Structure-efficacy relationships of immunostimulatory activity of CpG-containing oligodeoxynucleotides on mouse spleen cells1
Introduction
Certain molecular structures that are present in pathogens (pathogen-associated molecular patterns [PAMP]) are recognized by innate immune cells via pattern recognition receptors. The cells are activated upon recognition of PAMP and trigger the generation of optimal adaptive immune responses. The bacterial genome, compared to vertebrate DNA, contains a higher frequency of unmethylated deoxy-cytidyl-deoxyguanosine (CpG) dinucleotides. Small oligodeoxynucleotides (ODN) with unmethylated CpG dinucleotides (CpG ODN) are able to perfectly mimic the immunostimulatory activity of bDNA[1,2]. CpG ODN are known to stimulate innate and adaptive immunity because of their interesting immunostimulatory properties in a number of vertebrate, such as stimulating B-cell proliferation[3–5], enhancing the expression and synthesis of cytokines[6], and promoting natural killer (NK) cell cytotoxicity[7]. Multiple studies have shown that CpG ODN-induced activation is presumably a 2-step process. First, CpG ODN, independent of their sequence, are recognized by different receptors[1,2,8–10]; Mac-1 surface immunoglobulins and scavenger receptors are possibly involved in this process. The cellular uptake of ODN is sequence independent, but can be influenced dramatically by backbone modifications. Phosphorothioated modifications can enhance cellular uptake and increase immunostimulatory activity and half-time of CpG ODN[9,11]. In addition, runs of at least 4 guanosines have been reported to enhance the cellular uptake of ODN due to the fact that base-quartet-stabilized, 4-stranded helices called tetraplexes are formed[10,12]. Second, CpG ODN trigger the motif-dependent recognition of Toll-like receptor 9 (TLR-9) within the endosome and initiate immunocellular activation.
Immunostimulatory activities of CpG ODN depend on their structural and chemical characteristics. A number of researchers have studied the relationship between the CpG ODN structure and their immunostimulatory properties. CpG ODN were structurally and functionally divided into 3 types in previous studies for humans: A type (also known as D type), B type (also known as K type), and C type. A-type CpG ODN is capable of activating human plasmacytoid dendritic cells (pDC) to produce large amounts of type I IFN (α/β) and strongly activates NK cells. It has mixed phosphodiester/phosphorothioate backbones and contains a single hexameric purine/pyrimidine/CG/purine/pyrimidine motif flanked by self-complementary bases that form a stem-loop structure capped at the 3'-end by a poly G tail[13]. B-type CpG ODN primarily activates B cells, resulting in their proliferation and antibody secretion. It has phosphorothioate backbones and encodes multiple TCGTT and/or TCGTA motifs[13,14]. C-type CpG ODN optimally consist of a stimulatory hexameric CpG motif (5'-TCGTCGTT-3') linked by a T spacer to GC-rich palindromic sequences (5'-CGGCGCGCG-CCG-3')[15]. The C-type CpG ODN very strongly stimulates B cells as well as type I IFN secretion in vitro in human peripheral blood mononuclear cells. In vivo, it is a strong, Th1-inducing adjuvant. In addition, another C-type CpG ODN is reported to display a B-type CpG ODN structure feature at the 5'-end and A-type CpG ODN structure feature at the 3'-end and shares the activities of both A- and B-type CpG ODN[16]. Furthermore, the sequence of the optimal human hexamer motif was reported to be 5'-GTCGTT-3'[17] and the optimal mouse motif was 5'-GACGTT-3' [1].
Despite the previously mentioned reports, more detailed and systematic structure-activity relationship (SAR) studies of CpG ODN are still lacking. In this study, we tried to reveal some changes of the immunostimulatory properties of CpG ODN induced by the modifications of the ODN structure, for instance, additions and deletions of the functional CpG motifs, modification of the distance and the sites of CpG motifs, and the addition of self-complementary sequences. We recruited CpG 1826, an effective immuno-stimulatory B-type CpG ODN for murine, as the positive control and the template of the SAR study. This ODN was a strong B-cell stimulator, but a weaker inducer of IFN-α[1,18,19]. Various modifications on the first-class structural level were performed on the basis of ODN1826, and the immuno-stimulatory activities of the structurally-modified CpG ODN were comprehensively investigated, including the ability to stimulate mouse B cell proliferation, the ability to induce cytokines (interleukin [IL]-6, IL-12, and IFN-α) secretion and the ability to induce NK cell killing activity, and the changes of the expression of various lymphocyte surface molecules. Finally, the relationship between the CpG ODN structures and their immunostimulatory properties were primarily concluded.
Materials and methods
CpG ODN Purified, single-stranded, phosphorothioated ODN containing CpG motifs were synthesized by Sangon Biotech Company (Shanghai, China). The sequences used in this study are listed in Table 1, where ODN1 was the intact 1826, positive control, and the template; ODN 2–12 were the sequences modified based on the structure of 1826; and ODN13–15 were negative controls.
Full table
Cells and cell culture Spleen cells from 4- to 8-week-old C57BL/6 mice were cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum (GIBCO, Carlsbad, CA, USA) and antibiotics (100 IU/mL penicillin and 100 µg/mL streptomycin). Mouse B cells were obtained by centrifugation over Lympholyte M (CEDARLANE Laboratories, Hornby, Ontario, Canada) as described previously[1]. Yeast artificial chromosome-1 (YAC-1) cells were kindly provided by Prof Wen-xia ZHOU (Beijing Institute of Pharmacology and Toxicology, Beijing, China).
Cytokine ELISA The mouse spleen cells were plated onto 24-well dishes (3×106 per well). The CpG ODN, dissolved in TE buffer, were added to the cultured cells at a final concentration of 0.05, 0.25, and 1.0 µmol/L, respectively. The cells were cultured at 37 °C in a humidified incubator with 5% CO2, and the culture supernatants (SN) were collected at the appointed time-points. If not used immediately, the SN were stored at -20 °C until the assay. The amounts of murine IL-6, IL-12, and IFN-α in the SN were measured using commercially available ELISA kits (R&D Systems, Minneapolis, MN, USA). The experiments were performed 2 or 3 times for each CpG ODN in triplicate for each concentration.
Proliferation assays The proliferation of mouse B cells was determined by a [3H]thymidine incorporation assay as described previously[1]. Briefly, the spleen cells (5×105/well) were plated onto Costar 96-well plates (Corning Incorporated Corning, New York, NY, USA) and stimulated with CpG ODN at various concentrations (0.05, 0.25, and 1.0 µmol/L) or controls for 56 h, and then 0.5 µCi of [3H] thymidine was added to each well. The cells were harvested after incubation for another 16 h and the radioactivity was measured using a MicroBeta liquid scintillation counter (PerkinElmer, Boston, MA, USA). All assays were performed 4 times.
Measurement of NK-mediated cytotoxicity The NK cytotoxicity of mouse spleen cells stimulated by CpG ODN was assessed by standard 4 h 51Cr-release assays as previously described[7]. Briefly, the mouse spleen cells were incubated with CpG ODN (0.25 μmol/L) for 36 h and then harvested as effector cells. One million YAC-1 cells, used as target cells, were incubated with 50 µCi of 51Cr for 1 h at 37 °C, then washed several times and incubated for 4 h with the effector cells (E:T ratio, 50:1). Thereafter, the SN were harvested and the radioactivity was measured using a MicroBeta liquid scintillation counter (PE, Boston, MA, USA). The results were expressed as the percentage of specific lysis in terms of mean±SD of the results read in triplet wells. The following formula was used: percent specific lysis (%)=(experimental counts-target cell spontaneous release counts)/(maximal release counts-target cell spontaneous release counts)×100. Spontaneous lysis was measured from wells containing only target cells, whereas maximum lysis was measured from the wells containing target cells incubated with 10% SDS.
The cytotoxicity of mouse spleen cells stimulated by CpG ODN to the B16 cells was measured using B16 cells as target cells as described earlier.
Abs and flow cytometry The mouse spleen cells were incubated with or without CpG ODN (0.25 µmol/L) for 48 h, and then the B cells, pDC, and NK cells were analyzed by two-color staining on a FACS Calibur flow cytometer (BD Pharmingen, Franklin lakes, New Jersey, USA). For B cells, FITC-labeled anti-CD19 and PE-labeled anti-CD80 mAbs were used. For pDC, FITC-labeled anti-CD11c and PE-labeled anti-Iab mAbs were used. For the NK cells, FITC-labeled anti-CD94 fmAbs were used (BD Pharmingen, Franklin lakes, NJ, USA).
Animals Female BALB/c mice (6–8 weeks old) were used for all the experiments and were purchased from Experimental Animal Center of Academy of Military Medical Sciences (Beijing, China; certificate N
Statistical analysis Data are shown as mean±SD. Statistically significant differences were determined by Student’s t-test. Differences were considered statistically significant when P<0.05.
Results
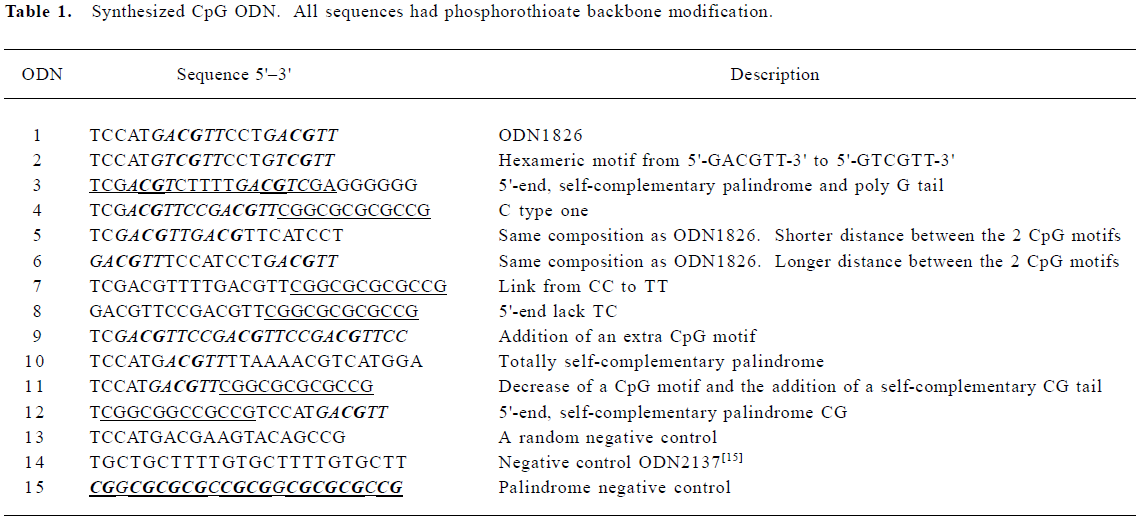
Production of cytokines induced by CpG ODN In the present study, we investigated the production of several cytokines, including murine IL-6, IL-12 (P40), and IFN-α in immunocytes stimulated by CpG ODN. The positive control ODN1826 (ODN1) is a typical B-class CpG ODN. It consists of 2 stimulatory hexameric CpG motifs (5'-TC
With regards to IL-12, the tendency of sequence-dependent stimulatory activity was similar to that of IL-6. ODN4, ODN5, ODN7, ODN10, ODN11, as well as ODN1826, induced relatively high levels of IL-12 P40 (Figure 1B). However, ODN1826 was no longer the strongest stimulator for IL-12 production. At the highest studied concentration of 1.0 μmol/L, ODN1826 treatment upregulated IL-12 P40 secretion to 145±12 pg/mL. At the same dose, ODN4, ODN7, ODN10, and ODN11 stimulated IL-12 P40 production to 172±14, 188±14, 229±17, and 184±16 pg/mL, respectively (P<0.05).
ODN1826 was a relatively weaker IFN-α stimulator (90±7 pg/mL at the dose of 1.0 µmol/L) compared to its IL-6-indu-ing activity in this study. Our findings were consistent with those reported in other studies (Figure 1C)[15]. Among all the ODN studied, the strongest IFN-α stimulators (the ODN were at a dose of 1.0 µmol/L) were partly similar to the induction of IL-6 and IL-12 production. The IFN-α stimulators were ODN4 (14 ±15 pg/mL), ODN5 (97±10 pg/mL), ODN7 (180±14 pg/mL), ODN8 (115±13 pg/mL), ODN10 (135±6 pg/mL) and ODN11 (150±14 pg/mL). Structural information (Table 1) demonstrated that, ODN4, ODN7, ODN10, and ODN11 all had a 3'-end, self-complementary palindrome motif, indicating that a 3'-end palindrome structure is important for CpG ODN-induced IL-12 and IFN-α production.
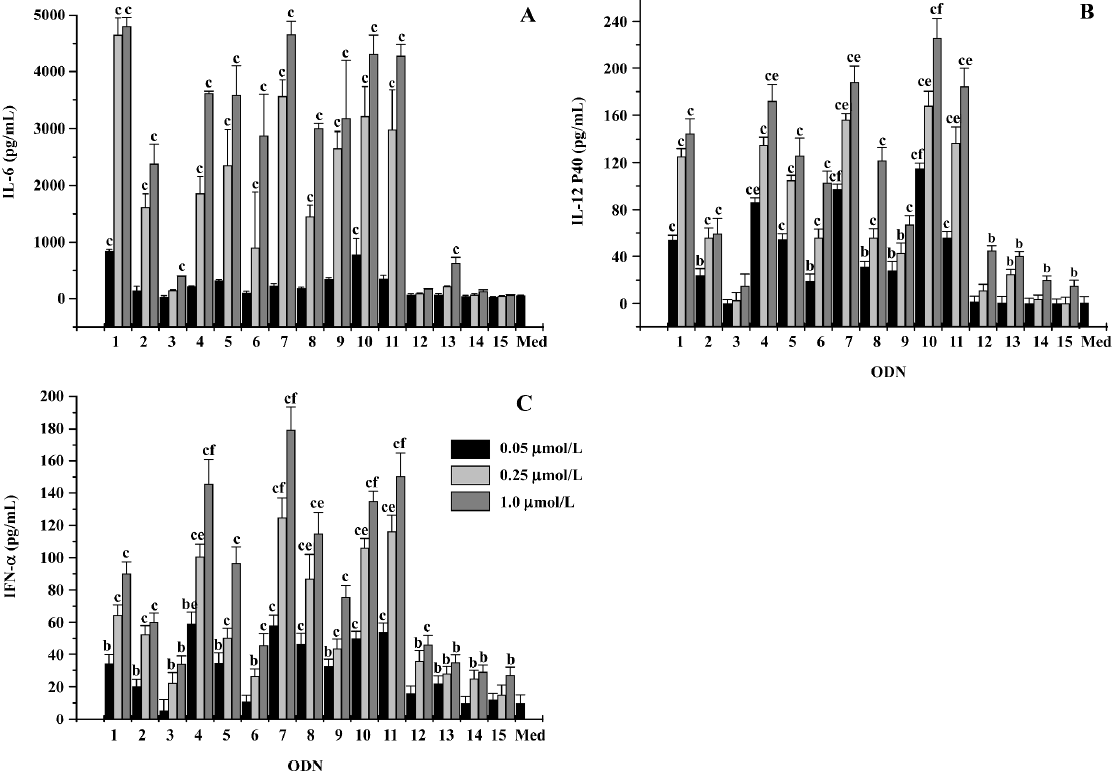
Effect of CpG ODN on B cell proliferation According to Krug et al, A-class ODN were poor stimulators of human B cell proliferation. In contrast, B- and C-class ODN efficiently stimulated B cell proliferation[20]. In our study, B-class ODN1826 induced the upregulation of B cells to a moderate extent. ODN5, ODN6, ODN8, and ODN9 stimulated B cell proliferation with comparable efficiency and in a dose-dependent manner. However, ODN4, ODN7, ODN10, and ODN11 had a more obvious effect on stimulating the proliferation of B cells than ODN1826 (Figure 2).
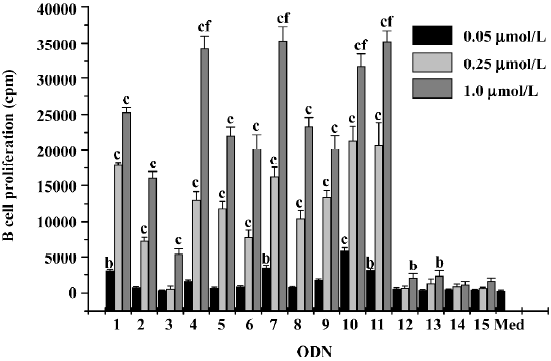
NK cell activation Another important activity of CpG ODN is the activation of NK cells[7]. ODN with a 3'-end, self-complementary palindrome, such as ODN4, ODN7, ODN10, and ODN11 induced more efficient NK cytotoxicity (Figure 3).
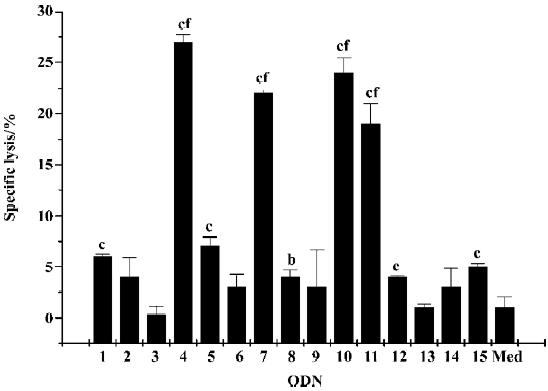
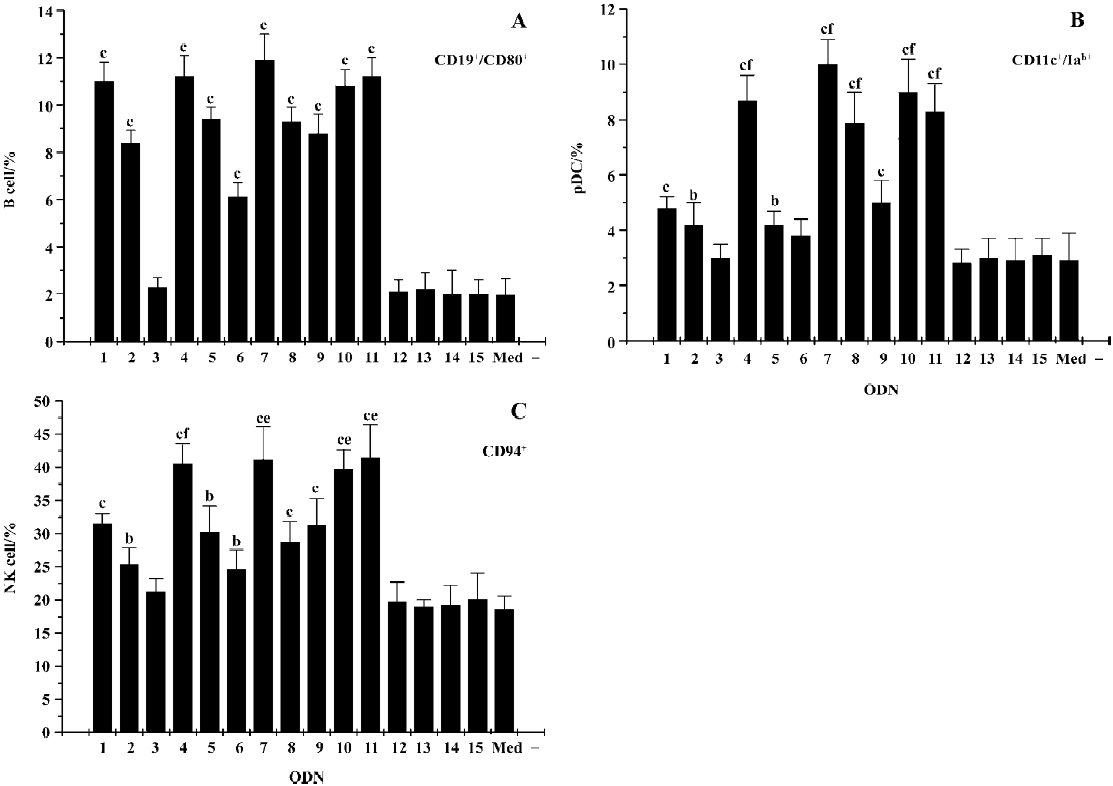
Flow cytometry analysis CD19 is the characteristic surface marker of B cells, and the activation of B cell proliferation is characterized by the upregulation of cell surface molecules, such as costimulatory molecules CD80. CpG ODN can also activate pDC and promote its maturation, which can determined by the upregulation of MHC II molecules (Iab) on the membrane of pDC. CD11c can be detected as the characteristic surface marker of pDC. In the present study, we determined the effect of CpG ODN on different immune cells by flow cytometry analysis on the basis of those marker molecules (Figure 4). The results showed that all CpG ODN, with the exception of ODN3, upregulated the expression level of CD80 on CD19-positive cells, in which ODN1826, ODN4, ODN7, ODN10, and ODN11 were especially efficient. As to the expression of Iab on CD11c-positive cells and CD94 expression on NK cells, the status was similar; the most potent CpG ODN were still ODN4, ODN7, ODN10, and ODN11.
SAR analysis The results showed that the optimal stimulatory hexameric CpG motif for murine was 5'-TC
Owning to lack of a TpC dinucleotide[21] at the 5'-end, the immune-stimulatory effects of ODN6 and ODN8 were weakened. The optimal distance between 2 hexameric CpG motifs seemed to be 3 nucleotides. Modification of the distance to a shorter (ODN5, the 2 motifs were connective) or a longer (ODN6, the 2 motifs spaced by an octanucleotide) one both weakened the comprehensive immunostimulatory activity.
The palindrome was essential to the immunostimulatory potency of a CpG ODN. The results showed that all the ODN with the best general immunostimulatory activity were that with self-complementary palindrome sequences, such as ODN4, ODN7, ODN10, and ODN11. However, the palindrome structures had to be at the 3'-end of the sequence. Once the palindrome moved to the 5'-end, like ODN3, ODN12, and ODN15, immunostimulatory activity was almost totally lost.
Discussion
In the present study, we tried to explore a new role of the SAR of CpG ODN and find the optimal structure for mouse spleen cells. The positive control and the template ODN1826 was the optimal B-class CpG ODN reported in previously published studies. It consists of 2 stimulatory hexameric CpG motifs (5'-TC
Various structural modifications were preformed on the basis of the template ODN1826. Some modifications introduced remarkable activity promotion to the CpG ODN, for instance, adding the palindrome to the sequence. However, some structural changes failed to improve the immunostimu-latory potency, such as modifications of the CpG number and the distance between functional CpG motifs or simply changing the motif sites. Previous studies[17,19,21], in which conclusions was drawn that a TpC dinucleotide at the 5'-end was important for the potency of a CpG ODN, was partly supported in our study. ODN6 lacked the 5'-TpC dinucleo-tide, which had weaker stimulatory potency than ODN1826, but the lack of potency could be compensated by an addition of a 3’-end CpG-rich palindrome tail, this is why ODN8 possess comparable immunostimulatory activity vs 1826.
All ODN with a palindrome sequence could be classified as C-class CpG ODN[15]. Our results indicated that C-class ODN possessed combined immune effects of A- and B-class CpG ODN[7,13,20]. C-class ODN strongly stimulated B cell proliferation, NK cell activation, and IFN-α production. However, not all C-class CpG ODN had immunostimulatory potency. It was very important to arrange the site of the palindromes; self-complementary palindrome at the 3'-end normally accompanied with satisfactory immunostimulatory potency. In contrast, a 5'-end, self-complementary palindrome is usually useless, like ODN3 and ODN12. A previous study[22] showed that the free 5'-end of the CpG ODN sequence was important for immunostimulatory potency. The 5'-end palindrome destroyed the free end; this may have been responsible for the lost of the immunostimulatory activity of ODN3 and ODN12.
However, ODN10, one of the most potent ODN involved in our study, possessed a holistic, self-complementary palindrome structure (5'-TCCATGACGTTTT
The analysis of cytokine secretion is a traditional method for the SAR of CpG ODN. Generally, a certain cytokine can be secreted by several immune cells, for example IFN-α secretion by pDC or cells of the mononuclear phagocyte system, so we could not identify which cells were influenced by CpG ODN. Thus, the more efficient and accurate method of flow cytometry was used as a powerful tool in the study for the determination of the cell-specific activation of ODN. As we known, CD19 was the characteristic surface marker of B cells, and the activation of B cell was characterized by the upregulation of cell-surface molecules, such as costimulatory molecules CD80. CD11c was the characteristic surface marker of pDC, and one of the mature markers of pDC was the upregulation of Iab expression on pDC. The results showed that both B-class CpG ODN and C-class CpG ODN could upregulate the level of CD80 expression on CD19-positive cells. C-class CpG ODN induced a much higher level of Iab expression on CD11c-positive cells than B-class CpG ODN. The CD94 expression on NK cells was similar to the Iab expression on pDC. The results obtained via flow cytometry and cytokine analysis were consistent.
All of the cellular immune effects of CpG ODN in murine were believed to result directly and indirectly from activating TLR-9-expressing pDC and B cells[23–28]. Never-theless, B cell and pDC activation seemed to need different structure characteristics[28,29]. An explanation for this is that pDC activation was associated with ODN that can form secondary structures, such as the dimeric C-class and the multimeric A-class. These higher-ordered structures might induce TLR-9 cross-linking, promote the recruitment of one or more additional cofactors or adaptor proteins into the TLR-9-signalling complex, and/or alter the intracellular compartmentalization of the ODN[30,31]. Thus, the relationship between the primary and higher-level structure of CpG ODN and its accessibility, recognition, and combination with TLR-9 still need further study.
References
- Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995;374:546-9.
- Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 2002;20:709-60.
- Hartmann G, Weeratna RD, Ballas ZK, Payette P, Blackwell S, Suparto I, et al. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J Immunol 2000;164:1617-24.
- Krieg AM. An innate immune defense mechanism based on the recognition of CpG motifs in microbial DNA. J Lab Clin Med 1996;128:128-33.
- Walker PS, Scharton-Kersten T, Krieg AM, Love-Homan L, Rowton ED, Udey MC, et al. Immunostimulatory oligodeoxy-nucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-gamma-dependent mechanisms. Proc Natl Acad Sci USA 1999;96:6970-5.
- Krieg AM, Yi AK, Hartmann G. Mechanisms and therapeutic applications in DNA vaccines. Trends Microbiol 1999;6:23-7.
- Ballas ZK, Rasmussen WL, Krieg AM. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J Immunol 1996;157:1840-5.
- Macfarlane DE, Manzel L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine, and structurally related compounds. J Immunol 1998;160:1122-31.
- Zhao Q, Matson S, Herrera CJ, Fisher E, Yu H, Kreig AM. Comparison of cellular binding and uptake of antisense phosphodiester, phosphorothioate, and mixed phosphorothioate and methylphos-phonate oligonucleotides. Antisense Res Dev 1993;3:53-66.
- Dalpke AH, Zimmermann S, Albrecht I, Heeg K. Phospho-diester CpG oligonucleotides as adjuvants: polyguanosine runs enhance cellular uptake and improve immunostimulative activity of phosphodiester CpG oligonucleotides in vitro and in vivo. Immunology 2002;106:102-12.
- Sester DP, Naik S, Beasley SJ, Hume DA, Stacey KJ. Phosphoro-thioate backbone modification modulates macrophage activation by CpG DNA. J Immunol 2000;165:4165-73.
- Lee SW, Song MK, Baek KH, Park Y, Kim JK, Lee CH, et al. Effects of a hexameric deoxyriboguanosine run conjugation into CpG oligodeoxynucleotides on their immunostimulatory poten-tials. J Immunol 2000;165:3631-9.
- Verthelyi D, Ishii KJ, Gursel M, Takeshita F, Klinman DM. Human peripheral blood cells differentially recognize and respond to two distinct CpG motifs. J Immunol 2001;166:2372-7.
- Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c type II dendritic precursor and CD11c+ dendritic cells to produce type I IFN. J Immunol 2001;166:2291.
- Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol 2004;34:251-62.
- Bao MS, Zhang Y, Wan M, Dai L, Hu XP, Wu XL, et al. Anti-SARS-CoV immunity induced by a novel CpG oligodeoxy-nucleo-tide. Clin Immunol 2006;118:180-7.
- Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol 2000;164:944-53.
- Rankin R, Pontarollo R, Ioannou X, Krieg AM, Hecker R, Babiuk LA, et al. CpG motif identification for veterinary and laboratory species demonstrates that sequence recognition is highly conserved. Antisense Nucleic Acid Drug Dev 2001;11:333-40.
- Ashman RF. CpG oligodeoxyribonucleotides rescue mature spleen B cells from spontaneous apoptosis and promote cell cycle entry. J Immunol 1998;160:5898-906.
- Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur J Immunol 2001;31:2154-63.
- Vollmer J, Weeratna R, Jurk M, Samulowitz U, McCluskie MJ, Payette P, et al. Oligodeoxynucleotides lacking CpG dinucleotides mediate Toll-like receptor 9 dependent T helper type 2 biased immune stimulation. Immunology 2004;113:212-23.
- Yu D, Zhu FG, Bhagat L, Wang H, Kandimalla ER, Zhang R, et al. Potent CpG oligodeoxynucleotides containing phosphodiester linkages: in vitro and in vivo immunostimulatory properties. Biochem Biophys Res Commun 2002;297:83-90.
- Zhao Q, Temsamani J, Iadarola PL, Jiang Z, Agrawal S. Effect of different chemically modified oligodeoxynucleotides on immune stimulation. Biochem Pharmacol 1996;51:173-82.
- Yu D, Zhao Q, Kandimalla ER, Agrawal S. Accessible 5'-end of CpG-containing phosphorothioate oligodeoxynucleotides is essential for immunostimulatory activity. Bioorg Med Chem Lett 2000;10:2585-8.
- Kandimalla ER, Bhagat L, Yu D, Cong Y, Tang J, Agrawal S. Conjugation of ligands at the 5'-end of CpG DNA affects immuno-stimulatory activity. Bioconjug Chem 2002;13:966-74.
- Yu D, Kandimalla ER, Bhagat L, Tang JY. ‘Immunomer’-novel 3'-3'-linked CpG oligodeoxynucleotides as potent immunomodulatory agents. Nucleic Acids Res 2002;30:4460-9.
- Bhagat L, Zhu FG, Yu D, Tang J, Wang H, Kandimalla ER, et al. CpG penta- and hexadeoxyribonucleotides as potent immuno-modulatory agents. Biochem Biophys Res Commun 2003;300:853-61.
- Ballas ZK, Kreig AM, Warren T, Rasmussen W, Davis HL, Waldschmidt M, et al. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol 2001;167:4878-86.
- Vollmer J, Weeratna R, Payette P, Jurk M, Schetter C, Laucht M, et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur J Immunol 2004;34:251-62.
- Hemmi H, Kaisho T, Takeda K, Akira S. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol 2003;170:3059-64.
- Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 2005;434:1035-40.
