Embryo-chondrocyte expressed gene 1, downregulating hypoxia-inducible factor 1α, is another marker of lung tumor hypoxia
Introduction
Hypoxia is common in solid tumors and is associated with an aggressive phenotype, and is resistant to therapy[1]. Hypoxic regions are also common features within rapidly growing malignant tumors, such as non-small cell lung cancer (NSCLC)[2]. Cancer cells undergo genetic and adaptive changes that allow them to survive and even proliferate in the hypoxic environment[3]. The transcriptional complex hypoxia-inducible factor (HIF) has emerged as a key regulator mediating many cellular responses necessary to adapt to changes in the hypoxic environment[4–6]. In normoxia, the HIF-1α units are unstable since 2 prolyl residues within the oxygen-dependent degradation domains of HIF-1α subunits are hydroxylated by prolyl hydroxylases and dioxygen as a co-substrate[6]. However, in hypoxia, as frequently occurs within tumors, there is insufficient oxygen to allow this process resulting in HIF-1α stabilization and translocation to the nucleus, where it is able to bind HIF-1α. The complex then recruits co-activators that bind to specific DNA hypoxia response elements (HRE), resulting in increased mRNA transcription[7]. Overexpression of HIF-1α, a “master” gene in hypoxia, is a frequent occurrence in many tumors, including NSCLC[8]. However, the pathways that regulate the HIF-1α expression are unclear today.
Reports have shown several pathways are regulated by hypoxia and many of the known oncogenic signaling pathways[9] overlap with hypoxia-induced pathways. One of the most consistently hypoxia-inducible genes has been identified as embryo-chondrocyte expressed gene 1 (DEC1)[6].
DEC1[10], also called the enhancer of split and hairy related protein-2[11] or stimulated with retinoic acid-13[12], has been identified independently by 3 research laboratories, each thoroughly studied on a different system of mammalian differentiation, which is located at chromosome 3p25.3–26[13]. Although the role of DEC1 in human physiology is not thoroughly clear, it has been reported to have roles in proliferation[12], apoptosis[14] and cell differentiation[15]. Recent studies[16,17] have also supposed there might be a possible relationship between DEC1 and HIF-1α expression by immunohistochemistal detection of the 2 proteins in many solid tumors, such as breast cancer and non-small lung cancer. Thus, DEC1, which might associate with HIF-1α and its expression in human tumors, might be a direct marker of tumor hypoxia.
DEC1 expression in normal and tumor lung tissues and cancer cells has rarely been reported. The potential role of DEC1 in A549 cells and whether DEC1 is linked to HIF-1α are unclear. Therefore, in order to further characterize the significance of DEC1 in normal and neoplastic lung tissues and A549 cells, we investigated the expression of DEC1 and its mechanism in lung adenocarcinoma.
Materials and methods
Patients and resources Ethical approval was obtained for this study from the Local Trials Committee. Paraffin wax-embedded material lung adenocarcinoma samples (n=82) and normal lung specimens (n=15) were obtained from Renmin Hospital of Wuhan University (Wuhan, China) between year 2002 and 2004 and were studied by using serial sections of 4 µm thickness. The patients’ ages ranged from 35 to 72 years.
Immunohistochemistal detection of DEC1 and HIF-1α DEC1 protein was detected using the rabbit polyclonal antiserum CW27[16]. CW27 antibody was a gift from Dr SB FOX[7] (Nuffield Department of Clinical Laboratory Sciences, John Radcliffe Hospital, Headley Way, UK). The CW27 and HIF-1α antibodies (Boster, Wuhan, China) were applied to the sections at dilution of 1:1000 and 1:200, respectively. The primary antibody was applied for 1 h at 37 °C. The sections were incubated with a secondary mouse anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 15 min at 37 °C. Then the sections were performed by the 3,3'-diaminobenzidine (DAB) method. All positive controls displayed an extensive and intensive positive combined nuclear and cytoplasmic staining in more than 80% of cells. All negative controls lacked cells displaying an immune reaction. The immunohistochemical results for the protein were classified as follows: –, no staining; +/++, nuclear staining in 1%–50% of cells and/or with weak cytoplasmic staining; +++, nuclear staining in more than 50% of cells and/or with strong cytoplasmic staining.
Cell culture The human lung adenocarcinoma A549 cell line, obtained from Wuhan University Cell Collection (Wuhan, China), was cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS) (Gibco, Grand Island, USA) and was passaged at 80%–90% confluence with PBS containing 0.25% trypsin and 1% EDTA. The cell was maintained at 37 °C in a humidified atmosphere with 21% O2, 74% N2, and 5% CO2. For the hypoxic condition, cell culture dishes were transferred to a Galaxy R CO2 incubator (RS Biotech Co, Ayrshire, Scotland, England), which was cultured with 1% O2, 5% CO2, and 94% N2 for 12 h, 24 h, and 48 h.
Plasmid construction A cDNA fragment coding for the full open reading frame of human DEC1 was cloned by RT-PCR from A549 cells at hypoxia for 48 h. Two specific primers for DEC1 were extended to include appropriate endonuclease sites to facilitate cloning (Table 1). The sense and antisense DEC1 cDNA were respectively ligated into the plasmid pcDNA3.1 (Invitrogen, Carlsbad, California, USA) to construct the pcDNA-DEC1+ and pcDNA-DEC1– plasmids through the T4 DNA ligase (TaKaRa, Dalian, China). The resulting constructs were subjected to sequencing analyses.
Stable translation A549 cells (1×108 cells/L) were seeded into 24-well cell culture plates. The cells were transfected with 1 µg DNA per well using Lipofectamine 2000 (Invitrogen, Carlsbad, California, USA) in serum-free Opti-MEMI medium (Invitrogen, Carlsbad, California, USA), according to the manufacturer’s protocol. Selection for stably transfected cells with 0.8 g/L G418 started 2 d after transfection. After 4 weeks of screening, these polyclonal, stably transfected cells were trypsinized. Monoclones were picked into a 6-well plate and cultured in RPMI-1640 medium with 10% FCS.
HIF-1α antisense oligonucleotide (AS-ODN) treatment The effect of HIF-1α in epithelial cells was accomplished by antisense oligonucleotide loading as described previously[18], using phosphorothioate derivatives of antisense (5'-GCC GGC GCC CTC CAT-3') or control sense (5'-ATG GAG GGC GCC GGC-3') oligonucleotides. The stably transfected pcDNA-DEC+/– A549 cells were relatively washed in serum-free medium. Then phosphorothioate control or antisense oligonucleotides (10 µmol/L) were transfected into the cells. After 4 h incubation at 37 °C, the transfected cells were exposed to hypoxia for 24 h.
MTT assays A549 cells and the stably transfected pcDNA-DEC+/– A549 cells in the exponential phase of growth were relatively plated into 96-well plates at 1×104 cells per well. After normoxia and hypoxia for 12 h, 24 h, and 48 h, The cell viability was determined by MTT assay[19].
Flow cytometric detection of apoptotic cells After the A549 cells and the stably transfected pcDNA-DEC+/– A549 cells were incubated with hypoxia for 0 h, 12 h, 24 h, and 48 h, the cells were analyzed by flow cytometry using a FACScan (Beckman Coulter, Chaska, MN, USA).
RT-PCR detection of DEC1 and HIF-1α Reverse transcription of total RNA was performed using M-MLV reverse transcriptase (Promega, San Luis Obispo, CA), and each PCR reaction was performed with a TaKaRa TaqTM hot start version (TaKaRa). The specific primers of HIF-1α, anti-sense DEC1, and β-actin synthesized by Sangon Co (Shanghai, China) are shown in Table 1. RT-PCR detection of the HIF-1α transcript was performed using 2 µg cDNA at 94 °C for the 5 min initial denaturation step, followed by 32 cycles at 94 °C for 50 s, 57.6 °C for 50 s, and 72 °C for 50 s. The antisense DEC1 reaction condition of PCR was as follows: 94 °C for the 5 min initial denaturation step, followed by 30 cycles at 94 °C for 50 s, 57.2 °C for 50 s, and 72 °C for 50 s. As a control for the equal amount of template, the β-actin gene was amplified using 2 µg cDNA as a template in 32 PCR cycles at 59.5 °C annealing temperature. Each band was analyzed on GDS8000 image analysis system (American UVP Co, Upland, USA). β-actin staining served as the internal standard.

Full table
Western blotting detection of HIF-1α and DEC1 Western blotting was performed as previously described with some modifications[20]. The cell pellets were homogenized in extraction buffer (50 mmol/L Tris-HCl, pH6.8, 0.1% SDS, 150 μmol/L NaCl, 100 mg/L phenylmethylsulfonyl fluoride, 1 mg/L aprotinin, 1% NP-40 and 0.5% sodium orthovanadate), and incubated at 4 °C for 30 min, and centrifuged 20 min at 12 000 g/min. The total protein in the cell lysate was measured with the Bio-Rad colorimetric kit (Bio-Rad, Hercules, CA, USA). For Western blotting, 50 µg protein lysate was separated in 10% SDS-PAGE and transferred onto nitrocellulose membranes (0.45 µm, Millipore, Billerica, MA, USA) The membranes were incubated for 24 h at 4 °C with the antibody CW27 and anti-HIF-1α (Santa Cruz Biotechnology, USA), respectively. The CW27 and HIF-1α antibodies were used at the concentration of 1: 500 and 1:450. Detection of monoclonal and polyclonal antibodies was performed using horseradish peroxidase-conjugated goat anti-rabbit immunoglobulins for 1 h at room temperature, respectively. Signals were detected with an enhanced chemiluminescence kit (Amersham Pharmacia, Buckinghamshire, UK). β-actin staining served as the internal standard for all membranes.
Immunocytochemistal detection of DEC1 and HIF-1α A549 cells (1×108 cells/L) were seeded into 6-well cell culture plates in 2 mL RPMI-1640 medium with 10% FCS to attain 70%–80% confluence. These cells were divided into 4 groups treated for: 0 h, 12 h, 24 h, and 48 h group at hypoxia, respec-tively. These cells were respectively fixed in 4% paraformaldehyde for 10 min, and stained for immunocytochemistry by following the procedures used for immunohistochemistry. The primary CW27 and HIF-1α antibodies were applied to sections at dilutions of 1:1000 and 1:150, respectively.
Statistical analysis Results were shown as mean±SD of the number of experiments indicated in the figure legends. Statistical analysis was done by Student’s t-tests, ANOVA, and Tukey’s post-hoc tests. Statistical significance was set at a level of P<0.05.
Results
Expression of DEC1 and HIF-1α in normal and tumor lung tissue DEC1 was weakly expressed in the normal lung tissues and specifically located in the nuclei and cytoplasm in 48.8% lung adenocarcinoma. HIF-1α was expressed in 3 (20%) normal human lung tissues, and was evaluated in 43 (52.4%) lung adenocarcinomas (Figure 1). There was a statistically significant correlation between the expression of HIF-1α and DEC1 (Table 2, P<0.05).

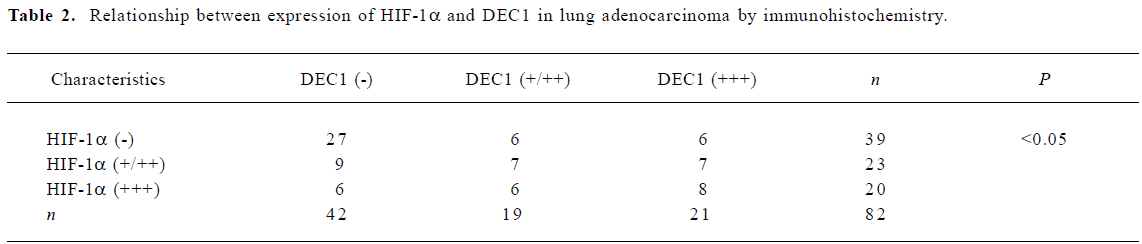
Full table
Inhibitory effect of hypoxia on the growth of A549 cells and the stably transfected pcDNA-DEC+/– A549 cells No changes of the growth of A549 cells were observed in the normoxic groups. However, the prolioferation of A549 cells at hypoxia was strongly inhibited in a time-dependent manner (Figure 2, Table 3, P<0.05). Moreover, the growth of the stably transfected pcDNA-DEC+ A549 cells was higher than those of A549 cells at the same time point at hypoxia. The growth of the stably transfected pcDNA-DEC– A549 cells was lower than those of A549 cells at the same time point at hypoxia (P<0.05).

Full table
Hypoxia induced apoptosis in A549 cells and the stably transfected pcDNA-DEC+/− A549 cells When exposed to hypoxemic environment for 0 h, 12 h, 24 h, and 48 h, apoptotic A549 cells were detected by flow cytometry. Compared to the normoxic groups, these apoptosis ratios in A549 cells obviously increased in a time-dependent manner (Figures 3,4,5, Table 4, P<0.05). Moreover, the lower apoptosis ratio of the stably transfected pcDNA-DEC+ A549 cells and the higher apoptosis ratio of the stably transfected pcDNA-DEC– A549 cells were detected at the same time point at hypoxia (P<0.05).




Full table
Hypoxia increased DEC1 and HIF-1α gene transcription in A549 cells The gene transcription levels of both DEC1and HIF-1α at hypoxia were higher than those under normoxia conditions (Figure 6, P<0.01). Through the linear correlation analysis about the aboved-mentioned results, DEC1 mRNA probably strongly related with HIF-1α mRNA for 24 h at hypoxia (r=0.822, P<0.01).
Hypoxia increased DEC1 and HIF-1α expression in A549 cell line Under a normoxic environment, DEC1 expression was detected at a low level, while HIF-1α was expressed in the nuclei and cytoplasm of A549 cells. And both DEC1 and HIF-1α expression were induced by hypoxia in A549 cells in a time-dependent manner (Figures 7, 8). The staining intensity was stronger in the 48 h group than that of the 12 h and 24 h group (P <0.05).


HIF-1α mRNA and protein expression in the stably transfected pcDNA-DEC+/– A549 cells There was no obvious difference of HIF-1α expression between the non-transfected and empty plasmid transfected groups. However, HIF-1α showed low gene transcription level in the pcDNA-DEC+ group (Figure 9, P<0.05), and high gene transcription level of HIF-1α was detected in the pcDNA-DEC– group compared to the non-transfected group (P<0.05). After normalization with β-actin, the HIF-1α protein significantly decreased in the cells transfected with plasmid pcDNA-DEC+ (Figure 10, P<0.05). On the contrary, HIF-1α protein increased in the cells transfected with plasmid pcDNA-DEC– (P<0.05). These results were in accordance with the mRNA expression.


DEC1 mRNA and protein expression in the stably transfected pcDNA-DEC+/– A549 cells with or without HIF-1α AS-ODN treatment This densitometric analysis revealed there was no obvious different in the intensity of DEC1 mRNA and protein levels with or without HIF-1α AS-ODN in the stably transfected pcDNA-DEC+ A549 cells (Figure 11, 12, P>0.05). The similar results were also found in the stably transfected pcDNA-DEC– A549 cells.


Discussion
In this study, the HIF-1α protein was strongly expressed in the lung adenocarcinoma and A549 cells. The growth of A549 cells was inhibited at hypoxia in a time-dependent manner through the MTT test. Moreover, hypoxia could obviously induce A549 cells apoptosis. We also found the mRNA and protein levels of the HIF-1α increased at hypoxia. These results reveal that HIF-1α plays an important role in the growth of A549 cells at hypoxia.
We also studied a new factor, DEC1. DEC1 is a new and structurally different class of the helix-loop-helix (HLH) protein. We examined the expression of the DEC1 and HIF-1α protein, which were both induced by hypoxia. These results were accorded with the previous study[21]. The DEC1 protein was variably expressed in lung adenocarcinomas. DEC1 was weakly expressed in the normal lung tissues. Previous study using immunohistochemical methods have reported a variable positivity of DEC1, ranging from 0 to 70% in the non-small cell lung cancer[16]. The data indicate that DEC1 play an important role in the process of lung adenocarcinoma progression. The data also reveals DEC1 in the lung adenocarcinoma directly links to HIF-1α, providing strong evidence that DEC1 is indeed another marker of tumor hypoxia.
Our results show that hypoxia significantly increases the mRNA and protein levels of DEC1 and HIF-1α in the lung adenocarcinoma A549 cells in a time-dependent manner. We observed the strong protein expression of DEC1 until 48 h at hypoxia, and the HIF-1α protein was strongly expressed from 12 h at hypoxia. The upregulation of DEC1 and HIF-1α reveals that DEC1 and HIF-1α probably act as the key mediators of the hypoxia-regulated process. Moreover, we found DEC1 mRNA was significantly associated with HIF-1α mRNA at hypoxia for 24 h. This data further indicate that DEC1 regulates HIF-1α or that HIF-1α has an effect on DEC1 at hypoxia.
We constructed the pcDNA-DEC+ and pcDNA-DEC– plasmids and selected the stably transfected pcDNA-DEC+ and pcDNA-DEC– A549 cells lines. The MTT test showed hypoxia could inhibit the growth of the stably transfected pcDNA-DEC+ and pcDNA-DEC– A549 cells in the time-dependent manner. Moreover, the growth of the stably transfected pcDNA-DEC+ A549 cells was faster than those of A549 cells. On the contrary, the growth of the stably transfected pcDNA-DEC– A549 cells was lower than those of A549 cells. We also found hypoxia could induce the apoptosis of the stably transfected pcDNA-DEC+ and pcDNA-DEC– A549 cells in a time-dependent manner. Compared to the A549 cells, the stably transfected pcDNA-DEC+ A549 cells showed a lower percentage of apoptosis while the stably transfected pcDNA-DEC– A549 cells showed a higher percentage of apoptosis.
To investigate the interaction between DEC1 and HIF-1α, we carried out RT-PCR and Western blotting to detect the mRNA and protein levels of HIF-1α. HIF-1α showed low expression level in the pcDNA-DEC+ group, but a high level of HIF-1α was detected in the pcDNA-DEC– group. Thus, it suggests that DEC1 might be the target gene of HIF-1α, and negative feedback exists between DEC1 and HIF-1α.
To confirm this conclusion, we designed the antisense and control sense oligonucleotides of HIF-1α. At mRNA and protein levels, there was no obvious different intensity of DEC1 with or without HIF-1α AS-ODN in the pcDNA-DEC+ or pcDNA-DEC– groups. Through the treatment with the HIF-1α AS-ODN, we found that HIF-1α AS-ODN had no effect on DEC1expression in the stably transfected pcDNA-DEC+/– A549 cells. So we identified that DEC1 may down-regulate HIF-1α at both mRNA and protein levels at hypoxia in A549 cells. However, more research on the relationship between DEC1 and HIF-1α is still required in the future.
In conclusion, our results reveal that DEC1 has significant roles in the process of lung adenocarcinoma progression and is another marker of tumor hypoxia associated with HIF-1α. DEC1 downregulates HIF-1α at both mRNA and protein levels at hypoxia in the lung adenocarcinoma A549 cells.
Acknowledgments
We are grateful for excellent technical assistance from State Key Laboratory of Virology, Medical School, Wuhan University, Wuhan, China.
References
- Brown JM. The hypoxic cell: a target for selective cancer therapy-eighteenth Bruce F. Cain memorial award lecture. Cancer Res 1999;59:5863-70.
- Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumor hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 2003;29:297-307.
- Harris AL. Hypoxia-a key regulatory factor in tumor growth. Nat Rev Cancer 2002;2:38-47.
- Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet 2001;11:293-9.
- Goonewardene TI, Sowter HM, Harris AL. Hypoxia-induced pathways in breast cancer. Microsc Res Tech 2002;59:41-8.
- Pugh CW, Ratcliffe PJ. The von Hippel-Lindau tumor suppressor, hypoxia-inducible factor-1 (HIF-1) degradation, and cancer pathogenesis. Semin Cancer Biol 2003;13:83-9.
- Chakrabarti J, Turley H, Campo L, Han C, Harris AL, Gatter KC, et al. The transcription factor DEC1 (stra13, SHARP2) is associated with the hypoxic response and high tumor grade in human breast cancers. Br J Cancer 2004;91:954-8.
- Dagnon K, Pacary E, Commo F, Antoine M, Bernaudin M, Bernaudin JF, et al. Expression of erythropoietin and erythropoietin receptor in non-small cell lung carcinomas. Clin Cancer Res 2005;11:993-9.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70.
- Shen M, Kawamoto T, Yan W, Nakamasu K, Tamagami M, Koyano Y, et al. Molecular characterization of the novel basic helix-loop-helix protein DEC1 expressed in differentiated human embryo chondrocytes. Biochem Biophys Res Commun 1997;236:294-8.
- Rossner MJ, Dorr J, Gass P, Schwab MH, Nave KA. SHARPs: mammalian enhancer-of-split- and hairy-related proteins coupled to neuronal stimulation. Mol Cell Neurosci 1997;9:460-75.
- Boudjelal M, Taneja R, Matsubara S, Bouillet P, Dolle P, Chambon P. Overexpression of Stra13, a novel retinoic acid-inducible gene of the basic helix-loop-helix family, inhibits mesodermal and promotes neuronal differentiation of P19 cells. Genes Dev 1997;11:2052-65.
- Teramoto M, Nakamasu K, Noshiro M, Matsuda Y, Gotoh O, Shen M, et al. Gene structure and chromosomal location of a human bHLH transcriptional factor DEC1. Stra13. SHARP-2/BHLHB2. J Biochem 2001;129:391-6.
- Li Y, Zhang H, Xie M, Hu M, Ge S, Yang D, et al. Abundant expression of Dec1/stra13/sharp2 in colon carcinoma: its antagonizing role in serum deprivation-induced apoptosis and selective inhibition of procaspase activation. Biochem J 2002;367:413-22.
- Ivanova AV, Ivanov SV, Danilkovitch-Miagkova A, Lerman MI. Regulation of STRA13 by the von Hippel–Lindau tumor suppressor protein, hypoxia, and the UBC9/ubiquitin proteasome degradation pathway. J Biol Chem 2001;276:15306-15.
- Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Wykoff CC, Gatter KC, et al. DEC1 (STRA13) protein expression relates to hypoxia-inducible factor 1-alpha and carbonic anhydrase-9 overexpression in non-small cell lung cancer. J Pathol 2003;200:222-8.
- Turley H, Wykoff CC, Troup SS. The hypoxia-regulated transcription factor DEC1 (Stra13, SHARP-2) and its expression in human tissues and tumors. J Pathol 2004;203:808-13.
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest 2000;105:577-87.
- Behar-Cohen FF, David T, Buechler Y, Nova MP, Houston LL, Pouliquen YM, et al. Cytotoxic effects of FGF2-saporin on bovine epithelial lens cells in vitro. Invest Ophthalmol Vis Sci 1995;36:2425-33.
- Han SW, Lei ZM, Rao CV. Up-regulation of cyclooxygenase-2 gene expression by chorionic gonadotropin during the differentiation of human endometrial stromal cells into decidua. Endocrinology 1996;137:1791-7.
- Wykoff CC, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Identification of novel hypoxia dependent and independent target genes of the von Hippel-Lindau (VHL) tumor suppressor by mRNA differential expression profiling. Oncogene 2000;19:6297-305.


