β-Sitosterol sensitizes MDA-MB-231 cells to TRAIL-induced apoptosis1
Introduction
The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), a member of the TNF family, is considered a promising anticancer agent due to its ability to induce apoptosis in various tumor cell types, but is not cytotoxic to many normal cell types in vitro or in vivo[1,2]. Cellular sensitivity to TRAIL is dependent on the expression of cell membrane TRAIL receptors and caspase-8[3,4]. Caspase-8 is activated in response to TRAIL and released cytochrome c, where it initiates a protease cascade that activates effector caspases, including caspase-3 and caspase-9[5]. Although the majority of breast cancer cell lines are resistant in TRAIL-induced apoptosis, TRAIL with chemotherapeutic drugs resulted in the synergistic induction of cell death[6]. A previous study reported that more than 250 ng/mL TRAIL induces 30% apoptosis in MDA-MB-231 cells[7]. These results suggest that anticancer agents can be used in synergistic treatment with TRAIL to sensitize resistant cells to TRAIL-mediated apoptosis[8]. A better understanding of the molecular mechanisms underlying identification of the sensitizing agents capable of overcoming TRAIL resistance may facilitate the establishment of TRAIL-based combination regimens for the improved treatment of breast cancers.
Phytosterols or plant sterols are the counterparts of animal cholesterol[9] and are not synthesized endogenously in humans, but are derived solely from the diet through intestinal absorption[10,11]. Among them, β-sitosterol (SITO) is one of the most common dietary phytosterols in the blood. Previous studies revealed that SITO reduces carcinogen-induced cancer of the colon in rats[12] and exhibits anti-inflammatory[13,14], anti-angiogenic[15], and immune-modulating properties[16,17]. SITO also induces apoptosis in hormone-insensitive and metastatic MDA-MB-231 human breast cancer cells through the activation of caspases[18–21]. When the cells are treated with high dosages of SITO (≥16 µmol/L) or long time points (more than 3 d), the cells can become more sensitive to SITO-induced apoptosis. To overcome these problems, the synergistic treatment of chemopreventive agents and TRAIL is a promising approach to selectively induce apoptosis and regression of breast cancer with minimal toxicity to normal cells[6,7].
In the present study, we first investigated whether SITO is a potent sensitizer for TRAIL-induced apoptosis in TRAIL-resistant MDA-MB-231 breast cancer cells. Moreover, we determined the first evidence that synergistic treatment with SITO and TRAIL regulates the activation of caspases and pro-apoptotic proteins, leading to a significant induction of TRAIL-mediated signaling and cell death in MDA-MB-231 human breast cancer cells.
Materials and methods
Reagents SITO (Cat N
Antibodies Antibodies against Bax, Bad, Bcl-2, Bcl-XL, Fas, FasL, cIAP-1, cIAP-2, XIAP, DFF40/CAD, DFF40/ICAD, poly(ADP-ribose) polymerase [PARP], lamin A, capase-3, capase-8, and capase-9 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The antibody against â-actin came from Sigma. Peroxidase-labeled donkey antirabbit and sheep antimouse immunoglobulin were purchased from Amersham.
Cell culture Human breast cancer MDA-MB-231 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured at 37 °C in a 5% CO2 humidified incubator and maintained in RPMI-1640 culture medium containing 10% heat-inactivated FBS.
Cell viability and growth The cells were treated with the indicated concentrations of SITO, TRAIL, or synergistic treatment (SITO+TRAIL). Control cells were supplemented with 5 mmol/L CD for 48 h. Following the treatment, cell viability was determined by MTT assay.
Nuclear staining After treatment with the indicated compounds for 48 h, the cells were harvested, washed in ice-cold phosphate-buffered saline (PBS), and fixed with 3.7% paraformaldehyde for 10 min at room temperature. The fixed cells were permeabilized with 0.5% Triton X-100 and stained with DAPI solution for 10 min at room temperature. The nuclear morphology of the cells was examined by fluorescence microscopy.
Annexin V analysis For the analysis of apoptosis, the cells were stimulated with the indicated compounds, and apoptosis was analyzed over time by the staining of phosphatidylserine translocation with fluorescein-isothiocyanate-Annexin V (PharMingen, SinDiego, CA, USA) according to the manufacturer’s instructions.
Protein extraction and Western blot analysis The cells were harvested, washed once with ice-cold PBS, and gently lysed for 10 min in 80 µL ice-cold lysis buffer (20 mmol/L sucrose, 1 mmol/L EDTA, 20 µmol/L Tris-Cl [pH 7.2], 1 mmol/L dithiothreitol, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 5 µg/mL pepstatin A, 10 µg/mL leupeptin, and 2 ìg/mL aprotinin). The supernatants were collected and the protein concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). The samples were stored at –80 °C or immediately used for the Western blot analysis. Aliquots containing 50 µg of total protein were separated on SDS–PAGE and transferred to nitrocellulose membranes (Schleicher & Schuell, Keene, NH, USA) for the Western blot analysis using the indicated primary antibodies. Peroxidase-conjugated secondary antibodies were detected using an ECL detection system.
Lactate dehydrogenase assay To determine plasma membrane integrity loss, lactate dehydrogenase (LDH) release into the extracellular medium was measured using the cyto-tox96 nonradioactive assay from Promega (Madison, WI, USA) in order to determine cytotoxicity. This assay measures the formation of a red formazan product after the conversion of lactate and nicotinamide adenine dinucleotide to pyruvate and NADH. The assay was used according to the manufacturer’s instructions. Briefly, the maximum release of LDH was obtained by adding 100 µL of 2% Triton X-100 to the untreated cells. In total, 100 mL of each sample were incubated with 100 µL of LDH assay reagents for 10 min, and the absorbance of the samples was measured at 490 nm. The percentage of LDH release was determined by dividing the amount of LDH released by the cells under each condition by the maximum amount of LDH release, and then multiplying the fraction by 100.
Determination of caspase activity Caspase activities were determined by colorimetric assays using caspases-3, -8, and -9 activation kits according to the manufacturer’s protocol. Briefly, the cells were lysed in the supplied lysis buffer. The supernatants were collected and incubated with the supplied reaction buffer containing dithiothreitol and substrates at 37 °C. The caspase activities were determined by measuring changes in absorbance at 405 nm using the microplate reader.
Statistical analysis All data from the MTT assays, FACS analyses, Western blot analysis, and caspase activity experiments were derived from at least 3 independent experiments. The images were visualized with Chemi-Smart 2000 (Vilber Lourmat, Marine, Cedex, France). The images were captured using Chemi-Capt (Vilber Lourmat, France) and transported into Photoshop. All data are presented as mean±SD. Significant differences among the groups were determined using one-way ANOVA with Scheffe’s test. A value of P<0.05 was accepted as an indication of statistical significance.
Results
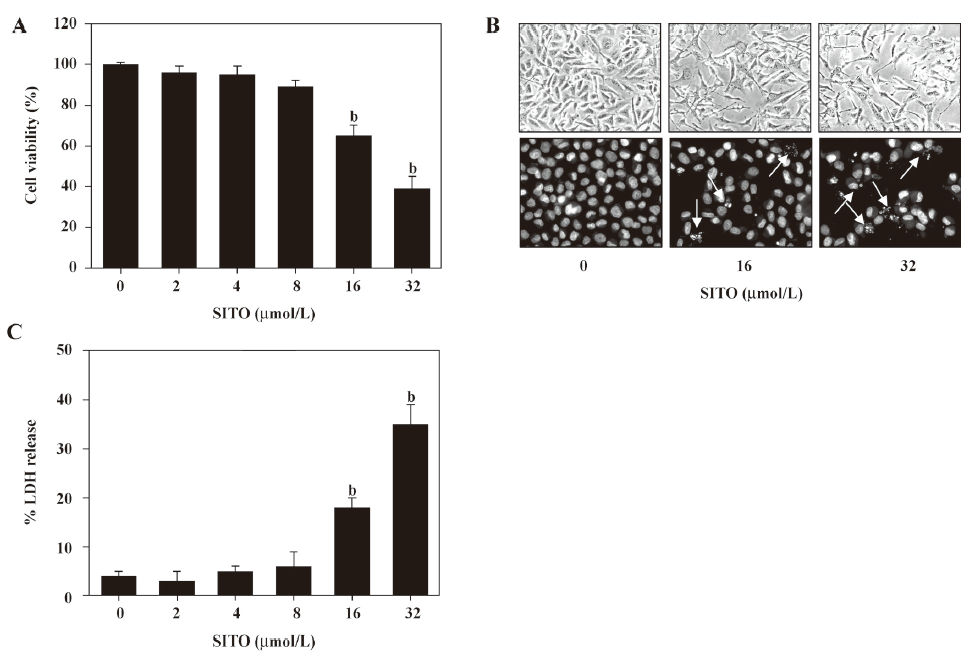
High dosages of SITO (≥ 16 µmol/L) induces apoptosis in MDA-MB-231 breast cancer cells To investigate whether SITO decreases cell viability, the MDA-MB-231 cells were stimulated with the various concentrations of SITO for 48 h and then subjected to MTT assay. As shown in Figure 1A, the inhibition of cell viability was dramatically observed at 16 µmol/L (65%±5%), which in turn decreased to 39%±5% when the cells were exposed to 32 µmol/L SITO at 48 h. CD (5 mmol/L), used as a vehicle control, did not affect cell proliferation and viability. We next examined whether SITO-induced cell death was followed by morphological features. As shown in Figure 1B, 16 or 32 µmol/L SITO induced distinct morphological change and the formation of the apoptotic body of the cells (white arrow), while the vehicle control displayed intact morphology and nuclear structure. Additionally, SITO (16 or 32 µmol/L) resulted in a significant increase of LDH release to 18%±2% or 35%±4%, respectively (Figure 1C). These results indicate that a high dose of SITO (≥ 16 µmol/L) inhibits cell proliferation and induces cell death in MDA-MB-231 cells.
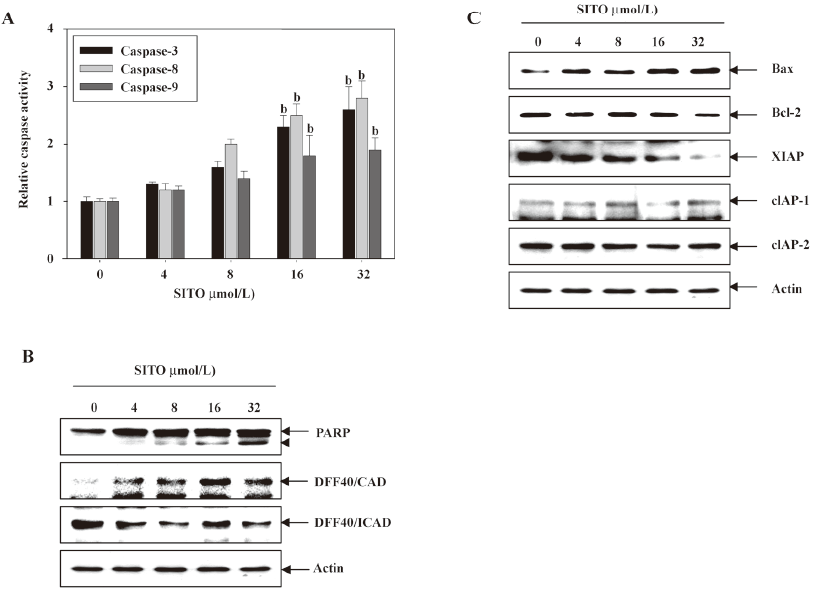
SITO induces apoptosis through upregulation of caspases and pro-apoptotic proteins, and downregulation of anti-apoptotic proteins In order to investigate whether caspases are likely to be involved in the apoptotic response observed in Figure 1, the cells were treated with the indicated concentrations of SITO for 48 h, and the caspase activity from the cell lysates was determined. As shown in Figure 2A, caspases-3, -8, and -9 were activated at concentrations greater than 16 µmol/L SITO. As shown by the Western blot analysis, SITO significantly increased the cleavage of PARP and the expression of DFF40/CAD with the downregulation of DFF40/ICAD at concentrations of 16 or 32 µmol/L SITO. These results indicate that treatment with more than 16 µmol/L SITO increases the activation of caspases-3, -8, and -9 followed by increases in PARP cleavage and DFF40/CAD expression (Figure 2B). In a parallel experiment, to determine the expression of anti- and pro-apoptotic proteins in SITO-mediated apoptosis, we analyzed the expressions of Bcl-2 and inhibitor of apoptosis (IAP) family proteins by Western blot analysis. As shown in Figure 2C, SITO dose dependently increased the Bax level and slightly decreased the Bcl-2 level. We further revealed that more than 16 µmol/L SITO decreased IAP family proteins, such as XIAP, but not cIAP-1 and cIAP-2 in the MDA-MB-231 cells. The SITO-treated cells resulted in a dose-dependent downregulation of IAP family members. These results suggest that the apoptotic actions of SITO are closely related with the upregulation of the Bax/Bcl-2 ratio, the downregulation of the IAP family, and caspase activation.
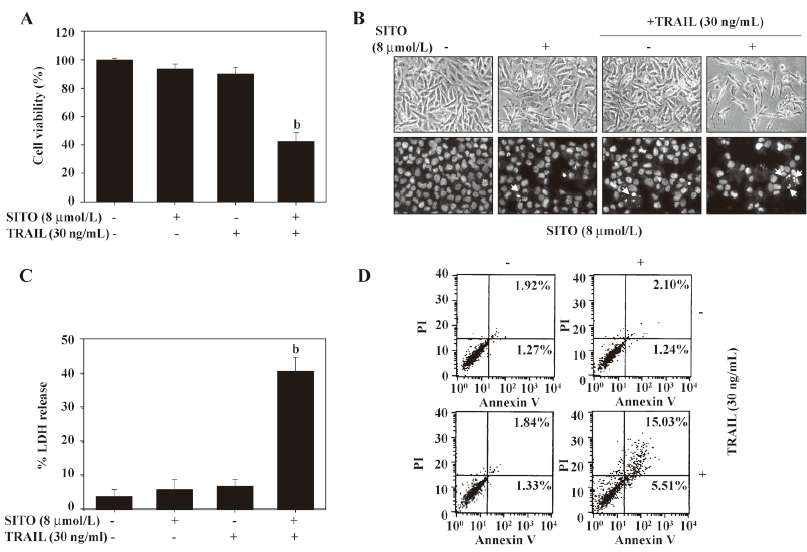
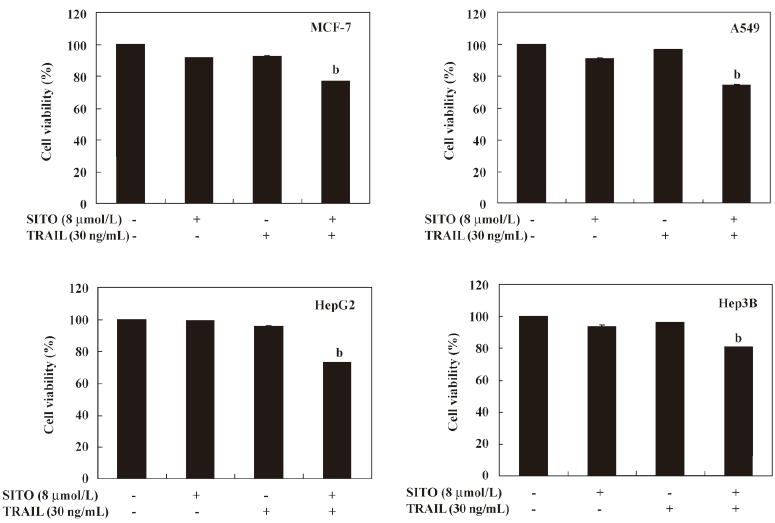
Non-toxic dose of SITO significantly triggers TRAIL-mediated apoptosis in a low dose of TRAIL-resistant MDA-MB-231 cells To investigate the synergistic effects, the MDA-MB-231 cells were treated with subtoxic concentration of SITO and TRAIL for 48 h and the cell viability was assessed using the MTT assay. As shown in Figure 3A, treatment with SITO (8 µmol/L) or TRAIL alone (30 ng/mL) for 48 h was not able to reduce cell viability in the MDA-MB-231 cells. Notably, the MDA-MB-231 cells treated with a synergistic treatment of 8 µmol/L SITO and 30 ng/mL TRAIL significantly reduced cell viability (42%±6%), which was similar to treatment with more than 32 µmol/L SITO alone. For an assessment of apoptosis, we analyzed chromatin condensation and apoptotic bodies. Treatment with SITO (8 μmol/L) or TRAIL (30 ng/mL) alone did not induce any morphological features or apoptotic bodies indicative of cell death (Figure 3B). However, the synergistic treatment with SITO and TRAIL induced significant decreased membrane shrinkage, cell rounding, and the appearance of apoptotic bodies in MDA-MB-231 cells. Additionally, LDH release was also increased more than 4 times than that of the control in the synergistic treatment with SITO and TRAIL (Figure 3C). Next, we analyzed the apoptotic effects using flow cytometric analysis to detect Annexin V+ cells. The synergistic treatment resulted in a markedly increased accumulation of Annexin V+ cells, whereas treatment with SITO or TRAIL alone did not (Figure 3D). We also examined whether SITO could sensitize various resistant tumor cells to the apoptotic effects of TRAIL. The treatment of MCF-7, A549, HepG2, and Hep3B cells with 30 ng/mL and 8 µmol/L SITO reduced cell viability in all of the tested cells, but the effects were weaker than the MDA-MB-231 cells. TRAIL alone or SITO alone had no significant effect on the viability (Figure 4). Taken together, these results indicate that SITO significantly sensitizes TRAIL-induced apoptosis in a low dose of TRAIL-resistant MDA-MB-231 cells.
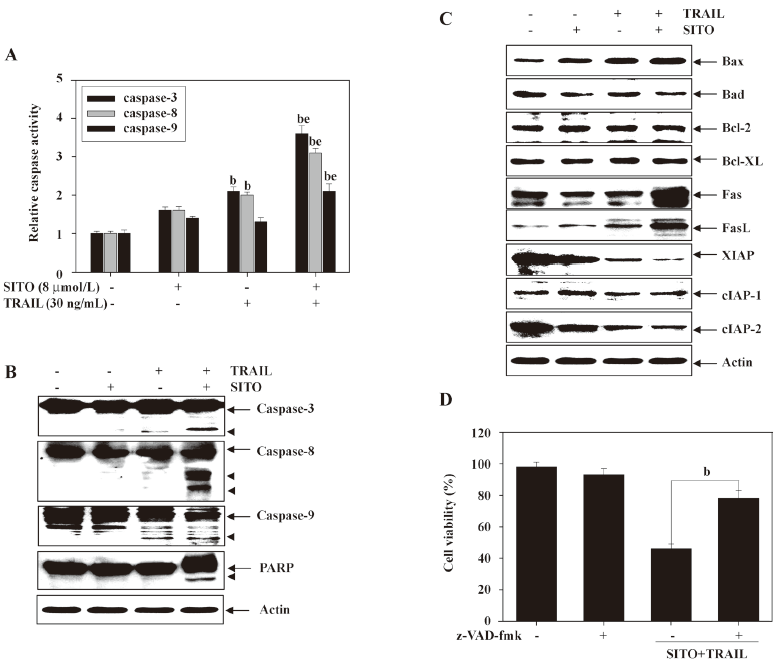
Synergistic treatment with SITO and TRAIL upregulates pro-apoptotic proteins and downregulates anti-apoptotic proteins Caspases are known to act as important mediators of apoptosis and contribute to the overall apoptotic morphology by cleavage of various cellular substrates. Therefore, we investigated the activation and expression of caspases-3, -8, and -9 in MDA-MB-231 cells treated with SITO and TRAIL for 48 h. Cell lysates containing equal amounts of total protein from cells treated with SITO and TRAIL were assayed for in vitro caspase activity. As shown in Figure 5A, treatment with TRAIL alone slightly increased caspase activity; however, the synergistic treatment with SITO (8 ìmol/L) and TRAIL (30 ng/mL) significantly increased the activity of caspases-3, -8, and -9 in MDA-MB-231 cells. The Western blot analysis also revealed that the synergistic treatment markedly induced cleavage of caspases in MDA-MB-231 cells (Figure 5B). To evaluate the expression of anti- and pro-apoptotic family proteins in the synergistic treatment, we analyzed the expression of these proteins using Western blot analysis. As shown in Figure 5C, the pro-apoptotic Bax expression was significantly induced in the synergistic treatment, whereas the levels of anti-apoptotic Bcl-2 and Bcl-XL remained unchanged. One of the IAP family members, anti-apoptotic XIAP was markedly downregulated, and the expressions of Fas and FasL were significantly augmented by the synergistic treatment. As shown in Figure 5D, synergistic treatment-induced apoptosis was significantly suppressed in the presence of pan-caspase inhibitor z-VAD-fmk, indicating that the TRAIL-induced apoptosis sensitized by SITO was mediated through caspase activation in the MDA-MB-231 cells. These results indicate that caspases are crucial regulators of apoptosis by the combined treatment of SITO and TRAIL in MDA-MB-231 cells.
Discussion
TRAIL has aroused great interest in cancer therapy because it can selectively induce cancer cells, and transformed cells can undergo apoptosis with no toxicity to normal cells[1,2]. These observations indicate that TRAIL may prove to be a safe and effective biological agent for cancer therapy in humans. However, recent studies have shown that many cancer cells, including human breast carcinoma cells, are resistant to the apoptotic effects of TRAIL[18–20]. Recent studies have also reported that SITO, as a chemotherapeutic agent, can induce apoptosis in metastatic MDA-MB-231 breast cancer cells[21–24]. If the breast cancer cells are treated with high concentrations of SITO for more than 2 or 3 d, SITO can significantly induce apoptosis. To overcome these problems, in the present study, we investigated whether synergistic treatment with SITO and TRAIL triggers apoptosis in MDA-MB-231 breast cancer cells that are normally resistant to either agent alone. The synergistic treatment induced cell death followed by cell shrinkage and apoptotic body formation, which are hallmark features of apoptosis. Our results also revealed that the synergistic treatment with SITO and TRAIL enhances apoptosis through the activation of caspases in MDA-MB-231 cells. This synergistic chemotherapy is very efficient to sensitize TRAIL-induced apoptosis in TRAIL-resistant MDA-MB-231 breast cancer cells.
Recently, several researchers reported that some chemopreventive agents sensitize TRAIL-mediated apoptosis through the activation of caspase-3 in TRAIL-resistant cancer cells[25,26]. Caspases belong to a family of cysteine proteases that are integral parts of the apoptotic pathway. In particular, activated caspases have many cellular targets, that when severed and/or activated, produce the morphological features of apoptosis[27]. Many studies have determined that a variety of chemotherapeutic agents induce apoptosis through the activation of caspases and the degradation of PARP and lamin A[28,29]. Our findings indicate that caspases are critical protease mediators of apoptosis triggered by the synergistic treatment of TRAIL and SITO. Furthermore, downstream in the TRAIL-induced apoptotic pathway, mutations to the pro-apoptotic protein Bax and increased expression of IAP family members such as XIAP and cIAP, can contribute to the resistance to TRAIL-mediated apoptosis[30–32]. In the present study, we found that the synergistic treatment increases the expression of the pro-apoptotic Bax, Fas, and FasL protein and results in a decrease of the anti-apoptotic XIAP, but not cIAP-1 and cIAP-2. The ability to induce apoptosis makes synergistic treatment with SITO and TRAIL a potentially effective preventive and therapeutic agent to combat malignant breast cancer cells.
Thus, the results of this study suggest that SITO sensitizes TRAIL-mediated apoptosis by the upregulation of apoptotic proteins, including Bax and caspases. Therefore, the use of TRAIL with low concentrations of SITO may provide an effective therapeutic strategy for safely treating TRAIL-resistant breast cancer cells.
References
- Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity or recombinant soluble Apo2 ligand. J Clin Invest 1999;104:155-62.
- Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 1999;5:157-63.
- Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997;277:815-8.
- Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science 1997;276:111-3.
- Srivastava RK. TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia 2001;3:535-46.
- Singh TR, Shankar S, Chen X, Asim M, Srivastava RK. Synergistic interactions of chemotherapeutic drugs and tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand on apoptosis and on regression of breast carcinoma in vivo. Cancer Res 2003;63:5390-400.
- Shao R, Lee DF, Wen Y, Ding Y, Xia W, Ping B, et al. E1A sensitizes cancer cells to TRAIL-induced apoptosis through enhancement of caspase activation. Mol Cancer Res 2005;3:219-26.
- Butler LM, Liapis V, Bouralexis S, Welldon K, Hay S, Thai LM, et al. The histone deacetylase inhibitor, suberoylanilide hydroxamic acid, overcomes resistance of human breast cancer cells to Apo2L/TRAIL. Int J Cancer 2006;119:944-54.
- Awad AB, Fink CS. Phytosterols as anticancer dietary components: evidence and mechanism of action. J Nutr 2000;130:2127-30.
- Vanhanen HT, Miettinen TA. Effects of unsaturated and saturated dietary plant sterols on their serum contents. Clin Chim Acta 1992;205:97-107.
- Muti P, Awad AB, Schunemann H, Fink CS, Hovey K, Freudenheim JL, et al. A plant food-based diet modifies the serum β-sitosterol concentration in hyperandrogenic postmenopausal women. J Nutr 2003;133:4252-55.
- Raicht RF, Cohen BI, Fazzini EP, Sarwal AN, Takahashi M. Protective effect of plant sterols against chemically induced colon tumors in rats. Cancer Res 1980;40:403-5.
- Gupta MB, Nath R, Srivastava N, Shanker K, Kishor K, Bhargava KP. Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med 1980;39:157-63.
- Garcia MD, Saenz MT, Gomez MA, Fernandez MA. Topical anti-inflammatory activity of phytosterols isolated from Eryngium foetidum on chronic and acute inflammation models. Phytother Res 1999;13:78-80.
- Choi S, Kim KW, Choi JS, Han ST, Park YI, Lee SK, et al. Angiogenic activity of β-sitosterol in the ischemia/reperfusion-damaged brain of Mongolian gerbil. Planta Med 2002;68:330-5.
- Bouic PJ, Lamprecht JH. Plant sterols and sterolins: a review of their immune-modulating properties. Altern Med Rev 1999;4:170-7.
- Bouic PJ. The role of phytosterols and phytosterolins in immune modulation: a review of the past 10 years. Curr Opin Clin Nutr Metab Care 2001;4:471-5.
- Clodi K, Wimmer D, Li Y, Goodwin R, Jaeger U, Mann G, et al. Expression of tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) receptors and sensitivity to TRAIL-induced apoptosis in primary B-cell acute lymphoblastic leukemia cells. Br J Haematol 2000;111:580-6.
- Fulda S, Jeremias I, Debatin KM. Cooperation of betulinic acid and TRAIL to induce apoptosis in tumor cells. Oncogene 2004;23:7611-20.
- MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, et al. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukemia. Oncogene 2002;21:6809-18.
- Awad AB, Roy R, Fink CS. β-Sitosterol, a plant sterol, induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol Rep 2003;10:497-500.
- Awad AB, Williams H, Fink CS. Phytosterols reduce in vitro metastatic ability of MDA-MB-231 human breast caner cells. Nutr Cancer 2001;40:157-64.
- Awad AB, Downie A, Fink CS, Kim U. Dietary phytosterol inhibits the growth and metastasis of MDA-MB-231 human breast cancer cells grown in SCID mice. Anticancer Res 2000;20:821-4.
- Awad AB, Downie AC, Fink CS. Induction of growth and stimulation of apoptosis by β-sitosterol treatment of MDA-MB-231 human breast cancer cells in culture. Int J Mol Med 2000;5:541-45.
- Sprick MR, Weigand MA, Rieser E, Rauch CT, Juo P, Blenis J, et al. FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 are essential for apoptosis mediated by TRAIL receptor 2. Immunity 2000;12:599-609.
- Bratton SB, MacFarlane M, Cain K, Cohen GM. Protein complexes activate distinct caspase cascades in death receptor and stress-induced apoptosis. Exp Cell Res 2000;256:27-33.
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997;326:1-16.
- Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta 1998;1387:17-31.
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 1999;68:383-424.
- Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-κB, survivin, Bcl-2 and caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol 2004;10:22-5.
- LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med 2002;8:274-81.
- Kim EH, Kim SU, Shin DY, Choi KS. Roscovitine sensitizes glioma cells to TRAIL-mediated apoptosis by downregulation of survivin and XIAP. Oncogene 2004;23:446-56.
