for the suppression of invasive growth through the reversal of EMT in cancer cells. Although the chemopreventive antiproliferative effects of genistein have been well documented in many types of human cancers[14–18], our results provide evidence to suggest that they may also be potential agents for the treatment of metastatic PCa, which justifies the potential use of soybean foods as a practical chemopreventive approach for patients with PCa.
References
- Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst 2005;97:1407-7.
- Bracarda S, de Cobelli O, Greco C, Prayer-Galetti T, Valdagni R, Gatta G, et al. Cancer of the prostate. Crit Rev Oncol Hematol 2005;56:379-96.
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54.
- Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A, et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate 2006;66:1664-73.
- Whitbread AK, Veveris-Lowe TL, Lawrence MG, Nicol DL, Clements JA. The role of kallikrein-related peptidases in prostate cancer: potential involvement in an epithelial to mesenchymal transition. Biol Chem 2006;387:707-14.
- Kasper S, Cookson MS. Mechanisms leading to the development of hormone-resistant prostate cancer. Urol Clin North Am 2006;33:201-10.
- Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer 1994;21:113-31.
- Parnes HL, Figg WD. Chemoprevention in prostate cancer. Pharmacotherapy 2006;26:1533.
- Clinical development plan: genistein. J Cell Biochem Suppl 1996;26:114-26.
- Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res 1989;49:1857-60.
- Schleicher RL, Lamartiniere CA, Zheng M, Zhang M. The inhibitory effect of genistein on the growth and metastasis of a transplantable rat accessory sex gland carcinoma. Cancer Lett 1999;136:195-201.
- Valachovicova T, Slivova V, Bergman H, Shuherk J, Sliva D. Soy isoflavones suppress invasiveness of breast cancer cells by the inhibition of NF-kappaB/AP-1-dependent and -independent pathways. Int J Oncol 2004;25:1389-95.
- Iishi H, Tatsuta M, Baba M, Yano H, Sakai N, Akedo H. Genistein attenuates peritoneal metastasis of azoxymethane-induced intestinal adenocarcinomas in Wistar rats. Int J Cancer 2000;86:416-20.
- Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer 1998;32:123-31.
- Kyle E, Neckers L, Takimoto C, Curt G, Bergan R. Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity. Mol Pharmacol 1997;51:193-200.
- Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr 1999;129:1628-35.
- Santibanez JF, Navarro A, Martinez J. Genistein inhibits proliferation and in vitro invasive potential of human prostatic cancer cell lines. Anticancer Res 1997;17:1199-204.
- Davis JN, Muqim N, Bhuiyan M, Kucuk O, Pienta KJ, Sarkar FH. Inhibition of prostate specific antigen expression by genistein in prostate cancer cells. Int J Oncol 2000;16:1091-7.
- Crowell JA, Holmes CJ. Agent identification and preclinical testing. Cancer Treat Res 2001;106:1-30.
- Luo Y, He DL, Ning L, Shen SL, Li L, Li X. Hypoxia-inducible factor-1alpha induces the epithelial-mesenchymal transition of human prostatecancer cells. Chin Med J (Engl) 2006;119:713-8.
- Luo Y, He DL, Ning L, Shen SL, Li L, Li X, et al. Over-expression of hypoxia-inducible factor-1alpha increases the invasive potency of LNCaP cells in vitro. BJU Int 2006;98:1315-9.
- Zhau HY, Chang SM, Chen BQ, Wang Y, Zhang H, Kao C, et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci USA 1996;93:15152-7.
- Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, et al. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol 2007;14:1034-9.
- Romijn JC, Verkoelen CF, Schroeder FH. Application of the MTT assay to human prostate cancer cell lines in vitro: establishment of test conditions and assessment of hormone-stimulated growth and drug-induced cytostatic and cytotoxic effects. Prostate 1988;12:99-110.
- Hempstock J, Kavanagh JP, George NJ. Growth inhibition of prostate cell lines in vitro by phyto-oestrogens. Br J Urol 1998;82:560-3.
- Bhatia N, Agarwal R. Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. Prostate 2001;46:98-107.
- Peterson G, Barnes S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 1993;22:335-45.
- Onozawa M, Fukuda K, Ohtani M, Akaza H, Sugimura T, Wakabayashi K. Effects of soybean isoflavones on cell growth and apoptosis of the human prostatic cancer cell line LNCaP. Jpn J Clin Oncol 1998;28:360-3.
- Geller J, Sionit L, Partido C, Li L, Tan X, Youngkin T, et al. Genistein inhibits the growth of human-patient BPH and prostate cancer in histoculture. Prostate 1998;34:75-9.
- Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet 1993;342:1209-10.
