Herbal medicine Yin Zhi Huang induces CYP3A4-mediated sulfoxidation and CYP2C19-dependent hydroxylation of omeprazole1
Introduction
Yin Zhi Huang (YZH), a decoction of Yin Chin (Artemisia capillaris) and 3 other herbs, is widely used in Asia to prevent and treat jaundice[1–3]. Recently, Huang et al demonstrated that YZH activated the constitutive androstane receptor (CAR, NR1I3), a key regulator of bilirubin clearance in the liver[4–6]. CAR leads to the increased expression of several CYP450 enzymes, including cytochrome P450 (CYP) 3A4 and CYP2C19[7,8]. Therefore, we suppose that YZH may be a modulator of CAR-targeted CYP450 isoenzymes. As a result, in clinical situations, herb-drug interactions may occur between YZH and substrate drugs of these CYP450 enzymes.
Omeprazole is a well-known CYP2C19 substrate which undergoes hydroxylation to form its major metabolite 5-hydroxyomeprazole. CYP3A4 mediates the sulfoxidation of omeprazole to form the second major metabolite omeprazole sulfone. CYP2C19 is a genetic polymorphism enzyme. A person exhibits either extensive or poor CYP2C19 enzyme activity. The latter phenotype occurs in 1%–3% of Caucasians and 13%–23% of Asians[9]. The most common CYP2C19 allelic variants causing the poor meta-bolizer (PM) phenotype contain a splicing defect (CYP2C19*2) or premature stop codon (CYP2C19*3)[10]. The polymorphism of CYP2C19 was reported to exhibit a marked effect on the pharmacokinetics of omeprazole[11].
The pharmacokinetics of omeprazole is also prone to be affected by CYP450 inducers, such as rifampicin[12,13] and some herbal medicines like St John’s wort and Ginkgo-biloba[14,15].
Until now, the effects of YZH on CYP450 subtypes and their clinical significance have still not been determined; herb–drug interactions for this herbal remedy are generally not known or are poorly studied. Thus, we carried out the present study to observe the impacts of YZH on CYP2C19 and CYP3A4 enzyme activities, and the potential herb-drug interaction involving YZH and omeprazole related to different CYP2C19 genotypes.
Materials and methods
Drugs and reagents YZH oral liquid was purchased from Shuang He (Beijing, China, N
Patients Eighteen unrelated, healthy adult men, from a total of 110 healthy Chinese volunteers, who had been screened for the CYP2C19 genotype, were recruited for this study (6 CYP2C19*1/CYP2C19*1, 5 CYP2C19*1/*2, 1 CYP2C19*1/*3, and 6 CYP2C19*2/CYP2C19*2). Genotyping procedures for identifying CYP2C19 wild-type gene (CYP2C19*1) and the 2 mutated alleles, CYP2C19*2 in exon 5 and CYP2C19*3 in exon 4, were performed by the PCR–restriction fragment length polymorphism method as described previously[9]. This study was approved by the Ethics Committee Board of Central South University, Hunan, China. The patients gave written informed consent to participate. All of the patients finished the entire protocol of this study. The participants were required between the ages of 18 and 28 years, non-smokers, and have a standard body mass index between 18 and 30 kg/m2. The patients were healthy with no clinically relevant conditions identified from medical history, physical examinations, electro-cardiogram, and routine laboratory tests (blood chemistry, hematology, and urine analysis). All the patients were refrained from use of any prescription or non-prescription medication 2 weeks before and throughout the study. They were also abstained from grapefruit juice, herbal dietary supplements, and caffeine-containing beverages, including coffee and green tea, 2 weeks before the study and during the study period. The volunteers were served standard meals and were monitored during the experimental period for the development of any possible adverse effects.
Study design and clinical protocol The study was carried out in a 2-phase crossover manner with a 5 week washout period between phases. In each phase, 18 volunteers received placebo or YZH oral liquid 10 mL 3 times daily for 14 d. On the 15th day, all the patients were given a single oral dose of omeprazole (20 mg capsule) with 250 mL of warm water, after an overnight fast. Two to four hours later, they had access to water and received breakfast. Omeprazole was given at 8:00 h, and 5 mL blood samples were collected into heparinized tubes from the antecubital vein immediately before (0 min) and at 0.25, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, and 12 h after drug administration. Blood samples were centrifuged at 2000×g for 10 min, and plasma was separated and stored at -80 °C until analyzed.
Analytic method of omeprazole and its metabolites Plasma concentrations of omeprazole and its metabolites, 5-hydroxyomeprazole and omeprazole sulfone, were quantified by validated HPLC with UV detection at a wavelength of 302 nm according to the method previously described[14] with minor modifications. The HPLC system (SHIMADZU 2010C HPLC series, Kyoto, Japan) was composed of a solvent-delivery system, an online degasser, an automatic sample injector, and a variable-wavelength detector. The data analysis included Class VP systems. In brief, a mixture of 1000 µL of a plasma aliquot and 100 µL propranolol (internal standard) were extracted with 5 mL dichloromethane. Each tube was blended in a vortex mixer vigorously for 3 min and centrifuged at 2500×g for 10 min. The organic layer was decanted and evaporated to dryness under nitrogen. The residue was reconstituted with 100 µL of the mobile phase, and 20 µL of this solution was injected into an HPLC column. A Hypersil BDS C18 column (particle size, 5 µm; 4.6 mm×200 mm; Thermo Co, Phenomenex, US) was used as an analytic column. The mobile phase was a mixture of 10 mmol/L ammonium formate (pH 4), methanol, and acetonitrile (60:25:15, v/v/v). The average recoveries of omeprazole, 5-hydroxy-omeprazole, omeprazole sulfone, and the internal standard were 89%, 73%, 87%, and 85%, respectively. The interday coefficients of variation for omeprazole, 5-hydroxy-omeprazole, and omeprazole sulfone were 5.1%, 5%, and 10%, respectively, indicating high reproducibility. The validated limit of quantification was 2.656 ng/mL for omeprazole. The retention times were 5.25, 8.69, 9.86, and 11.52 min for the internal standard, 5-hydroxy-omeprazole, omeprazole sulfone, and omeprazole, respectively, and the flow rate was 1 mL/min.
Analytic method of YZH components The concentrations of 4 main components in YZH oral liquid baicalin, geniposide, chlorogenic acid, and 6,7-dimethylesculetin (scoparone) were quantified by validated HPLC with UV detection at a wavelength of 276, 230, 335, and 280 nm, respectively, according to the method previously described[29–32] with minor modifications. The HPLC system (Agilent 1100 series, Agilent Co, California, USA) was composed of a solvent-delivery system, an online degasser, an automatic sample injector, and a variable-wavelength detector. The data systems included a HP Chem Station controller (Hewlett-Packard, Houston, Florida, USA). A ZORBAX Eclipse XDB-C18 column (particle size, 5 µm; 4.6×150 mm; Agilent, California, USA) was used as an analytic column. The mobile phase was a mixture of 0.625% acetic acid and acetonitrile (85:15, v/v) for chlorogenic acid, 0.625% acetic acid and methanol (75:25, v/v) for 6,7-dimethylesculetin, 0.625% acetic acid and acetonitrile (75:25, v/v) for baicalin, and a mixture of distilled water and acetonitrile (80:20, v/v) for geniposide. The average recoveries of baicalin, geniposide, chlorogenic acid, and 6,7-dimethylesculetin were 100.1%, 100.15%, 93.6%, and 97.27%, respectively. The interday coefficients of variation for baicalin, geniposide, chlorogenic acid, and 6,7-dimethylesculetin were 1.66%, 1.63%, 3.5%, and 1.96%, respectively, indicating high reproducibility.
Pharmacokinetic analysis The area under the plasma concentration-time curves (AUC) of omeprazole and its metabolites were calculated by the linear trapezoidal method and further extrapolated to infinity by dividing the last experimental concentration by the terminal slope (λ). The terminal elimination rate constant (λ) was determined by loglinear regression, and the terminal elimination half-life (T1/2) was determined by the following relationship: T1/2=0.693/λ. The peak plasma concentration (Cmax) and time to peak concentration (tmax) were assigned by visual inspection of the data. The AUC from time 0 to infinity (0–∞) ratios of omeprazole to 5-hydroxyomeprazole (AUCOPZ/AUCOH-OPZ) and omeprazole to omeprazole sulfone (AUCOPZ/AUCOPZ-SUL) were calculated as the apparent markers of CYP2C19 and CYP3A4 activities, respectively[14,16,17].
Statistical analysis The pharmacokinetic parameters of omeprazole and its metabolites treated by placebo and YZH were analyzed using the paired Student’s t-test. The mean changes in pharmacokinetic parameters among the 3 CYP2C19 genotype groups were compared, using one-way ANOVA. P<0.05 was considered statistically significant for all tests. All statistical analyses were performed using SPSS software (version 11.0, SPSS, Chicago, IL, USA). Data are expressed as mean±SEM.
Results
YZH components analysis Qualitative and quantitative measurement by HPLC of 4 components of YZH including baicalin, chlorogenic acid, 6,7-dimethylesculetin, and geniposide are presented in Figure 1. In every 10 mL YZH oral liquid, there is 442.49±75.38 mg 6,7-dimethyles-culetin, 29.7±5.64 g baicalin, 39.64±0.002 mg geniposide, and 476.22±58.16 mg chlorogenic acid.
Plasma omeprazole pharmacokinetics The mean pharmacokinetic parameters of omeprazole and its metabolites after 14 d of treatment by placebo and YZH are summarized in Table 1. After YZH administration, the plasma concentrations of omeprazole significantly decreased in comparison to the control (placebo; Figure 2A). The Cmax decreased by 38.84%±25.72% (P=0.030), 30.33%±18.22% (P=0.019), and 15.69%±5.18% (P=0.001) in the CYP2C19*1/CYP2C19*1, CYP2C19*1/CYP2C19*2 or *3 and CYP2C19*2/CYP2C19*2 patients, respectively; the AUC(0–∞) of omeprazole decreased by 46.69%±17.82% (P=0.007), 41.38%±14.04% (P=0.002), and 16.25%±12.18% (P=0.039) in CYP2C19*1/CYP2C19*1, CYP2C19*1/CYP2C19*2 or *3 and CYP2C19*2/CYP2C19*2 patients, respectively. No significant difference in tmax was observed for omeprazole between the placebo and YZH treatment phases.
Full table
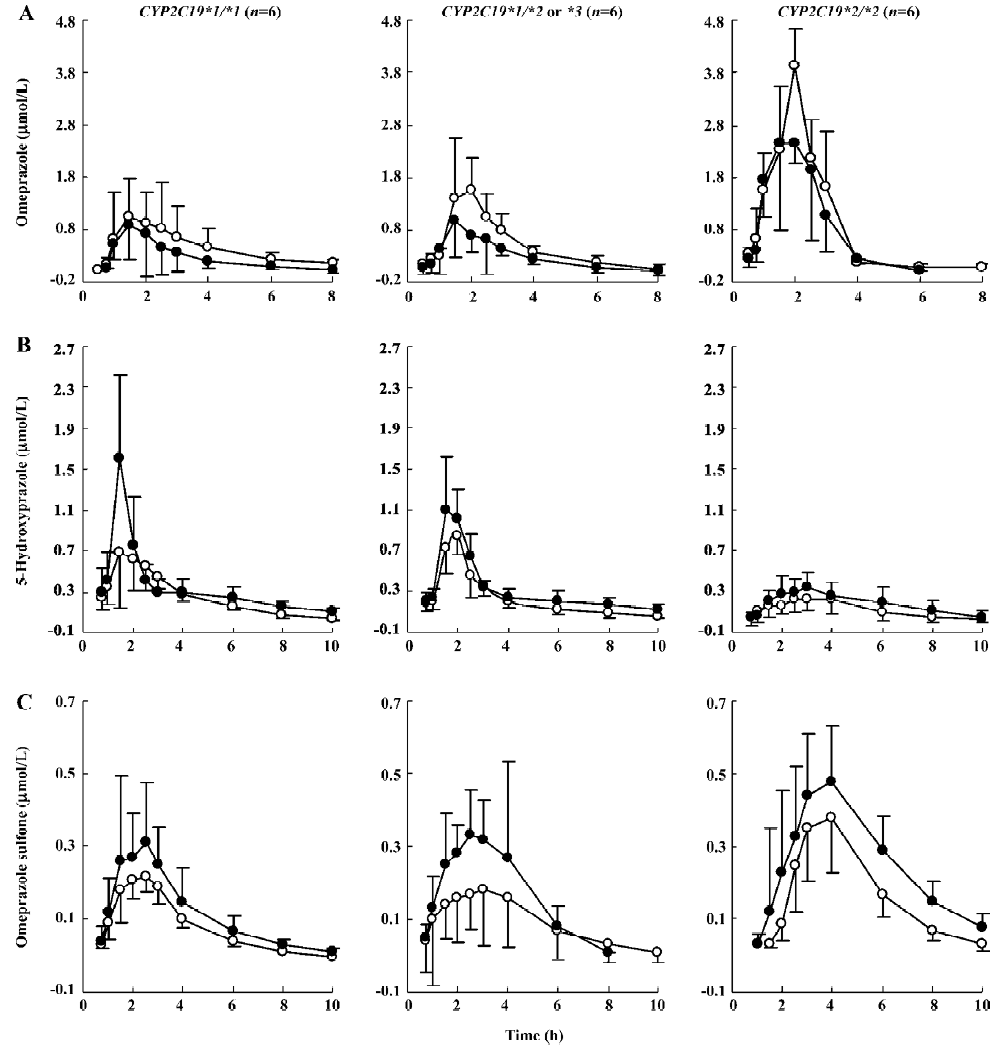
Plasma pharmacokinetics of omeprazole metabolites YZH greatly increased the plasma concentrations of 5-hydroxyomeprazole and omeprazole sulfone (Figure 2B, 2C). The Cmax and AUC(0–∞) of 5-hydroxyomeprazole increased by 81.43%±41.54% (P=0.001), 54.11%±33.57% (P=0.001), 42.82%±16.83% (P=0.001), 49.56%±46.79% (P=0.027), 31.93%±22.97% (P=0.012), and 40.07%±33.56% (P=0.024) in CYP2C19*1/CYP2C19*1, CYP2C19*1/CYP2C19*2 or *3 and CYP2C19*2/CYP2C19*2 patients, respectively,whereas the Cmax and AUC(0–∞) of omeprazole sulfone increased by 73.25%±31.09% (P=0.002), 57.44%±30.33% (P=0.000), 45.25%±41.27% (P=0.026), 40.14%±39.36% (P<0.05), 22.54%±15.81% (P=0.012), and 59.92%±36.69% (P=0.007) in CYP2C19*1/CYP2C19*1, CYP2C19*1/CYP2C19*2 or *3 and CYP2C19*2/CYP2C19*2 patients, respectively.
Omeprazole hydroxylase activity Based on the changes in the omeprazole and 5-hydroxyomeprazole plasma concentrations, the AUCOPZ/AUCOH–OPZ ratios (an index of omeprazole hydroxylase activity) decreased by 64.80%±12.51% (P=0.001), 57.98±14.80% (P=0.002), and 37.74%±16.07% (P=0.004) in CYP2C19*1/CYP2C19*1, CYP2C19*1/CYP2C19*2 or *3 and CYP2C19*2/CYP2C19*2 patients, respectively (Figure 3A). The decrease in the AUCOPZ/AUCOH–OPZ ratio was significantly greater in CYP2C19*1/CYP2C19*1 and CYP2C19*1/CYP2C19*2 or *3 groups than those in the CYP2C19*2/CYP2C19*2 group (P=0.047 or 0.009, Figure 3A).
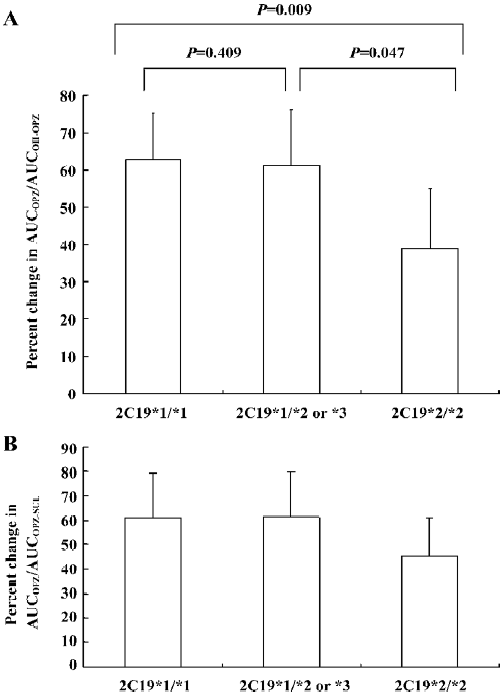
Omeprazole sulfoxidation activity The AUCOPZ/AUCOPZ–SUL ratios (an index of omeprazole sulfoxidation activity) also significantly decreased after YZH administration by 63.31%±18.45% (P=0.004), 54.87%±18.42% (P=0.003), and 45.16%±15.54% (P=0.003) in the CYP2C19*1/CYP2C19*1, CYP2C19*1/CYP2C19*2 or *3 and CYP2C19*2/CYP2C19*2 patients, respectively (Figure 3B). No significance was observed in the change degrees of the AUCOPZ/ AUCOPZ–SUL ratio among the 3 2C19 genotypes (P>0.05, Figure 3B).
Discussion
Since there may be variability in the YZH products currently on the market[18], in order to be better controlled, the YZH oral liquid used in our study was of the same batch number. Moreover, we quantified 4 main components of YZH oral liquid using HPLC with UV detection, which might help explain the results and conclusions extrapolated from our study in some ways. Baicalin accounts for 97.69% for every 10 mL YZH oral liquid used in our experiment, which is in agreement with the manufacture standard of YZH to contain above 90% baicalin.
Because the first and last time points of quantifiable concentrations for the 3 analytic compounds varied among individuals, and to keep the data comparable, we analyzed data by using AUC(0–∞).
In this study, we demonstrate that YZH significantly induces the CYP2C19-dependent omeprazole hydroxylation. We use the AUC ratio of omeprazole to 5-hydroxyomeprazole to evaluate the phenotypic activity of omeprazole hydroxylation, which is recognized to be a valid representation of the hydroxylation phenotype activity[14,17]. Moreover, a recent study indicated that AUCOPZ/AUCOH–OPZ was likely to be a more reliable and sensitive method for probing CYP2C19 than those previously used[9]. In this study, we show that YZH treatment caused a 16.08%–79.36% decrease in AUCOPZ/AUCOH–OPZ. The change percentage of AUCOPZ/AUCOH–OPZ following YZH administration in CYP2C19*1/CYP2C19*1 and CYP2C19*1/CYP2C19*2 or *3 are 1.5 and 1.7 times greater than that of CYP2C19*2/CYP2C19*2, respectively, exhibiting a gene-dosage-inductive effect of YZH on CYP2C19 activity. In our current study, CYP2C19 *2 homozygotes display a large degree of induction. Nevertheless, PM have no or low CYP2C19 activity, and thus would not be expected to be markedly induced[12]. Two possible explanations for this discrepancy are that CYP2C19 still has some level of activity in the PM with *2 mutation and thus can be induced, or the induced hydroxylation is catalyzed by a non-CYP2C19 hydroxylase[13,14].
We also observed a potent inductive action of YZH on the CYP3A4-mediated metabolic pathway of omeprazole sulfoxidation. YZH treatment produced a marked decrease in the AUCOPZ/AUCOPZ–SUL ratios of all the patients, indicating that YZH is also a potential in vivo inducer of CYP3A4.
The effects of the components of YZH on CYP450 have previously been studied. Baicalin could induce several CYP450 in rat liver microsomes[19], while chlorogenic acid has no obvious effects on CYP450[20]. Due to a high concentration of baicalin in YZH oral liquid, the inductive effect of baicalin on CYP3A4 and CYP2C19 might be one of the mechanisms for our study. Moreover, the nuclear receptor CAR is involved in molecular mechanisms of CYP450 induction by binding to the promoters of both CYP2C19 and CYP3A4[7,8]. Therefore, 6,7-dimethylesculetin, an agonist for CAR, according to Huang et al[4], might increase CYP2C19 and CYP3A4 expression in the mRNA level and further increases CYP2C19 and CYP3A4 enzyme activities, which also helps explain the results of our study. Similarly, rifampin and St John’s wort induce CYP450 enzymes and interact with other drugs in clinical situations by activating another nuclear receptor, the pregnane X receptor[21,22]. Since CAR transcriptionally regulates the expression of other CYP450 subtypes, such as CYP2B6 and CYP2C9[23–25], the relationships between YZH and other CYP450 enzymes are worth further exploration.
As we mentioned earlier, like other herbal remedies, YZH contains many components which may affect various drug metabolic enzymes, drug transporters, and receptors[4–6,20]. Thus, herb-drug interactions are likely to be more complicated than drug-drug interactions. Herbal medicine St John’s wort was reported to exert a biphasic effect on CYP enzymes starting with a parent inhibition followed by a significant induction during long-term use due to its enzyme inhibiting-, expression inducing-, and drug transport-promoting properties[26,27]. However, we do not know whether or not YZH affects the activities of the 2 enzymes in a similar way.
In this study, YZH use caused more than a 15% of decrease in the Cmax and AUC(0–∞) of omeprazole. YZH is beginning to be taken as herbal tea by people around the world[26], and our current study emphasized that clinicians should be alert to other drugs catalyzed mainly by CYP2C19[27], such as the protease inhibitor nelfinavir, tricyclic antidepressants (imipramine, amitriptyline, and clomipramine), benzodiazepines (diazepam and flunitrazepam), proguanil and other proton pump inhibitors (lansoprazole and pantoprazole), and those catalyzed mainly by CYP3A4[28] to avoid possible clinically significant interactions with YZH. It takes time to arouse the attention of people to the adverse effects of a drug, especially for traditional Chinese medicine (TCM). However, with more and more adverse effects of TCM occurring[29], it is urgent for clinical pharmacologists to guide people to take TCM more safely and effectively.
In conclusion, YZH, acting as an inducer of both CYP3A4 and CYP2C19, increases the metabolism of omeprazole in a CYP2C19 genotype-dependent manner and results in decreased plasma omeprazole concentrations. Attention should be paid for clinicians to avoid related drug interactions when YZH is co-administrated with CYP2C19 and CYP3A4 substrate drugs like omeprazole.
References
- Yin J, Wennberg RP, Xia YC, Liu JW, Zhou HZ. Effect of a traditional Chinese medicine, yin zhi huang, on bilirubin clearance and conjugation. Dev Pharmacol Ther 1991;16:59-64.
- Yin J, Wennberg RP, Miller M. Induction of hepatic bilirubin and drug metabolizing enzymes by individual herbs present in the traditional Chinese medicine, yin zhi huang. Dev Pharmacol Ther 1993;20:186-94.
- Dong YS, Huang ZH, Wu LF. Treatment of infantile hepatitis syndrome with injection of yin zhi huang. Zhongguo Zhong Xi Yi Jie He Za Zhi 1992; 12: 26-7, 5–6.
- Huang WD, Zhang J, Moore DD. A traditional herbal medicine enhances bilirubin clearance by activating the nuclear receptor CAR. J Clin Invest 2004;113:137-43.
- Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, et al. Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci USA 2003;100:4156-61.
- Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, et al. Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA 2003;100:4150-5.
- Goodwin B, Hodgson E, D’costa JD, Liddle C. Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Mol Pharmacol 2002;62:359-65.
- Chen YP, Ferguson SS, Goldstein JA. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol 2003;64:316-24.
- de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of mephenytoin metabolism in humans. J Biol Chem 1994; 269: 15 419–22.
- Brosen K, de Morais SM, Meyer UA, Goldstein JA. A multifamily study on the relationship between CYP2C19 genotype and S-mephenytoin oxidation phenotype. Pharmacogenetics 1995;5:312-7.
- Chang M, Tybring G, Dahl ML, Gotharson E, Sagar M, Seensalu R, et al. Interphenotype differences in disposition and effect on gastrin levels of omeprazole-suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol 1995;39:511-8.
- Zhou HH, Anthony LB, Wood AJ, Wilkinson GR. Induction of polymorphic 4'-hydroxylation of S-mephenytoin by rifampicin. Br J Clin Pharmacol 1990;30:471-5.
- Feng HJ, Huang SL, Wang W, Zhou HH. The induction effect of rifampicin on activity of mephenytoin 4'-hydroxylase related to M1 mutation of CYP2C19 and gene dose. Br J Clin Pharmacol 1998;45:27-9.
- Yin OQP, Tomlinson B, Waye MMY, Chow AHL, Chow MSS. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics 2004;14:841-50.
- Wang LS, Zhou G, Zhu B, Wu J, Wang JG, Abd El-Aty AM, et al. St John’s wort induces both cytochrome P450 3A4-catalyzed sulfoxidation and 2C19-dependent hydroxylation of omeprazole. Clin Pharmacol Ther 2004;75:191-7.
- Sun H, Zuo Z, Yin OQ, Tomlinson B, Chow MS. A. ‘high-throughput’ cocktail method for screening the effect of herbal product on liver isozyme activities: experience with Ginkgo biloba. Clin Pharmacol Ther 2002;45:27-29.
- Palovaara S, Tybring G, Laine K. The effect of ethinyloestradiol and levonorgestrel on CYP2C19-mediated metabolism of omeprazole in healthy female subjects. Br J Clin Pharmacol 2003;56:232-7.
- Meng QH, Liu YS, Jing SM, Hu YZ. Study on the application of index of composite information of chromatographic fingerprints in quality control of traditional Chinese medicine. Chin J Nat Med 2004;2:359-64.
- Hao YN, Zhu XY, Cheng GF. Effects of baicalin on liver microsomal cytochrome P450 system. Pharm J Clin PLA 2000;16:68-71.
- Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St John’s wort, a herbal preparation used in the treatment of depression. J Pharmacol Exp Ther 2000;294:88-95.
- Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, et al. St John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci USA 2000;97:7500-2.
- Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol 1999;56:1329-39.
- Finch CK, Chrisman CR, Baciewicz AM, Self TH. Rifampin and rifabutin drug interactions: an update. Arch Intern Med 2002;162:985-92.
- Ferguson SS, Lecluyse EL, Megishi N, Goldstein JA. Regulation of human CYP2C9 by the constitutive androstane receptor: discovery of a new distal binding site. Mol Pharmacol 2002;62:737-46.
- Lamba V, Lamba J, Yasuda K, Strom S, Davila J, Hancock ML, et al. Hepatic CYP2B6 expression: gender and ethnic differences and relationship to CYP2B6 genotype and CAR (constitutive androstane receptor) expression. J Pharmacol Exp Ther 2003;307:906-22.
- Lazar MA. East meets west: a herbal tea finds a receptor. J Clin Invest 2004;113:23-5.
- Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P4502C19 genetic polymorphism. Clin Pharmacokinet 2002;41:913-58.
- Plant NJ, Gibson GG. Evaluation of the toxicological relevance of CYP3A4 induction. Curr Opin Drug Discov Devel 2003;6:50-6.
- Sparreboom A, Cox MC, Acharya MR, Figg WD. Herbal remedies in the United States: potential adverse interactions with anticancer agents. J Clin Ontol 2004; 22: 12 2489–503.
