Differential modulation of bradykinin-induced relaxation of endothelin-1 and phenylephrine contractions of rat aorta by antioxidants1
Introduction
Increased oxidative stress has been reported in vascular diseases and is implicated in the alteration of vascular function. Clinical and experimental studies have shown that endogenous or exogenous reactive oxygen species (ROS) can modulate vascular tone and perhaps mediate signal transduction[1–4]. In different vascular beds and under different conditions, ROS has been reported to mediate varied vascular functions, such as vasoreactivity, vascular proliferation, and vascular cell signaling[4–7]. Such actions depend on the particular pathophysiological condition. In cardiovascular diseases, increased oxidative stress and the increased production of vasoconstrictor agents, such as catecholamine, angiotensin, thromboxane, and endothelin-1 (ET-1) have been reported and implicated in the pathogenesis of vascular dysfunction[3,4,7–10]. Under such pathological conditions, there is the possibility that vasoactive agents can recruit different ROS components to modulate distinct vascular functions. Such interactions will depend on the vascular environment, type, and levels of vasoactive agents (cons-trictors/dilators) present in that particular vascular bed[10–14]. The origin of ROS produced in activated vascular cells, including superoxide and H2O2 has been a subject of several studies and the use of different oxidase inhibitors and/or substrates have identified membrane-associated oxidase inhibited by flavin oxidase inhibitors and stimulated by NADH or NADPH, suggesting that NAD(P)H oxidases are an important source of superoxide production in the vascular system of several animals[3,6,15,16]. In the cardiovascular system, NAD(P)H oxidases are membrane-associated enzymes which catalyze reduction of oxygen using electrons donated by NADH or NADPH. Upon stimulation by vasoactive agents, O2- is produced within minutes to hours by endothelial cells and vascular smooth muscle cells. Recent evidence further indicates that lipoxygenase, cyclooxy-genase, mitochondrial oxidases, xanthine oxidase, and nitric oxide (NO) synthases are also sources of ROS[3,4,6,16–18].
Bradykinin (BK) is an important vasoactive agent which is released by the vascular system especially during inflammatory processes, including ischemia reperfusion and other conditions which may involve the generation of ROS. BK can modulate vascular functions via numerous mechanisms, including the release of prostanoids, NO, the regulation of intracellular calcium ions, the activation of potassium channels, and ROS generation[9,19–23]. The varied vascular actions of BK and the mechanisms by which they are accomplished are vascular bed-dependent[19–24]. For example, in the brain, BK has been reported to cause cerebrovascular dilation through the activation of Ca2+-activated potassium channels, the endothelium-derived hyperpolarizing factor, and NO[22–24], while in the peripheral vessels it acts through the release of NO, prostanoids, activation of K+ channel, ROS generation, or the release of hyperpolarizing factors[19–22].
In this study, we investigated the hypothesis that ROS contributes to BK relaxation and that the relaxation is differentially modulated in the presence of phenylephrine (PE) and ET-1-induced contractions.
Superoxide dismutase (SOD; a superoxide scavenger), catalase (CAT; a hydrogen peroxide scavenger), and vitamin C (a non-selective antioxidant) were used to test the working hypothesis. We characterized the involvement of ROS in the BK relaxation of ET-1- and PE-induced contractions and compared the contribution of ROS to PE- and ET-1-generated tension.
Materials and methods
Drugs and chemicals SOD, CAT, vitamin C, bradykinin, PE, acetylcholine, and ET-1 were purchased from Sigma-Aldrich (St Louis, MO, USA).
Experimental animals Male Sprague-Dawley rats (250–300 g; purchased from Harlan, Houston, TX, USA), were used for this study and maintained according to the National Institute of Health NIH guidelines on the care and use of laboratory animals (Texas Southern University, Houston, TX, USA). The protocol used for this study was approved by the Animal Care Committee of the Texas Southern University.
Tissue preparation Following anesthesia with urethane (2 g/kg; ip), the chest cavity was opened and the thoracic aorta was removed and placed in a petri dish containing cold Kreb’s (4 oC) solution (in mmol/L, NaCl 113, KCl 4.7, NaHCO3 25.0, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, and glucose 5, pH 7.4) and continuously gassed with 95% O2 and 5% CO2. The aorta was cleansed of connective tissues and cut into 3–4 mm rings. The aortic ring was then mounted in a 10 mL jacketed bath (World Precision Instruments, Sarasota, FL, USA) at 37 °C. The ring was suspended in the bath solution by 2 hooks; the lower one fixed to the bottom of the bath while the upper one was connected via a transbridge (model TBM4, World Precision Instruments, USA) data-acquisition system (DataQ Instruments, Akron, OH, USA) for the recording of isometric tension developed to the application of vasoactive agents. The rings were subjected to a resting tension of 2 g and allowed to equilibrate for a period of 90 min while being rinsed every 15 min. During the equilibration period, the rings were subjected to 2 challenges of 1×10-7 mol/L PE 30 min apart and relaxed with 1×10-5 mol/L acetylcholine to test the functionality of the tissues. Tissues that did not produce 70%–80% relaxation of the tension generated were considered non-responsive and were excluded from the study. Changes in tension were monitored via a force displacement transducer connected to a DI-720 system (DATAQ software, USA).
Cumulative dose-response curves Following a 90 min equilibration, the aortic rings were preconstricted with PE (1×10-7 mol/L) or ET-1 (1×10-9 mol/L). The concentration of PE or ET-1 used was shown in preliminary experiments to produce about 70% of maximal contraction. The contraction to PE or ET-1 was allowed to reach a plateau and stabilize (5–10 min) before the relaxation studies com-menced. The relaxation responses to the cumulative concentrations of BK (1×10-9–1×10-5 mol/L) were determined in the absence or presence of antioxidants: SOD (300 U/mL; a superoxide scavenger), CAT (300 U/mL; a hydrogen peroxide scavenger), or vitamin C (1×10-4 mol/L; a scavenger of superoxide, hydroxyl radicals, and hydrogen peroxide). The concentrations of the antioxidants and other agents used were consistent with those reported by us and others[4,14,25].
The contribution of ROS to the contraction induced by PE or ET-1 was investigated by evaluating the dose-dependent contraction of the aortic ring to PE or ET-1 (1×10-10–1×10-6 mol/L) in the absence or presence of SOD, CAT, or vitamin C.
Statistical analysis Vascular relaxation responses are presented as percentage change in the relaxation of the aortic ring from preconstricted values following the addition of BK. The contraction responses to PE or ET-1 are presented as tension in grams. Data are reported as mean±SEM and subjected to two-way ANOVA followed by Student-Newman-Keul’s post-hoc test. P<0.05 was considered statistically significant.
Results
The application of PE (1×10-7 mol/L) to the aortic ring preparation resulted in the development of tension that attained a plateau in 3–5 min. The average tension developed to PE application was 1.69±0.19 g (n=10). ET-1 (1×10-9 mol/L) application resulted in slow-developing tension which attained plateau in 7–10 min with an average tension of 1.82±0.18 g (n=10).
BK (1×10-9–1×10-5 mol/L) dose-dependently relaxed PE- and ET-1-induced tension. BK-induced relaxation was significantly greater in the rings precontracted with ET-1 (1×10-9 mol/L) compared to those with PE (1×10-7 mol/L). For example, the maximum relaxation of ET-1- and PE-induced tension mediated by BK (1×10-5 mol/L) application was 75%±5% for ET-1 versus 35%±4% for PE, respec-tively.
Role of ROS in BK-induced relaxation of PE or ET-1 contraction
Incubation of aortic rings with SOD, CAT, or vitamin C did not have any significant effects on PE or ET-1-induced tension In Figure 1, BK (1×10-9–1×10-5 mol/L) dose-dependently relaxed PE-induced tension. Pretreatment of the ring with SOD (300 U/mL), CAT (300 U/mL), or vitamin C (1×10-4 mol/L) for 15 min significantly enhanced the BK relaxation of PE contraction. At the highest concentration of BK (1×10-5 mol/L) employed, the relaxation to BK was significantly increased from 35%±4% relaxation in the control to 56%±9%, 60%±5%, or 49%±6%, respectively, for SOD, CAT, or vitamin C (Figure 1, P<0.05, n=8, ANOVA).
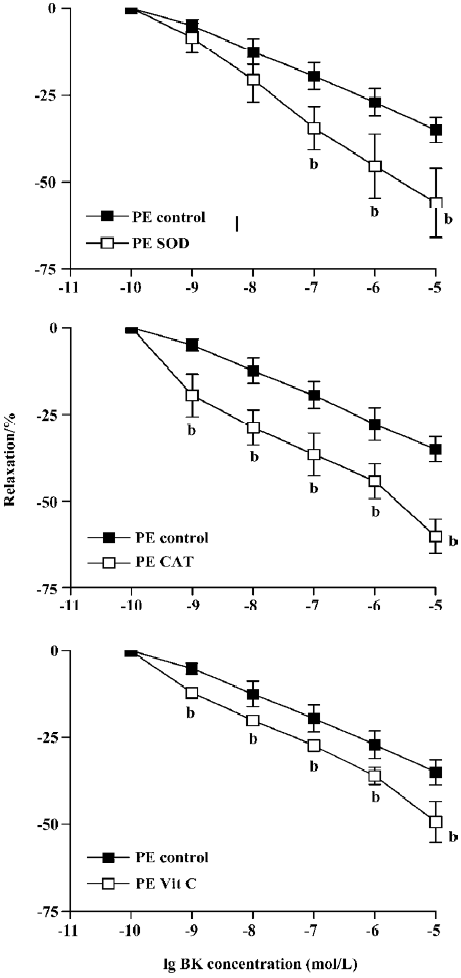
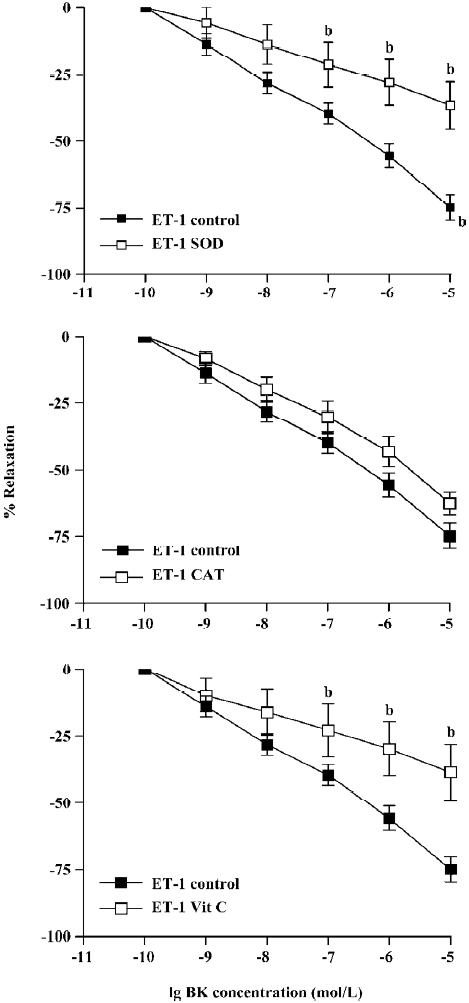
BK dose-dependently relaxed ET-1-induced contraction Unlike PE, the BK relaxation of ET-1 tension was significantly attenuated by pretreatment with SOD, CAT, or vitamin C (Figure 2). Thus, at the highest concentration (1×10-5 mol/L), BK-induced relaxation was reduced from 75%±5% (control) to 37%±9% (SOD), 63±3% (CAT), or 39%±7% (vitamin C) following 15 min pre-incubation (Figure 2, P<0.05, ANOVA, n=8).
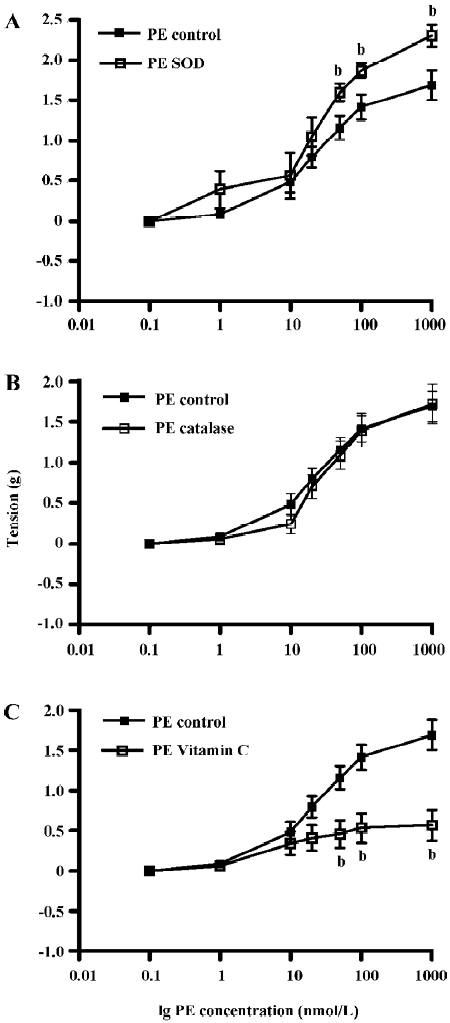
Contribution of ROS to PE- and ET-1-induced contraction of the aortic ring The effects of pre-incubation with SOD, CAT, or vitamin C on PE-induced contraction of the aortic ring was determined (Figure 3). PE dose-dependently contracted the aortic rings and pre-incubation with SOD (300 U/mL) significantly enhanced PE contraction by 36%. PE tension increased from 1.69±0.19 g (control) to 2.30±0.14 g (SOD) (Figure 3A, P<0.05, n=10, ANOVA). Pre-incubation with CAT (300 U/mL) had no significant effects on PE-induced contraction (1.69±0.19 g) in the control versus 1.72±0.25 g in CAT (Figure 3B, P>0.05, n=10). However, pre-incubation with vitamin C significantly attenuated PE-induced tension by 50%. Tension was reduced from 1.69±0.19 g in the control rings to 0.84±0.26 g in the vitamin C-treated rings (Figure 3C, P<0.05, n=8, ANOVA).
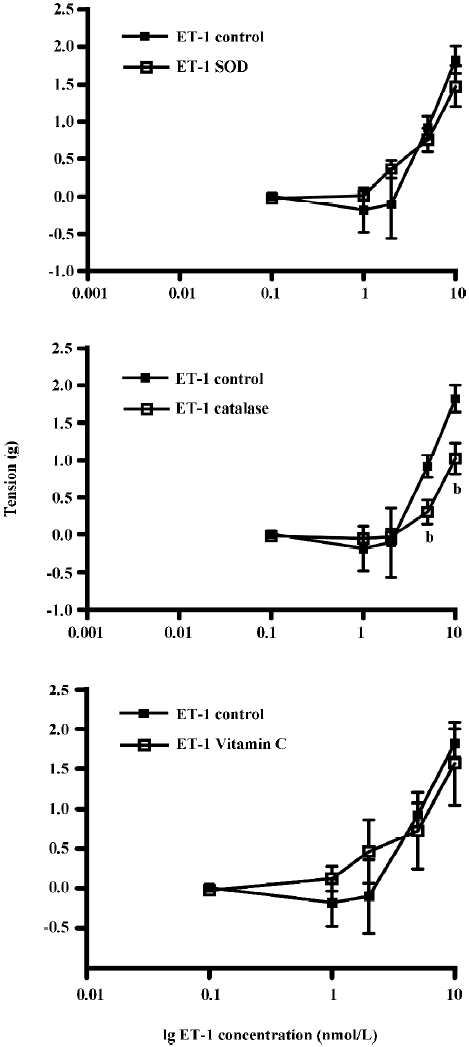
The effects of pre-incubation of the aortic ring with SOD, CAT, or vitamin C on ET-1-induced contraction was determined (Figure 4). Pre-incubation with SOD or vitamin C did not have any significant effects on ET-1 contraction. The tension generated by ET-1 (10 nmol/L) was 1.82±0.18 g (control), 1.48±0.27 g (SOD), or 1.57±0.52 g (vitamin C) (Figure 4A,4C, P>0.05, n=9). However, pre-incubation with CAT significantly attenuated ET-1-induced tension at higher (5 and 10 nmol/L), but not at the lower concentrations (Figure 4B, P<0.05, n=9, ANOVA). The tension generated by ET-1 after CAT treatment was reduced by 66% (from 0.92±0.15 g in the control to 0.31±0.16 g by 5 nmol/L) and by 44% (from 1.82±0.18 g in the control to 1.03±0.21 g by 10 nmol/L).
Discussion
This study revealed that: (i) BK evoked dose-dependent relaxation of ET-1- and PE-contracted aortic rings, producing a greater relaxation of ET-1-induced tension than that by PE; (ii) pretreatment with antioxidants enhanced BK relaxation of PE tension, but attenuated its relaxation of ET-1 tension; (iii) PE-induced contraction was enhanced by pretreatment of aortic rings with SOD, but not vitamin C or CAT; and (iv) ET-1-induced contraction was attenuated by pretreatment of the aortic rings with CAT, but not SOD or vitamin C. The results presented generally support a differential role for ROS in BK-induced vascular relaxation of PE- and ET-1-induced contraction and that the contribution of free radical species for the generation of tension by ET-1 or PE is agonist specific. O2– negatively modulated PE contraction while H2O2 positively modulated ET-1-induced contraction.
It is now well established that various stimuli can induce increased production of ROS in vascular cells[4,6,10,26,27]. ROS produced in activated vascular cells can come from different oxidases, for example, xanthine/xanthine oxidase, mitochondrial oxidase, and arachidonic acid oxygenases, including the NADPH oxidase in the vascular wall[3,6,7,28,29]. The ROS usually produced primarily is superoxide which undergoes dismutation to H2O2, another potent ROS. Results from various laboratories support the role of ROS in vascular function in response to different vasoconstrictors and vasodilators. For example, PE via its activation of α1-receptors or ET-1 via the activation of ETA receptors could potentially generate ROS via the stimulation of protein kinase C (PKC), Ca2+ channels, or arachidonic acid metabolites[4,7,10,27,30,31]. In view of the similarity in signaling mechanisms between different constrictor and relaxant agents, it is difficult to specifically identify the exact source(s) of ROS or their qualitative effects in a particular preparation. In the cardiovascular system, NAD(P)H oxidases are membrane-associated enzymes which catalyze the reduction of oxygen using electrons donated by NADH or NADPH. Upon stimulation by vasoactive agents, O2– is produced within minutes to hours by endothelial cells and vascular smooth muscle cells and is considered the main source of free radical generation in the vascular system[6,7,15,16].
In this study, we addressed the differential effects of ROS on PE- and ET-1 contraction of the rat aorta and characterized the relaxant effects of BK in tissues in which tension was generated with PE or ET-1 in order to evaluate the differential role of ROS. In the PE-contracted preparations, ROS appeared to contribute to BK-induced relaxation inasmuch as antioxidant treatment with SOD, CAT, or vitamin C resulted in enhanced relaxation. Although the exact mechanism by which this occurs is not clear, the observation tends to support the hypothesis that the O2– and SOD-facilitated conversion of O2– to H2O2 subserves a contractile function. The attenuation by O2– in BK relaxation of PE contraction suggests that PE may activate the production of O2– which may reduce the availability of NO that is potentially released by BK. Consistent with this observation is the reported involvement of ROS in vascular signaling via α1-adrenoceptors in which the inhibition by the SOD mimetic was accompanied by a decrease in ROS generation and release in vascular tissue as well as tone[31]. Also, it is possible that BK-induced relaxation may be blunted by a PE-induced increase in the O2– level leading to the neutralization of NO[1,3,7,10,31]. Thus, SOD mitigated this effect and preserved NO leading to the enhanced relaxation (Figure 1A). Consistent with this notion is the enhanced BK relaxation of PE tension following treatment of the aortic ring with vitamin C, an antioxidant and the reported attenuation of BK relaxation during high oxidative stress[3,4,19,22]. This observation finds support in the finding that vitamin C via its antioxidant properties can stabilize cofactors for eNOS (tetrahydrobio-pterin)[7,32], thereby preserving NO bioavailability and promoting relaxation. The degree of enhancement of the relaxation evoked by BK was similar in the tissues treated with SOD or CAT indicating that both O2– and H2O2 in the PE-contracted aorta are equally effective and negatively coupled to BK relaxation. Vitamin C, a non-selective antioxidant, enhanced the BK-induced relaxation of PE tension, but surprisingly, to a lower extent than that observed in the presence of SOD or CAT. The reason for this is not clear. How-ever, as ROS are known to produce contraction and/or relaxation in the same preparation[17,28,29,33–35], and because of its non-selective effects, we speculate that the final effect on vascular response will be the net effect of vitamin C on ROS that produces contractile or relaxant effects.
In the ET-1-contracted aorta, ROS produced effects opposite to that in the PE-contracted aorta and attenuation rather than enhancement of relaxation resulted when the ET-1-contracted aorta was challenged with antioxidants. Comparing the degree of attenuation, O2– exerted a greater role than H2O2 as SOD produced a greater (38%) attenuation of BK relaxation than CAT (10%). On the other hand, the effects produced by vitamin C were similar in magnitude to that produced by SOD (36%), suggesting that O2– is the predominant ROS playing a greater role in the BK relaxation of ET-1-contracted aorta. The minimal effect of CAT is consistent with the studies of Ellis et al[14] in the mouse isolated aorta. It thus appears that in an ET-1-contracted tissue, O2– produced relaxation while H2O2 produced contraction, a notion supported by studies that demonstrated relaxation and contractile effects to O2– or H2O2, respectively[17,33,34]. This being the case, BK may have produced relaxation via O2– in the process of a cyclooxygenase (COX)-dependent prostaglandin production, a known mechanism for the relaxant effects of BK. In preparations incubated with SOD and challenged with ET-1, the dismutation of O2– to a contractile superoxide anion may therefore have accounted for the enhanced tone to ET-1. Also, the vasoconstrictor actions of H2O2 has been attributed to its ability to increase intracellular Ca2+, the generation of arachidonic acid metabolites with vasoconstrictor activity, and to its direct Ca2+-independent tonic effects on vascular smooth muscle contractile apparatus[11,33,34,36,37].
Apart from relaxation of the aortic ring, the contribution of ROS to PE- or ET-1-induced contraction was also inves-tigated. Pretreatment with antioxidants resulted in the selective regulation of tension generated by PE and ET-1. Thus, PE-induced contraction was enhanced by an O2– scavenger (SOD) indicating that O2– contributes a negative tone to vessels challenged with PE. On the other hand, increased H2O2 level resulting from the SOD-induced dismutation of O2– did not influence PE contraction. However, ET-1-induced contraction was attenuated by CAT, but not by SOD or vitamin C, indicating that H2O2 contributed to ET-1-induced contraction, but not PE-induced contraction. This is consistent with studies that have reported that H2O2 causes vasoconstriction[17,34,35]. Thus, the contribution of free radical species to the generation of tension by ET-1 or PE is agonist specific and the contribution of free radicals to BK relaxation is also agonist specific, resulting in a differential modulation of its relaxation. This differential effect may be a reflection of the interaction of the multiple signaling processes, for example, phospholipase C, PKC, mitogen-activated protein kinases, and intracellular Ca2+ involved in BK relaxation, PE or ET-1 contraction, and ROS generation. These mechanisms are further complicated by the fact that PE and ET-1 are potent stimulators of vascular superoxide generation[6,26,28,29,31,37,38]. It therefore appears that different mechanisms involved in ROS generation and agonist-induced signaling mechanisms will define the resulting vascular responses to vasoconstrictors and dilators in a particular tissue.
References
- Dhalla NS, Temsah RW, Netticaden T. Role of oxidative stress in cardiovascular disease. J Hypertens 2000;18:655-73.
- Touyz RM. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypetension: what is the clinical significance? Hypertension 2004;44:248-52.
- Touyz RM. Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal 2005;7:1302-14.
- Yakubu MA, Sofola OA, Igbo I, Oyekan AO. Link between free radicals and protein kinase C in glucose-induced alteration of vascular dilation. Life Sci 2004;75:2921-32.
- Finkel T. REDOX-dependent signal transduction. FEBS Lett 2000;476:52-4.
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase. Role in cardiovascular biology and disease. Circ Res 2000;86:494-501.
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 2003;42:1075-81.
- Yakubu MA, Leffler CW. Role of ET-1A receptor in hematoma-induced alteration of cerebral microvascular responses in vivo. Brain Res 1996;734:149-56.
- Yakubu MA, Leffler CW. L-type voltage-dependent Ca2+ channels in cerebral microvascular endothelial cells and ET-1 biosynthesis. Am J Physiol Cell Physiol 2002;283:C1687-95.
- Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber J. Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res 2004;94:37-45.
- Nowicki PT, Flavahan S, Hassanain H, Mitra S, Holland S, Goldschmidt-Clermont PJ, et al. Redox signaling of the arteriolar myogenic response. Circ Res 2001;89:114-6.
- Channon KM, Guzik TJ. Mechanisms of superoxide production in human blood vessels: relationship to endothelial dysfunction, clinical and genetic risk factors. J Physiol Pharmacol 2002;53:515-24.
- Rathaus M, Bernheim J. Oxygen species in the microvascular environment: regulation of vascular tone and the development of hypertension. Nephrol Dial Transplant 2002;17:216-21.
- Ellis A, Pannirselvam M, Anderson TJ, Triggle CR. Catalase has negligible inhibitory effects on endothelium-dependent relaxations in mouse isolated aorta and small mesenteric artery. Br J Pharmacol 2003;140:1193-200.
- Babior BM. NADPH oxidase. An update. Blood 1999;93:1464-76.
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245-313.
- Leffler CW, Busija DW, Armstead WM, Mirro R. H. 2O2 effects on cerebral prostanoids and pial arteriolar diameter in piglets. Am J Physiol 1990;258:H1382-7.
- Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 2000;87:26-32.
- Ignarro LJ, Byrns RE, Buga GM, Wood KS. Mechanisms of endothelium-dependent vascular smooth muscle relaxation elicited by bradykinin and VIP. Am J Physiol 1987;253:H1074-82.
- Hall JM. Bradykinin receptors: pharmacological properties and biological roles. Pharmacol Ther 1992;56:131-90.
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 1995;268:C799-822.
- Ihara E, Derkach DN, Hirano K, Nishimura J, Nawata H, Kanaide H. Ca2+ influx in the endothelial cells is required for the bradykinin-induced endothelium-dependent contraction in the porcine interlobar renal artery. J Physiol 2001;534:701-11.
- Hernanz R, Briones AM, Alonso MJ, Vila E, Salaices M. Hypertension alters role of iNOS, COX-2, and oxidative stress in bradykinin relaxation impairment after LPS in rat cerebral arteries. Am J Physiol Heart Circ Physiol 2004;287:H225-34.
- Lacza Z, Puskar M, Kis B, Perciacannte JV, Miller AW, Busija DW. Hydrogen peroxide acts as an EDHF in the piglet pial vasculature in response to bradykinin. Am J Physiol Heart Circ Physiol 2002;283:H406-11.
- Itoh T, Kajikuri J, Hattori T, Kusama N, Yamamoto T. Involvement of H2O2 in superoxide-dismutase-induced enhancement of endothelium-dependent relaxation in rabbit mesenteric resistance artery. Br J Pharmacol 2003;139:444-56.
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994;74:1141-8.
- Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens 2004;22:1141-9.
- Wolin MS, Ahmad M, Gupte SA. Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 2005;289:L159-73.
- Wolin MS, Gupte SA, Oeckler RA. Superoxide in the vascular system. J Vasc Res 2002;39:191-207.
- Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev 1994;46:325-415.
- Hao L, Nishimura T, Wo H, Fernandez-Patron C. Vascular responses to α1-adrenergic receptors in small rat mesenteric arteries depend on mitochondrial reactive oxygen species. Arterioscler Thromb Vasc Biol 2006;26:819-25.
- Shi W, Meininger CJ, Haynes TE, Hatakeyama K, Wu G. Regulation of tetrahydrobiopterin synthesis and bioavailability in endothelial cells. Cell Biochem Biophys 2004;41:415-34.
- Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vasc Biol 2000;20:1430-42.
- Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 2003;138:1085-92.
- Gil-Longo J, González-Vázquez C. Characterization of four different effects elicited by H2O2 in rat aorta. Vasc Pharmacol 2005;43:128-38.
- Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and patho-physiology. Arterioscler Thromb Vasc Biol 2000;20:2175-83.
- Graham RM, Perez DM, Hwa J, Piascik MT. α1-Adrenergic receptor subtypes: molecular structure, function, and signaling. Circ Res 1996;78:737-49.
- Liu Y, Guyton KZ, Gorospe M, Xu Q, Lee JC, Holbrook NJ. Differential activation of ERK, JNK/SAPK and P38/CSBP/RK MAP kinase family members during the cellular response to arsenite. Free Radic Biol Med 1996;21:771-81.
