Differentiations of transplanted mouse spermatogonial stem cells in the adult mouse renal parenchyma in vivo1
Introduction
Stem cells exist in many tissues and are responsible for generating differentiated cells during an animal’s lifetime[1,2]. Understanding the cellular and molecular mechanisms of regulation for stem cell differentiation and for the development and functioning of gonads is of utmost importance to the future use of stem cells in transplantation or cell-based therapies and regenerative medicine, as well as understanding aging and tumor formation[3–6]. Recent advances in the establishment of male germ line stem cells have provided researchers with the ability to identify, isolate, maintain, expand, and differentiate the spermatogonia, the primitive male germ cells, as cell lines under in vitro conditions.
Although the potential of embryonic stem cells, which have the capacity to self-renew and undergo multilineage differentiation into a wide range of tissues, has been attested in numerous kinds of adult tissues[7–10], little is about the key regulatory mechanisms that control their differentiation. Moreover, whether germ line stem cells from neonatal mouse testes possess the characteristic of pluripotent of multilineage differentiation, similar to embryonic stem cells, has not been elucidated.
Researchers now believe that for most pluripotent stem cell types, the extracellular environment or niche provides signals necessary for differentiation and self-renewal[11]. However, until now there has been no evidence for the pluripotency and plasticity of adult spermatogonial stem cells, which are responsible for maintaining spermatogenesis throughout the male life. Given that evidence has shown that the transplantation of spermatogonial stem cells into specific tissue results in parenchyma formation[12], spermatogonial stem cells may have high proliferation activity, and their differentiation pathway may probably be altered. In the present study, we transplanted spermatogonial stem cells, isolated from neonatal mouse testes, into mouse renal parenchyma and evaluated the differentiation potential of spermatogonial stem cells in vivo.
Materials and methods
Purification and verification of mouse spermatogonial stem cells[13] In total, 5–7 d-old Balb/c neonatal male mice, obtained from Charles River Laboratories (Baltimore, MD, USA), were used for the isolation of spermatogonial stem cells. The testicles of the mice were obtained by scrotal insection under methoxyflurane anesthesia. Tunica albuginea were carefully denuded and digested with 1 g/L collagenase (1 mL; Sigma, St Louis, MO, USA) for 20 min, and then the supernatant were collected by centrifuging for 5 min. The supernatant were digested and centrifuged again by the same procedure as described earlier. The supernatant were cocultured with a digestive enzyme mix (containing 0.25% trypsin, 1 mL, and 1.5 g/L hyaluronidase, 1 mL) at 37 °C until the anatomical structure of the seminiferous tubule disappeared. The tissue pellet was then collected by centrifugation 3 min at 1000 r/min and suspended in 1.5 mL Dulbecco’s modified Eagle’s medium (DMEM) complete medium supplemented with 7.5% NBS, 7.5% fetal bovine serum (FBS), 1% glutamine, 1% non-essential amino acid, 1% penicillin, 1% streptomycin, and 1% pyruvic acid sodium. The tissue suspension (1.5 mL) was carefully layered on Percoll gradients centrifugate of different densities for 25 min at 1400 r/min. The cell suspension in the third and fourth layers of Percoll centrifugation were then aspirated using a clean Pasteur pipette, transferred to a clean centrifuge tube, washed once with 1.5 mL phosphate-buffered saline (PBS), and centrifuged at 1000 r/min for 10 min. The resultant cell pellet was suspended in DMEM complete medium and cultivated in a 60 mm culture flask at 5% CO2 at 37 °C for at least 3 h. After most of the testicular interstitial, peritubular myoid, and Sertoli cells were attached, the remnant cell suspension containing spermatogonial stem cells was collected and cultured.
The isolated spermatogonial stem cells were washed with PBS twice. The supernatant was discarded and the cells were added into 100 μL PBS. The cells were then stained according to the manufacturer’s recommendations with the monoclonal antibodies against fluorescein isothiocyanate (FITC)-conjugated a6-intergrin and c-kit (Biolegend). The incubations were performed at room temperature for 30 min. Control groups were incubated with FITC-conjugated mouse immunoglobulin G1 isotype antibodies. After incubation, the cells were washed with PBS containing 0.1% BSA. Approximately 1×106 cells were used for flow cytometry. Quantitative analyses were performed using a Beckman Coulter flow cytometer (Beckman Coulter).
Activity of mouse spermatogonial stem cells in vivo The spermatogonial stem cells were transplanted into the right testes of germ cell-depleted recipient mice by microinjection. The left testis served as an internal control. Recipients used were male Balb/c mice 5–7 weeks of age. The recipient mice were whole body irradiated with gamma rays from Co60 (10 Gy per mouse), which destroyed endogenous spermatogenesis and decreased immune activity. Two weeks after irradiation, the recipients were ready for transplantation. Approximately 20 μL spermatogonial stem cell suspension was transplanted at a concentration of 1×106 cells/mL via the rete testis into seminiferous tubules of the right testis of 30 animals. After 12 weeks, recipient mice were killed, and the testes were fixed in 4% paraformaldehyde (PFA) embedded in paraffin. The tissue sections were subjected to hematoxylin-eosin staining[14] . All of the experimental procedures complied with the National Regulations for the Care and Use of Laboratory Animals.
Establishment of the transplantation model The recipients were 30 female Balb/c mice 5–7 weeks of age, and were also whole body irradiated with gamma rays from Co60 (10 Gy per mouse). Twenty-four hours after irradiation, a dorsal incision was made in these recipient mice. When the kidney was exposed, spermatogonial stem cells at a density of 1×106 cells/mL were injected in parenchyma under the renal capsule by a TB syringe with a 29-gauge needle at 100 μL per mouse. The recipients were then kept under pathogen-free conditions in laminar flow boxes in accordance with established institutional guidelines and approved protocols. Twenty animals were transplanted with spermatogonial stem cells, and 10 animals were injected with DMEM culture medium and served as external controls. After 90 d transplantation, recipient mice were killed and the kidney tissue was taken out, fixed in 4% PFA, and embedded in paraffin. Tissues sections of each murine kidney were prepared for immunohistochemistry and fluorescence in situ hybridization (FISH) [15] .
Immunohistochemistry for the expression of ricinus communis agglutinin, vimentin, CD45, and F4/80 protein Serial four micron thick paraffin sections were dried and dewaxed. Sections were blocked with 10% normal goat serum at room temperature. All sections were developed with a 3,3'-diaminobenzidine-tetrachloride substrate (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and counterstained with hematoxylin. All sections were mounted, cover slipped, and examined with an Olympus microscope equipped with a digital camera. The primary antibodies used were as follows: goat antiricinus communis agglutinin (RCA) antibody (1:200 dilution) for the proximal convoluted tubule, mouse antivimentin antibody (1:500 dilution) for podocytes of the glomerulus, mouse anti-CD45 antibody (1:100 dilution) for leukocytes, and mouse anti-F4/80 protein (1:150 dilution) for macrophages. All primary antibodies were purchased from Santa Cruz Biotechnology, and diluted in PBS containing 0.3% Triton-X 100 and 10% FBS for use. All tissue sections were incubated with the primary antibodies overnight at 4 °C and incubated with the horseradish peroxidase-conjugated secondary antibodies. All the secondary antibodies were used at 1:200 dilutions with 2% FBS.
FISH for chromosome Y After being deparaffinized, the tissue slides were treated with Triton-X 100 for 90 s and digested with proteinase K for 30 min. The slides were then rinsed in PBS followed by rehydration. After drying, the sections were put in 30 μL denaturing solution (containing 70% formamide and 2× SSC, pH 7.2) at 72 °C for 5 min to allow nuclear denaturation. The specific painting probe for chromosome Y was labeled with fluorescence dye Cy3 (Cambio, UK). For each hybridization, approximately 100 ng probe, labeled with fluorescence dye Cy3, was used in a 10 μL hybridization mixture (containing 55% formamide, 2×SSC, and 10 μg human placenta DNA), which was denatured at 75 °C for 5 min. After the probes were prepared, the denatured slides were hybridized with probes in a moist chamber overnight at 42 °C. After incubation, the slides were washed twice in 2×SSC, 3 times in 2×SSC containing 5% formamide, and once in 0.1×SSC, respectively, at 42 °C. Finally, 10 μL 4,6-diamidino-2-phenylindole was added to counterstain the nuclei. The FISH signal of the Cy3-labeled probe was detected by an Olympus BX60 fluorescence microscope.
Spermatogonial stem cell differentiation into renal parenchyma cells We evaluated the transplanted spermatogonial stem cell differentiation into parenchyma cells in vivo. The Cy3- and RCA-positive (CD45 and F4/80 negative) cells or Cy3- and vimentin-positive (CD45 and F4/80 negative) cells were detected in 20 randomly chosen 1×1 mm areas of renal tissues. Data were presented as mean±SEM. Statistical analysis was performed using ANOVA. Duncan’s multiple range tests were used to evaluate difference between individual means (version 12; SPSS, Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
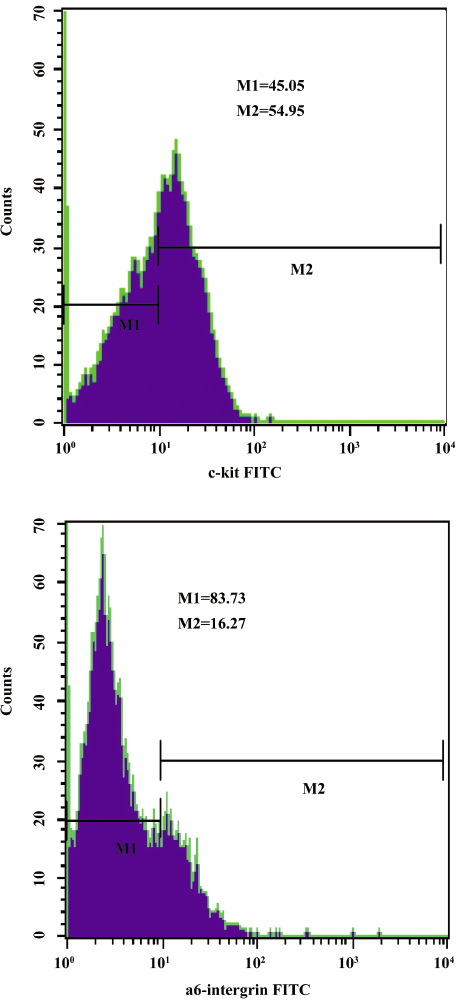
Purification and identification of mouse spermatogonial stem cells The isolated mouse spermatogonial stem cell phenotype was ascertained by staining with rat-specific monoclonal antibodies by flow cytometry. As shown in Figure 1, the c-kit expression was relative high and a6-intergrin was low. The results demonstrated that the cells we isolated were a crowd of undifferentiated stem cells, and the major component were type A spermatogonial stem cells (Figure 1).
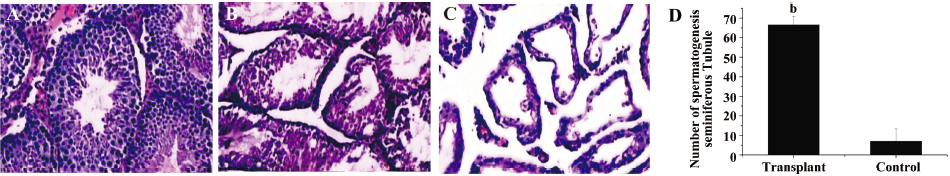
A histological analysis of the testes after 3 months of transplantation showed the appearance of germ cells with nuclear condensation in the inner cell layer of the tubules and sperm in the lumen. Spermatogonial stem cells migrated to the basement membrane and colonized the tubules. To assess the level of spermatogenesis in the recipient testes, the number of tubule cross-sections with evidence of spermatogenesis (defined as the presence of multiple layers of germ cells in the entire circumference of the seminiferous tubule) was recorded for 2 sections from each testis. The proliferation of seminiferous epithelial cells was distinctly observed in seminiferous tubules of the transplanted testes, whereas only a few seminiferous epithelial cells were observed in periphery of seminiferous tubules in non-transplanted control testes (Figure 2).
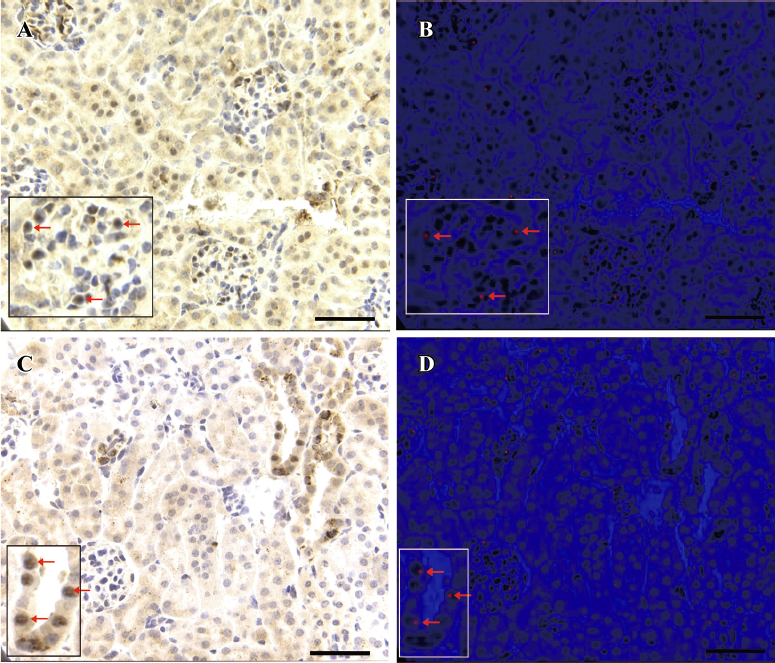
Characterization of the pluripotency of mouse spermatogonial stem cells in vivo Numerous Cy3-positive cells, which were a bright red fluorescent color, were detected in the translated kidney tissues at the site of injection through the FISH method. On the contrary, almost no red fluorescence signal was observed in the control kidneys. FISH combined with immunohistochemical staining of renal tissues indicated that 6.85%±0.31% RCA-positive cells expressing red fluorescence color in the brush border of the proximal convoluted tubule and epithelial cells located at the thin segment of the proximal convoluted tubule and collecting tubulus. Compared to the controls (0.79%±0.15%), the difference reached statistical significance (P<0.05). Additionally 11.08%±0.95% vimentin-positive cells expressed a red fluorescence color in the podocyte of the glomerulus or the basal lamina cells of renal tubulus. Compared to the controls (1.03%±0.45%), the difference reached statistical significance (P<0.05). According to the results of FISH and immunohistochemical staining, some podocytes of the glomerulus and epithelial cells of the renal tubules were identified to have chromosome Y (Figure 3).
Discussion
Spermatogonial stem cells, which normally divide asym-metrically, giving rise to 1 stem cell and one spermatogonium, can initiate differentiation to produce spermatozoa[16,17]. Spermatogonial stem cells maintain populations of highly differentiated, but short-lived cells or sperm through a critical balance between alternate fates: daughter cells either maintain stem cell identity or initiate differentiation[17]. The continuation of the spermatogenic process throughout life relies on a proper regulation of self-renewal and differentiation of the spermatogonial stem cells situated on the basal membrane of the seminiferous epithelium and accounting for only 0.03% of all germ cells.
The potential of embryonic stem cells to generate all lineages of embryos in vivo has been widely reported in the literature. However, several kinds of somatic stem cells have been thought to produce only the cell lineages characteristic of the tissues in which they reside for long time. Recent studies have suggested that some stem cells may have the potential for differentiation outside of their original tissues. Donor-derived cells have been found after transplantation in multiple non-hematopoietic tissues, including astrocytes in the brain[18], skeletal muscle[19], and bone[20]. Researchers[18,19,21,22] have demonstrated that all lineages of glia can be differentiated from stem cells in bone marrow. Conversely, stem cells derived from non-hematopoietic tissues, such as neural stem cells and muscle side population cells, have been found to differentiate into hematopoietic cells[20,22,23]. Some basic research has also confirmed that mesenchymal stem cells may differentiate into mesenchymal tissues, such as bone, cartilage, muscle, and adipose tissue[20]. One recent study showed that highly purified hematopoietic stem cells even have the potential to differentiate into muscle[19]. Moreover, Poulsom et al[24] found that bone marrow cells could differentiate into both epithelial cells of the renal tubule and podocytes of glomerulus in the adult mouse kidney.
Taken together, the pluripotency has been confirmed in most stem cells types. However, whether this process of multilineage differentiation does exist in spermatogonial stem cells has not been clarified. In this study, we transplanted the spermatogonial stem cells, isolated from neonatal mouse testis, into the renal parenchyma of female mice for observation of its differentiation. The results showed that some mature renal cells of female mice could express chromosome Y, the cell specific marker for spermatogonial stem cell origin. Furthermore, we demonstrated that cells with chromosome Y-positive expression in female mice were epithelial cells of the renal tubule and podocytes of the glomerulus, ruling out the possibility of macrophages or leukocytes.
To our knowledge, this is the first report that spermatogonial stem cells can not only restore damaged spermatogenesis, but can potentially differentiate into mature renal parenchyma cells in whole body irradiated adult mice. However, the exact mechanism of the pluripotency of spermatogonial stem cells is not clear. Previous studies have reported that spermatogonial stem cells can be pluripotentiality acquired and can be able to differentiate into derivatives of the embryonic germ layers[12] or cardiomyocytes[25]. Based on the current study and other published literature, we believed that spermatogonial stem cells may possess a high degree of plasticity, like other somatic stem cells. This multilineage differentiation may be attributed to local environmental change. When spermatogonial stem cells were transplanted into the injured renal parenchymal, some special factors (such as several transcriptional factors)[26] that exist in kidney may be involved the process of multipotent differentiation. Further studies are needed to determine the accurate mechanism.
References
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science 2000;287:1427-30.
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell 1997;88:287-98.
- Gage FH, Ray J, Fisher LJ. Isolation, characterization, and use of stem cells from the CNS. Annu Rev Neurosci 1995;18:159-92.
- Zhao GQ, Garbers DL. Male germ cell specification and differentiation. Dev Cell 2002;2:537-47.
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11.
- Donovan PJ, Stott D, Cairns LA, Heasman J, Wylie CC. Migratory and postmigratory mouse primordial germ cells behave differently in culture. Cell 1986;44:831-8.
- Rochette-Egly C, Chambon P. F9 embryo carcinoma cells: a cell autonomous model to study the functional selectivity of RARs and RXRs in retinoid signaling. Histol Histopathol 2001;16:909-22.
- Lehtonen E, Laasonen A, Tienari J. Teratocarcinoma stem cells as a model for differentiation in the mouse embryo. Int J Dev Biol 1989;33:105-15.
- McLaren A, Durcova-Hills G. Germ cells and pluripotent stem cells in the mouse. Reprod Fertil Dev 2001;13:661-4.
- Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development 1989;105:733-7.
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 2001;414:98-104.
- Guan K, Nayernia K, Maier LS, Wagner S, Dressel R, Lee JH, et al. Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 2006;440:1199-203.
- Izadyar F, Spierenberg GT, Creemers LB, den Ouden K, de Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reproduction 2002;124:85-94.
- Boettger-Tong HL, Johnston DS, Russell LD, Griswold MD, Bishop CE. Juvenile spermatogonial depletion (jsd) mutant seminiferous tubules are capable of supporting transplanted spermatogenesis. Biol Reprod 2000;63:1185-91.
- Gordeeva O, Zinovieva R, Smirnova Y, Payushina O, Nikonova T, Khrushchov N. Differentiation of embryonic stem cells after transplantation into peritoneal cavity of irradiated mice and expression of specific germ cell genes in pluripotent cells. Transplant Proc 2005;37:295-8.
- McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol 2000;163:3-9.
- de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol 1998;10:694-701.
- Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA 1997;94:4080-5.
- Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 1999;401:390-4.
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK. Multilineage potential of adult human mesenchymal stem cells. Science 1999;284:143-7.
- Nakano K, Migita M, Mochizuki H, Shimada T. Differentiation of transplanted bone marrow cells in the adult mouse brain. Transplantation 2001;71:1735-40.
- Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA 1999;96:14482-6.
- Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science 1999;283:534-7.
- Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, et al. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 2001;195:229-35.
- Guan K, Wagner S, Unsöld B, Maier LS, Kaiser D, Hemmerlein B, et al. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res 2007;100:1615-25.
- Kanatsu-Shinohara M, Lee J, Inoue K, Ogonuki N, Miki H, Toyokuni S, et al. Pluripotency of a single spermatogonial stem cell in mice. Biol Reprod 2008;78:681-7.
