Expression of PLK1 and survivin in non-Hodgkin’s lymphoma treated with CHOP
Introduction
Non-Hodgkin’s lymphoma (NHL) forms a highly heterogeneous group of diseases. Despite steady improvement in their classification, patients with the same diagnosis can have markedly different clinical courses. Since prognosis remains uncertain, several biologically-based prognostic indicators have been proposed in addition to the international prognostic index (IPI) scores. However, none have proven to be completely effective[1], so novel independent factors are required to better characterize the malignant potential of NHL.
Accumulating evidence suggests that abnormalities in cell cycle and apoptosis are hallmarks of clonal expansion leading to cancer emergence[2,3]. Polo-like kinase 1 (PLK1) has been implicated in various aspects of the progression of the cell cycle[4], including centrosome maturation[5], assembly of the mitotic spindle[6], regulation of the anaphase-promoting complex[7], and cytokinesis[8]. Survivin, a new inhibitor of the apoptosis protein (IAP) family, directly inhibits caspase 3 and caspase 7 and is one of the strongest anti-apoptosis factors by far[9]. Although PLK1 and survivin play completely different roles, they have some characteristics in common[10–13]: they are mostly expressed in the G2/M phase of the cell cycle and they are not expressed in most normal differentiated tissues; however, they are overexpressed in a broad spectrum of cancer types and the expression often correlates with poor outcomes. To assess the clinical significance of PLK1 and survivin in NHL, the expression of PLK1 and survivin were examined and correlated with clinical outcome.
Materials and methods
Patients A total of 88 patients (age: 5–86 years, median 47 years) were diagnosed with NHL at Union Hospital (Wuhan, China) between January 2001 and June 2006. None of the patients underwent radiotherapy or chemotherapy prior to diagnosis. Classification was established on standard hematoxylin-eosin-stained sections according to the guidelines of the World Health Organization 2001 Classification of Lymphoid Neoplasms[14]. Eighty-eight cases were treated with CHOP or E-CHOP or CHOP plus Rituximab (600 mg/m2 CTX, iv, d 1; 50 mg/m2 ADR, iv, d 1; 2 mg/m2 VCR, iv, d 1; 60 mg/m2 Pred, po, d 1–7; 100 mg/m2 etoposide, iv, d 1; 375 mg/m2 Rituximab, iv, d 1). Clinical follow-up data were available for all patients. The median follow up was 33 months (from 2 to 64 months). As a control for NHL, 5 cases of reactive lymph node proliferation were included in the study.
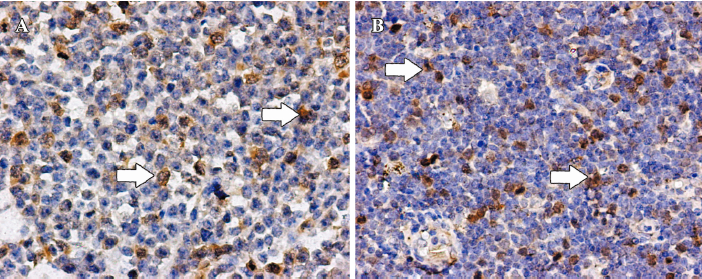
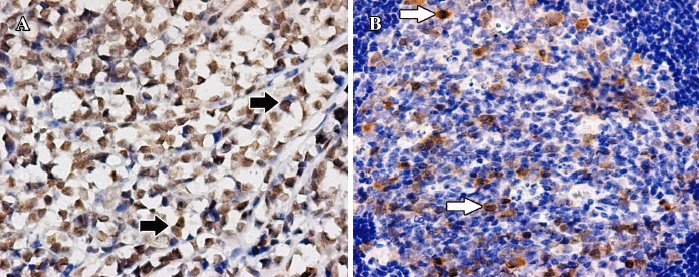
Immunohistochemistry on tissue slides Immunostaining was performed with primary antibodies against PLK1 (1:100; rabbit polyclonal antibody; Biolegend, San Diego, CA, USA) and survivin (1:150; rabbit polyclonal antibody; Neomarkers, Fremont, CA, USA). In brief, 4 µm thick formalin-fixed paraffin sections were dewaxed and rehydrated through a graded series of ethanols. After antigen retrieval with microwave heating, the sections were treated with 0.3% hydrogen peroxide followed by incubation in bovine serum albumin. The sections were then incubated with primary antibodies. After washing with phosphate-buffered saline, the sections were incubated with biotinylated anti-immunoglobulin G of the appropriate species and streptavidin peroxidase using a labeled streptavidin biotin kit (Zhongshan Biotechnology, Beijing, China). The immunoperoxidase reaction product was visualized with diaminobenzidine/hydrogen peroxide. The sections were counterstained with hematoxylin.
Evaluation of tissue staining The staining intensity of the tissue slides was evaluated independently by 2 pathologists who were blinded to the patients’ characteristics and survival. To assess the differences in staining intensity, an immunoreactivity scoring system (IRS) was applied. The intensity of staining was designated as negative (0), weak (1), moderate (2), or strong (3). Additionally, the percentage of positive cells was evaluated and scored as either no cells (0), less than 10% of cells (1), 10%–50% of cells (2), 51%–80% of cells (3), or over 80% of the cells stained (4). By multiplication of these 2 parameters, the IRS for each case was calculated. Finally, the cases were grouped as antigen negative (IRS 0–6) or antigen positive (IRS 7–12) for statistical analysis[15].
Statistical analysis Patient characteristics were compared by Fisher’s exact test or χ2-test. Generally, P<0.05 was considered statistically significant. For all statistical procedures, SPSS v11.5 software (SPSS, Chicago, IL, USA) was used.
Results
Expression of 2 proteins in NHL In all 88 NHL cases, 63.6% expressed PLK1 and 79.5% expressed survivin. The detailed distribution is listed in Table 1. The expression of PLK1 and survivin were then investigated in B-NHL and T-NHL, respectively. The PLK1 expression rate had no significance difference between B-NHL and T-NHL (57.8% vs 77.3%; P=0.103), as did survivin (76.6% vs 86.4%; P=0.544).
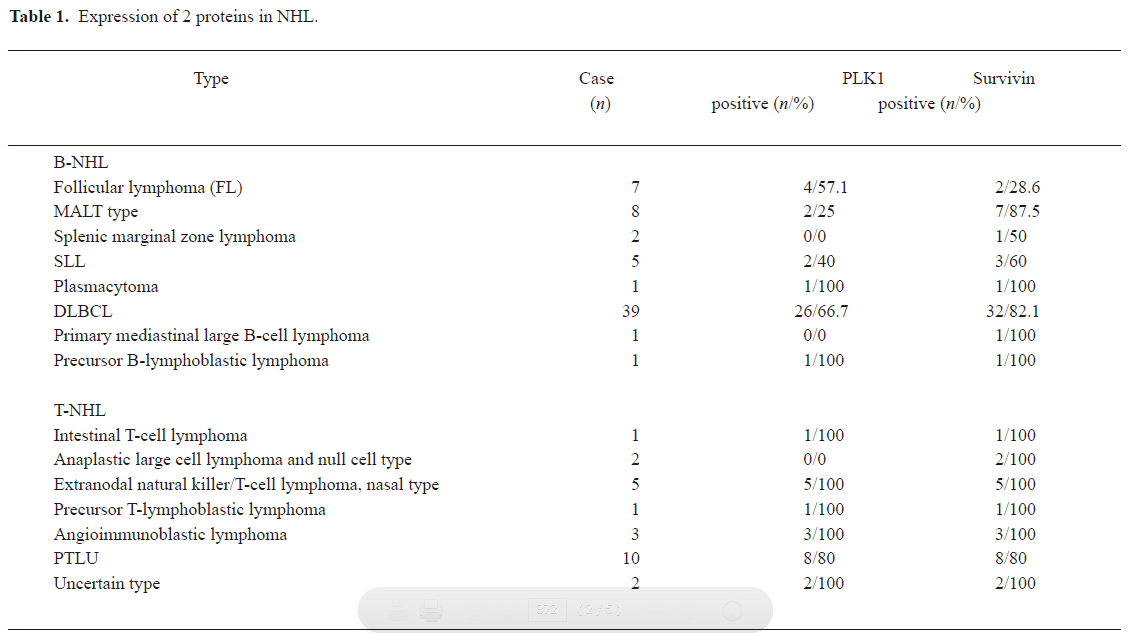
Full table
PLK1 was often strongly expressed in nuclei and seldom weakly in cytoplasm, while survivin was mainly strongly expressed in nuclei. None of the 5 reactive lymph node proliferation cases showed sufficient staining of PLK1 or survivin to be designated as positive (Figures 1, 2).
Relationship between the expression of 2 proteins and clinical characteristics in B-NHL and T-NHL In B-NHL, there was a highly significant positive correlation of PLK1 expression with age, Ann Arbor stage, lactate dehydrogenase (LDH) levels, systemic symptoms, IPI scores, and therapeutic effect. However, in T-NHL, the PLK1 expression was only associated with LDH levels, systemic symptoms, and IPI scores. There were no significant relationships between the survivin expression and these outcomes in either B-NHL or T-NHL (Table 2).

Full table
Discussion
In recent years, a growing spectrum of biochemical investigations has contributed to better characterize biological tumor behavior, thereby improving treatment strategies[16]. Among these studies, PLK1 and survivin were selected as the study objects, concerning the 2 important aspects of tumor biological behavior: proliferation and apoptosis.
PLK1 is a cell cycle-regulated, cyclin-independent serine/threonine protein kinase, and its protein level changes from very low level during the G1 phase to at least a 10-fold increase by the G2/M phase[17]. PLK1 is involved in mitotic spindle organization and cytokinesis and seems to play an essential role in regulating chromosomal segregation[18]. Furthermore, overexpressed PLK1 can cause the formation of oncogenic foci in NIH 3T3 cells, which are capable of forming tumors in nude mice[19]. The elevated expression of PLK1 had been found in non-small cell lung cancer[20], squamous cell carcinoma of the head and neck[21], esophageal cancer[22], gastric cancer[23], ovarian cancer[24], endometrial cancer[25], colon cancer[26], breast cancer[27], and malignant melanoma[28], prostate[29], papillary carcinoma[30], and oropharyngeal carcinomas[31]. The expression of PLK1 was correlated with an adverse clinical outcome in some tumors.
Survivin belongs to the IAP family and it suppresses apoptosis induced by Fas, Bax, caspases, and anticancer drugs[32]. Survivin is expressed in a strict cell cycle-dependent and tissue-restricted manner. It is expressed at high levels in the G2/M phase and in proliferating tissues[33]. Overexpressed survivin can be detected in almost all human tumors and is correlated with poor prognosis[34].
In the present study, the expression rate of PLK1 was lower in some less aggressive or indolent types, such as small lymphocytic lymphoma (SLL) and marginal zone B-cell lymphoma (MALT) than that in some more aggressive types, such as diffuse large B-cell lymphoma (DLBCL) and peropheral T-cell lymphoma unspecified (PTLU), while the expression rate of survivin seemed to have no such trend. This suggested that PLK1 overexpression was correlated with cell-proliferating activity, and survivin overexpression implied the common characterization of NHL: the dysregulation of apoptosis.
PLK1 overexpression was positively linked to age, Ann Arbor stage, LDH levels, systemic symptoms, IPI scores, and therapeutic effect in B-NHL, and was only linked to LDH levels, systemic symptoms, and IPI scores in T-NHL. The reason may be the highly heterogeneity and limited number of cases of T-NHL. There was no significant difference between the survivin-negative and -positive groups for these characteristics, either in B-NHL or in T-NHL. It verified again that apoptosis resistance may be the essence of NHL.
In conclusion, PLK1 and survivin were expressed at high levels in NHL cases, and PLK1 overexpression was associated with elderly patient, advanced tumor stage, systemic symptoms, elevated LDH levels, and poor therapeutic effect. The overexpression of survivin was not correlated with bad outcome.
Acknowledgements
The authors thank Dr Xiu NIE and Hua-xiong PAN for supplying the lymphoma tissues and for their skillful technical assistance.
References
- Fisher RI, Shah P. Current trends in large cell lymphoma. Leukemia 2003;17:1948-60.
- Corn PG, El-Deiry WS. Derangement of growth and differentiation control in oncogenesis. Bioessays 2002;24:83-90.
- Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411:342-8.
- Lowery DM, Lim D, Yaffe MB. Structure and function of Polo-like kinases. Oncogene 2005;24:248-59.
- Dai W, Wang Q, Traganos F. Polo-like kinases and centrosome regulation. Oncogene 2002;21:6195-200.
- McInnes C, Mazumdar A, Mezna M, Meades C, Midgley C, Scaerou F, et al. Inhibitors of Polo-like kinase reveal roles in spindle-pole maintenance. Nat Chem Biol 2006;2:608-17.
- Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol 2004;164:233-41.
- Zhou T, Aumais J P, Liu X, Yu-Lee LY, Erikson RL. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev Cell 2003;5:127-38.
- Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC, Hwang JI, et al. An anti-apoptotic protein human survivin is a direct inhibitor of caspase-3 and -7. Biochemistry 2001;40:1117-23.
- Duffy MJ, O’Donovan N, Brennan DJ, Gallagher W M, Ryan BM. Survivin: a promising tumor biomarker. Cancer Lett 2007;249:49-60.
- Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett 2006;244:164-71.
- Takai N, Hamanaka R, Yoshimatsu J, Miyakawa I. Polo-like kinases (Plks) and cancer. Oncogene 2005;24:287-91.
- Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene 2005;24:267-76.
- Jaffe ES, Harris NL, Stein H, editors. Pathology and genetics of tumours of aematopoietic and lymphoid tissues. World Health Organization Classification of Tumours, Lyon, France: IARC Press; 2001.
- Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Kobel M, et al. Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer 2004;90:815-21.
- Cheson BD. What is new in lymphoma? CA Cancer J Clin 2004;54:260-72.
- Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer 2006;6:321-30.
- van Vugt MA, van de Weerdt BC, Vader G, Janssen H, Calafat J, Klompmaker R. Polo-like kinase-1 is required for bipolar spindle formation but is dispensable for anaphase promoting complex/Cdc20 activation and initiation of cytokinesis. J Biol Chem 2004; 279: 36 841–54.
- Smith MR, Wilson ML, Hamanaka R, Chase D, Kung H, Longo DL, et al. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun 1997;234:397-405.
- Wolf G, Elez R, Doermer A, Holtrich U, Ackermann H, Stutte HJ, et al. Prognostic significance of polo-like kinase (PLK) expression in non-small cell lung cancer. Oncogene 1997;14:543-9.
- Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K. Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res 1999;59:2794-7.
- Tokumitsu Y, Mori M, Tanaka S, Akazawa K, Nakano S, Niho Y. Prognostic significance of polo-like kinase expression in esophageal carcinoma. Int J Oncol 1999;15:687-92.
- Kanaji S, Saito H, Tsujitani S, Matsumoto S, Tatebe S, Kondo A, et al. Expression of polo-like kinase 1 (PLK1) protein predicts the survival of patients with gastric carcinoma. Oncology 2006;70:126-33.
- Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Expression of polo-like kinase in ovarian cancer is associated with histological grade and clinical stage. Cancer Lett 2001;164:41-9.
- Takai N, Miyazaki T, Fujisawa K, Nasu K, Hamanaka R, Miyakawa I. Polo-like kinase (PLK) expression in endometrial carcinoma. Cancer Lett 2001;169:41-9.
- Takahashi T, Sano B, Nagata T, Kato H, Sugiyama Y, Kunieda K. Polo-like kinase 1 (PLK1) is overexpressed in primary colorectal cancers. Cancer Sci 2003;94:148-52.
- Wolf G, Hildenbrand R, Schwar C, Grobholz R, Kaufmann M, Stutte HJ, et al. Polo-like kinase: a novel marker of proliferation: correlation with estrogen-receptor expression in human breast cancer. Pathol Res Pract 2000;196:753-9.
- Kneisel L, Strebhardt K, Bernd A, Wolter M, Binder A, Kaufmann R. Expression of polo-like kinase (PLK1) in thin melanomas: a novel marker of metastatic disease. J Cutan Pathol 2002;29:354-8.
- Weichert W, Schmidt M, Gekeler V, Denkert C, Stephan C, Jung K, et al. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate 2004;60:240-5.
- Ito Y, Miyoshi E, Sasaki N, Kakudo K, Yoshida H, Tomoda C, et al. Polo-like kinase 1 overexpression is an early event in the progression of papillary carcinoma. Br J Cancer 2004;90:414-8.
- Knecht R, Oberhauser C, Strebhardt K. PLK (polo-like kinase), a new prognostic marker for oropharyngeal carcinomas. Int J Cancer 2000;89:535-6.
- Wheatley SP, McNeish IA. Survivin: a protein with dual roles in mitosis and apoptosis. Int Rev Cytol 2005;247:35-88.
- Li F. Role of survivin and its splice variants in tumorigenesis. Br J Cancer 2005;92:212-6.
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene 2003;22:8581-9.