Gum mastic increases maspin expression in prostate cancer cells1
Introduction
Maspin is a mammary serine protease inhibitor with tumor suppressive activity for prostate cancers[1–3], and its expression decreases with prostate cancer progression[4]. Functional studies have demonstrated that maspin inhibits tumor invasion and motility of human prostate cancer cells in vitro[5], as well as tumor growth and metastasis in the nude mice assay[6,7]. It is speculated that upregulation of maspin in the prostate tumors may offer great hope for reversing the tumor phenotypes[8,9]. Accumulating evidence has shown that maspin regulation is controlled at the transcriptional level, and some elements in the maspin promoter are identified. In our previous studies, we characterized a negative androgen-responsive element (ARE) element and a positive Sp1 element in the maspin promoter in prostate cancer cells[10]. In order to identify what agents can regulate maspin expression, we treated prostate cancer cells with different compounds to select the potential agent for prostate cancer therapy. Gum mastic, a natural resin, has been shown to dramatically increase maspin expression.
Known for centuries, gum mastic is a resinous exudate obtained from the stem and the main leaves of Pistacia lentiscus trees and is extensively used in Mediterranean and Middle Eastern countries, both as a dietary supplement and herbal remedy[11]. Medical trials have indicated that gum mastic has no side effects, and shows its protective effects on the gastrointestinal environment, such as relief of ulcers, reducing the intensity of gastric mucosal damage caused by anti-ulcer drugs and aspirin, and possessing anti-acid and cytoprotective qualities[12–14]. Recently, gum mastic has shown its antibacterial and antiviral action and antitumor effect in many traditional Chinese medicines[15,16]. In the present work, we ascertained the effect of gum mastic on maspin expression in human prostate cancer cells and discovered possible mechanisms involved in this regulatory system.
Materials and methods
Cell culture and treatments The human prostate cancer cell lines, LNCaP and DU-145, were obtained from the American Type Culture Collection (Manassas, VA, USA). The LNCaP cell line was established from a lymph node metastasis of a prostate cancer patient and expressed androgen-receptor (AR), and DU-145 was from a bone metastasis and was absent of AR. Cells were seeded in 35 mm culture dishes in RPMI -1640 medium supplemented with 10% fetal bovine serum (FBS) and 5% CO2 at 37 °C until reaching approximately 50%–70% confluence. The cells were maintained in serum-free RPMI-1640 medium for a further 8 h before experiments in order to synchronize cells. The cells were then treated with gum mastic at indicated concentrations in RPMI- 1640 medium containing 5% FBS (GIBCO BRL Grand Island, NY, USA). Gum mastic (Sigma, St Louis, MO, USA, N
Western blot analysis Treated LNCaP cells were harvested and lysed as described previously[4]. Cell extracts were quantified by BCA method. For the Western blot analysis, 40 µg cell extracts were separated on 10% SDS-PAGE and transferred to the nitrocellulose membrane, and then the nitrocellulose membrane was immediately blocked with 5% non-fat milk in PBS buffer for 1 h at room temperature. After blocking, the membrane was incubated with human specific anti-maspin antibodies (BD Biosciences, San Diego, CA, USA) at 4 °C for 12 h, followed by the incubation with peroxidase-labeled second antibody for 1 h, and immunoreactive bands were visualized by enhanced chemiluminescence (ECL, Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA). β-tubulin (Sigma, USA) was used to normalize the quantity of the protein on the blot. At least 3 independent Western blots were performed.
RT-PCR analysis Total RNA was isolated from the treated cells by TRIzol reagent (Invitrogen, Carlsbad, CA, USA). According to the manufacturer,s instructions, a portion of total RNA (2 µg) was transcribed reversibly with the M-Mulv reverse transcriptase in the presence of a random hexamer primer. The resulting cDNA preparation was subjected to PCR amplification using PCR kit from TaKaRa Biotech (DaLian, China). The primers used for maspin gene were: sense 5'-TGCTGCCTACTTTGTTGGCAAGT-3' and antisense 5'-TGATACTGTCAATGTTTCCCATACAGA-3', and for the housekeeping gene β-actin: sense 5'-GTGGGGCGCCCA-GGCACCACGATG-3' and antisense 5'-CTCCTTAATGTCA-CGCACGATTT-3'. PCR profiles consisted of first initial denaturation at 94 °C for 3 min, followed by 8 cycles of denaturation at 94 °C for 40 s, primer-annealing at 53 °C for 40 s, and primer extension at 72 °C for 40 s. Then the primers for β-actin were added into the reaction complex, and the reaction continued at the condition of denaturation at 94 °C for 40 s, primer-annealing at 53 °C for 40 s, and primer extension at 72 °C for 40 s for 16 cycles. The final primer extension was performed at 72 °C for 10 min. The PCR products were analyzed by electrophoresis on a 1.5% agarose gel containing ethidium bromide and photographed under UV light. At least 3 independent RT-PCR were performed.
Transient transfection assay pGL3-860 bp maspin promoters were described previously. Transient transfection was performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA). Briefly, for the firefly Lucifer’s reporter assay, the cells were transfected with 1 µg of pGL3-860 bp in combination with 0.05 µg pRL-TK (Promega, Madison, WI, USA) for the internal control. In following transfections, the cells were incubated with designated concentrations of gum mastic additionally for 12 h in RPMI-1640 medium containing 5% FBS, then the cell extracts were prepared and luciferase assays were performed according to the manufac-turer,s instruction of Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA). At least 3 independent transfections were performed, and standard deviations (SD) were calculated.
Nuclear extracts LNCaP cells were grown in the same conditions as described earlier, and cultured in RPMI-1640 with addition of gum mastic for 24 h. 2×109 cells were pelleted and resuspended in 1 mL cold hypotonic buffer (10 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L DTT, and 0.5 mmol/L PMSF). Following 15 min incubation on ice, the cells were lysed by adding 50 µL of 10% NP-40 and centrifuged at 6000×g for 5 min at 4 °C. Then the pellets were resuspended in 70 µL of cold hypertonic buffer (20 mmol/L HEPES, pH 7.9, 1.5 mmol/L MgCl2, 420 mmol/L NaCl, 0.2 mmol/L EDTA, 25% glycerol, 10 µg /mL aprotinin, and 0.5 mmol/L PMSF). The resuspended cells were stirred gently at 4 °C for 30 min and centrifuged at 12 000 rpm for 5 min at 4 °C. The supernatant was extensively dialyzed against the dialysis buffer (20 mmol/L HEPES, pH 7.9, 50 mmol/L KCl, 25% glycerol, 0.5 mmol/L DTT, and 0.5 mmol/L PMSF) for 2 h at 4 °C. The protein concentration of the dialyzed material was determined by BCA method and stored at -80 °C in small aliquots.
Electrophoretic mobility shift assay (EMSA) Equal amounts of sense and antisense oligonucleotides were mixed and annealed in 10 mmol/L Tris·HCl, pH 8.0, 200 mmol/L NaCl, and 1 mmol/L EDTA by heating to 95 °C for 5 min and cooling to room temperature for over 3 h. The corresponding oligonucleotides were labeled with digoxin (DIG). The following oligonucleotides were used for EMSA experiments: maspin Sp1: sense 5'-TGCCGCCGAGGCGGGGCGGGGCGGGGCGT-GGAG-3' and antisense 5'-GCTCCACGCCCCGCCCCGCCCC-GCCTCGGCGGCA-3'; maspin ARE: sense 5'-AAGAATGGA-GATCAGAGTACTT-3' and antisense 5'-AAGTACTCTGA-TCTCCATTCTT -3'.
Binding reactions were carried out at room temperature for 30 min in a mixture containing 4% glycerol, 1 mmol/L MgCl2, 0.2 mmol/L EDTA, 0.5 mmol/L dithiothreitol, 50 mmol/L NaCl, 10 mmol/L Tris·HCl, 2 µg poly (dI-dC), 10 µg nuclear extracts, and DIG-labeled oligonucleotide probe. Then the reaction mixture was subjected to electrophoresis in 5% non-denaturing polyacrylamide gels in 0.25×Tris/borate/EDTA buffer. Based on the instructions of the DIG Gel Shift Kit (Roche Co, Palo Alto, CA, USA), electroblotting and chemiluminescent detection were performed.
The specificity of ARE and Sp1 binding was confirmed by adding 125-fold in excess of unlabeled DNA probe to the assay. At least 3 independent EMSA were performed.
Results
Gum mastic increased maspin expression Maspin is a tumor-suppressing gene for prostate cancer. The aim of this study was to determine whether maspin protein levels change with gum mastic treatment in prostate cancer LNCaP cells. Western blotting was performed. As shown in Figure 1A, when the LNCaP cells were exposed to different concentrations of gum mastic for 24 h, gum mastic increased maspin expression in a dose-dependent manner. Gum mastic 8 μg/mL increased maspin expression about 1.5-fold. To further demonstrate the increased effect of gum mastic on the steady levels of maspin mRNA, RT-PCR was used. The results in Figure 1C and 1D show that maspin mRNA expression was significantly increased by gum mastic, which was consistent with the effect of gum mastic on maspin protein expression. To understand the potential mechanism by which the expression of maspin could be affected by gum mastic, transient transfections with a maspin promoter-luciferase construct was performed to determine if transcriptional activity of the maspin gene was changed by gum mastic. As shown in Figure 1E, the activity of the maspin promoter was increased by gum mastic, consistent with the results of Figure 1A–1D. Therefore, we may conclude that the increased effects of gum mastic on maspin expression mainly occur at the transcriptional level, which subsequently affects the levels of maspin mRNA and protein. At the same time, we also detected maspin protein levels in androgen-independent prostate cancer DU-145 cells treated with gum mastic. As illustrated in Figure 1F, gum mastic increased maspin expression in DU-145 cells.
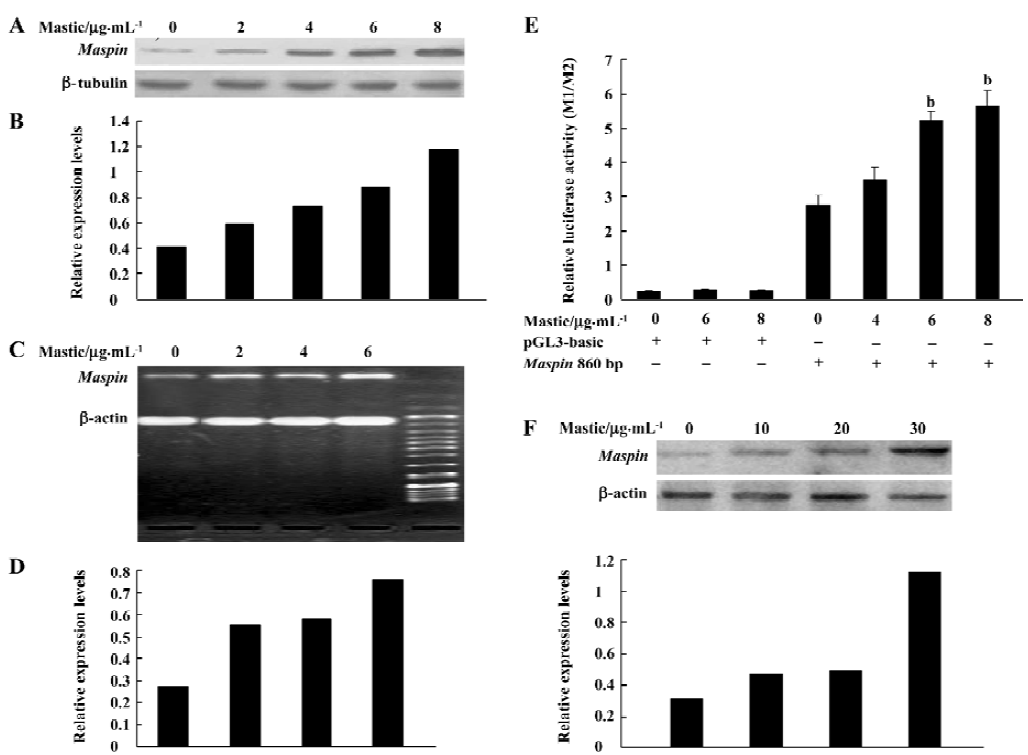
Gum mastic inhibited nuclear AR expression It has been demonstrated that maspin is an AR-mediated gene. AR binds to the ARE element in the maspin promoter to inhibit its expression. To determine whether the increased effect of gum mastic on maspin expression might be due to the alteration of AR expression, the effect of gum mastic on AR expression was studied by Western blotting. Since the AR was a nuclear protein and functioned in the nucleus, we prepared the nuclear extracts for Western blotting after the LNCaP cells were exposed to gum mastic for 24 h. The results from Figure 2 show that the expression of the AR protein was dramatically decreased by gum mastic in a dose-dependent manner.
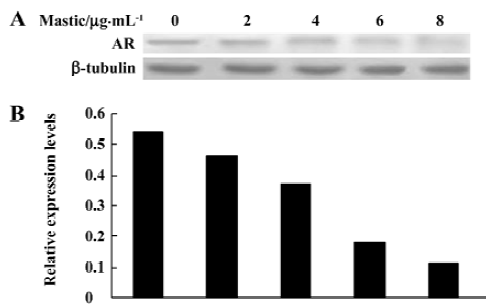
Gum mastic inhibited AR binding to ARE in the maspin promoter The above results led us to investigate whether the AR binding to ARE could be inhibited by gum mastic. We used the gel band-shift technique as an in vitro functional assay to determine the binding activity of ARE in the maspin promoter. The results in Figure 3 show that ARE binding activity was largely decreased by 8 µg/mL gum mastic after 24 h of treatment when compared with the control. The bands were confirmed to be a result of specific binding for ARE because the DNA-protein complex was competed out by a 125-fold molar excess of unlabeled ARE oligonucleo-tides. Furthermore, the bands could be reduced by a specific anti-AR antibody. Together with the earlier results, it was implicated that gum mastic increased maspin expression by inhibiting AR activity.

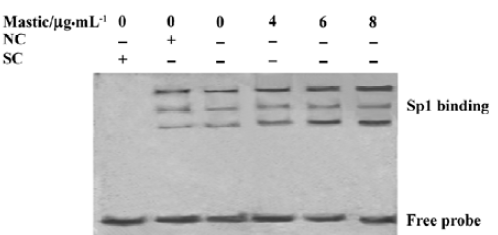
Gum mastic enhanced the binding activity of Sp1 element in the maspin promoter We have demonstrated that the Sp1 element in the maspin promoter plays a positive role in its transcription[10]. To further study the potential molecular mechanisms of gum mastic on maspin expression, the binding activity of the Sp1 element in the maspin promoter was detected after the LNCaP cells were exposed to different concentrations of gum mastic for 24 h. The results from Figure 4 show that gum mastic enhanced the binding activity of Sp1 element in the maspin promoter. The bands were confirmed to be a result of specific binding for Sp1 because the DNA-protein complex was competed out by a 125-fold molar excess of unlabeled Sp1 oligonucleotides, but not by a 125-fold molar excess of unlabeled E2F oligonucleotides.
Discussion
We have demonstrated that AR binds to the ARE element in the maspin promoter to inhibit its expression, and Sp1 plays a positive role in maspin transcription[10]. In this study, it was shown that gum mastic inhibited AR binding to ARE, and enhanced Sp1 binding activity. Moreover, gum mastic enhanced the activity of the maspin promoter, which might finally contribute to its upregulation for maspin expression.
Gum mastic is an affordable and safe natural supplement that protects the digestive system, heals peptic and duodenal ulcers, and eradicates Helicobacter pylori from the gut, while H pylori is a primary agent to promote the development of gastric cancer[17]. It is implicated that gum mastic may effectively prevent gastric cancer. Recently, it was reported that gum mastic induces the apoptosis of the human colon cancer HCT116 cells by activating caspase-8 and caspase-9[18]. Gum mastic has also been shown to inhibit LNCaP cell growth by inhibiting AR function[19]. AR is closely related to the development and progression of prostate cancer[20,21]. Androgen-responsive prostate cancer LNCaP cells require AR for continued proliferation and survival[22]. AR blockade may be an effective strategy in the fight against prostate cancer. Maspin is an AR target gene, specifically targeting AR, or its downstream signaling molecules will be potentially effective for achieving total AR blockade.
Maspin is often silenced in prostate cancer cells and exhibits suppressing activity against tumor growth and metastasis. It has also been shown to be involved in processes that are important to both tumor growth and metastasis such as cell invasion, angiogenesis, and more recently, apoptosis[5–9]. Therefore, maspin can be taken as a potential target for cancer therapy. Maspin expression is directly regulated by the p53 gene. p53 induces maspin expression in prostate cancer cells and suppresses tumor growth and metastasis[23]. γ-Linolenic acid, an essential fatty acid with anticancer properties, is reported to induce maspin expression and inhibit cell motility[24]. Peroxisome proliferator-activated receptor-gamma, nitric oxide, and the manganese-containing superoxide dismutase induced maspin expression in breast and prostate cancer cells. This effect correlated with a differentiated phenotype, decreased cell motility and invasiveness, and increased the apoptotic index[25–29]. Our study discovered another potential agent for inducing maspin expression and inhibiting the growth of prostate cancer cells.
Gum mastic inhibited AR function and increased the AR-mediated gene maspin expression in LNCaP cells. On the other hand, gum mastic also has been shown to inhibit the proliferation of androgen-refractory prostate cancer cells, DU-145 (data not shown), which express little of the AR. This implies that the inhibitory effect of gum mastic on prostate cancer is not limited in an androgen-responsive manner, and other signal pathways may be involved in the mastic-mediated regulatory system. Sp1 is a ubiquitous transcription factor that binds to consensus elements in the proximal promoters of a wide variety of genes. It is involved in the regulation of many aspects of physiological and pathological conditions, including cell growth, apoptosis, angio-genesis, and invasion[30,31]. Sp1 has 2 other closely related members of a gene family encoding proteins with very similar structures, but different molecular weights, Sp3 and Sp4. Sp1, Sp3, and Sp4 are highly conserved and can recognize the GC box with identical affinities[32]. Gum mastic increased Sp1 binding activity, which may contribute to its increased effect on maspin expression; the upregulation of maspin leads to its inhibition on prostate cancer cells.
References
- Abraham S, Zhang W, Greenberg N, Zhang M. Maspin functions as tumor suppressor by increasing cell adhesion to extracellular matrix in prostate tumor cells. J Urol 2003;169:1157-61.
- Sheng S, Pemberton PA, Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem 1994;269:30988-93.
- Sheng SJ, Carey J, Elisabeth AS, Lauren D, Mary JC, Hendrix SR. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. PNAS 1996;93:669-74.
- Zou Z, Anisowicz A, Hendrix MJC, Thor A, Neveu M, Sheng S, et al. Identification of a novel serpin with tumor suppressing activity in human mammary epithelial cells. Science 1994;263:526-9.
- Cher ML, Biliran HR Jr, Bhagat S, Meng Y, Che M, Lockett J, et al. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. PNAS 2003;100:7847-52.
- Schaefer JS, Zhang M. Role of maspin in tumor metastasis and angiogenesis. Curr Mol Med 2003;3:653-8.
- Zhang M, Volpert O, Shi YH, Bouck N. Maspin is an angiogenesis inhibitor. Nat Med 2000;6:196-9.
- Jiang N, Meng Y, Zhang S, Mensah OE, Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene 2002;21:4089-98.
- Li Z, Shi HY, Zhang M. Targeted expression of maspin in tumor vasculatures induces endothelial cell apoptosis. Oncogene 2005;24:2008-19.
- He ML, Jiang AL, Zhang PJ, Hu XY, Liu ZF, Yuan HQ, et al. Identification of androgen-responsive element ARE and Sp1 element in the maspin promoter. Chin J Physiol 2005;48:160-6.
- Al-Said MS, Ageel AM, Parmar NS, Tariq M. Evaluation of mastic, a crude drug obtained from Pistacia lentiscus for gastric and duodenal anti-ulcer activity. J Ethnopharmacol 1986;15:271-8.
- Marone P, Bono L, Leone E, Bona S, Carretto E, Perversi L. Bactericidal activity of Pistacia lentiscus mastic gum against Helicobacter pylori. J Chemotherapy 2001;13:611-4.
- Huwez FU, Al-Habbal MJ. Mastic in treatment of benign gastric ulcers. Gastroenterologia Japonica 1986;21:273-4.
- Al-Habbal MJ, Al-Habbal Z, Huwez FU. A double-blind controlled clinical trial of mastic and placebo in the treatment of duodenal ulcer. Clin Exp Pharmacol P 1984;11:541-4.
- Huwez FU, Thirlwell D, Cockayne A. Ala’Aldeen DA. Mastic gum kills Helicobacter pylori. New Engl J Med 1998;339:1946-8.
- Will Block. Mastic is more than an antibacterial. Available from URL: http://www.life-enhancement.com/article_template.asp?ID=770
- Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology 2005;128:1567-78.
- Balan KV, Demetzos C, Prince J, Dimas K, Cladaras M, Han Z, et al. Induction of apoptosis in human colon cancer HCT116 cells treated with an extract of the plant product, Chios mastic gum. In Vivo 2005;19:93-102.
- He ML, Yuan HQ, Jiang AL, Gong AY, Chen WW, Zhang PJ, et al. Gum mastic inhibits the expression and function of the androgen receptor in prostate cancer cells. Cancer 2006;12:2547-55.
- Pascale VN, Jian X, Yuan YJ, Paul H, Daniel H, Sharon A, et al. Identification of genetic pathways activated by the androgen receptor during the induction of proliferation in the ventral prostate gland. J Biol Chem 2004;279:1310-22.
- Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med 2000;6:703-6.
- Yang Q, Fung KM, Day WV, Kropp BP, Lin HK. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell Int 2005;5:8-18.
- Ines IT, Carmelo F, Elena L, Ileana F, Francesco B, Massimo PC. Binding of Sp1 to the proximal promoter links constitutive expression of the human uPA gene and invasive potential of PC3 cells. Blood 2002;100:3325-32.
- Zou Z, Gao C, Nagaich AK, Connell T, Saito S. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem 2000;275:6051-4.
- Jiang WG, Hiscox S, Horrobin DF, Bryce RP, Mansel RE. γ-Linolenic acid regulates expression of maspin and the motility of cancer cells. Biochem Biophys Res Commun 1997;237:639-44.
- Khalkhali-Ellis Z, Hendrix MJ. Nitric oxide regulation of maspin expression in normal mammary epithelial and breast cancer cells. Am J Pathol 2003;162:1411-7.
- Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, et al. Terminal differentiation of human breast cancer through PPARγ. Mol Cell 1998;24:465-70.
- Li JJ, Colburn NH, Oberley LW. Maspin gene expression in tumor suppression induced by overexpressing manganese-containing superoxide dismutase cDNA in human breast cancer cells. Carcinogenesis 1998;19:833-9.
- Duan H, Zhang HJ, Yang JQ, Oberley LW, Futscher BW, Domann FE. MnSOD up-regulates maspin tumor suppressor gene expression in human breast and prostate cancer cells. Antioxid Redox Sign 2003;5:677-88.
- Luster TA, Johnson LR, Nowling TK, Lamb KA, Philipsen S, Rizzino A. Effects of three Sp1 motifs on the transcription of the FGF-4 gene. Mol Reprod Dev 2000;57:4-15.
- Zhang X, Li Y, Dai C, Yang J, Mundel P, Liu Y. Sp1 and Sp3 transcription factors synergistically regulate HGF receptor gene expression in kidney. Am J Physiol-Renal 2003;284:82-94.
- Hagen G, Muller S, Beato M., Suske G. Cloning by recognition site screening of two novel GT box binding proteins: a family of Sp1 related genes. Nucleic Acids Res 1992;20:5519-25.
