Ability of alpha-lipoic acid to reverse the diabetic cystopathy in a rat model1
Introduction
Diabetic cystopathy (DC) is one of the most common complications in diabetes mellitus (DM) and is increasingly becoming a concern in DM patients [1,2]. More than half of DM patients present with this debilitating and costly complications characterized by loss of sensation, increased bladder capacity, impaired bladder contractility, and an increase in residual urine[3], as well as urgency and incontinence[4]. DC may develop insidiously even at an early stage of DM, and eventually progresses to an atonic bladder accompanying urinary tract infection, vesicoureteral reflux, and uremia. Bladder smooth muscle, urothelium, and nerves are all involved, and a variety of mechanisms, including the upregulation of endothelins A and B[31] and impaired control of nitric oxide[32], are attributed to the occurrence and development of DC; however, the exact mechanism still needs to be explored[2]. It has been proven to be difficult to restore bladder function in diabetic patients clinically [5]. Therefore, there is a great need to develop new therapeutic approaches for DC.
Alpha-lipoic acid (α-LA) is a nutritional dithiol compound and an essential cofactor in oxidative metabolism in the mitochondria[6]. α-LA acts with its reduced form, dihydrolipoate, as a potent antioxidant to scavenge free radicals, chelate metal ions, and recycle antioxidants[7]. Therapeutic approaches using α-LA with definite effects in both the prevention and treatment of diabetes-induced oxidative stress have been reported[8,9]. Clinically, α-LA has been used in Germany for patients with diabetic neuropathy for more than 30 years, reducing neuropathic deficits by a clinically-meaningful degree[10,11]. The fact that exogenous α-LA supply has been reported to be effective in preventing and reversing the development of diabetic complications indicates a variety of effects of α-LA on the dysfunction of endothelia, neurons, and muscles. Therefore, it is likely that α-LA supplement therapy may represent a reasonable approach for treating diabetic cystopathy.
In the present study, we investigated the ability of α-LA to reverse cystopathy induced by diabetes in a streptozotocin (STZ)-induced diabetic rat model and explored the possible mechanism underlying this effect.
Materials and methods
Animal model Male Sprague–Dawley rats weighing 200–220 g (The Laboratory Animal Center of China Medical University, Shenyang, China) were fasted overnight (n=32). Diabetes were randomly induced in 22 rats by administering a single intraperitoneal (ip) injection of 65 mg/kg body weight STZ freshly dissolved in 1% citrate buffer (pH 4.2) at 4 °C before injection. The other 10 rats that served as the control group received the same volume of vehicle. The blood glucose level was measured by a commercial glucose analyzer (Accu-Chek complete system, Roche, Indianapolis, IN, USA) using the glucose-oxidase method. In total, 20 rats with blood glucose levels more than 300 mg/dL[5,21,25] 4 weeks after STZ administration were considered diabetic. Six weeks after the induction of diabetes, the 20 rats were randomly divided into 2 groups consisting of 10 animals each: DM/vehicle group and DM/α-LA group, receiving daily ip injections of a vehicle or 100 mg/kg body weight α-LA [33] for 6 weeks, respectively. They were kept under identical conditions with a 12 h light–dark cycle and free access to food and water. All animals were alive at the end-point. Body weight and blood glucose level were measured weekly. The total voided urine volume per 24 h of all 3 groups were determined using the metabolic cage method at the end-point, then the rats were subjected to in vitro cystometry and the bladders were harvested for further studies.
The care and handling of animals were in accordance with institutional guidelines and approved by the Institutional Animal Care and Use Committee of China Medical University (Shenyang, China).
Cystometry Cystometry was performed under anesthetized condition. The rats (n=6, randomly selected from each group) were anesthetized with urethane subcutaneously injected (1200 mg/kg body weight). The bladder was exposed with a low midline abdominal incision, and a 27-gauge needle with polyethylene tubing was inserted into the bladder through the dome. The intravesical catheter was connected via a 3-way stopcock to a pressure transducer (TP-200T, Nihon Kohden, Tokyo, Japan) joined to an amplifier (AP-600G, Nihon Kohden, Tokyo Japan) and a micro-infusion pump (OT701, JMS, Hiroshima, Japan). The 2 ureters were cut near the entrances to the bladder, and the distal ends of the ureters were tied using non-absorbable suture. Bladder urine was drained outside before the test. After being equilibrated for at least 1 h, warm saline (37 °C) was infused at a rate of 0.08 mL/min. Then cystometric parameters were measured during the saline infusion for 1–2 h to evaluate bladder function. Data was recorded by a portable computer via a multiport controller (MedLab-U/4C, MedEase, Nanjing, China). Saline voided from the urethral meatus was collected and measured to determine voided volume. Post-void residual volume was measured by withdrawing intravesical fluid through the catheter after constant voided volumes were collected. The following urodynamic parameters were compared among the groups: bladder capacity (BC, volume of infused saline at micturition), maximal intravesical pressure (pv, max, peak bladder pressure during micturition), voided volume for per micturition (VV), post-void residual volume, and voiding efficiency (VE). VE was estimated as (VV/BC) ×100.
Bladder homogenates preparation The whole bladders harvested from all rats were chopped into small pieces and homogenized on ice in HEPES-buffered saline. The homogenates were separated into aliquots and frozen at -80 °C until used.
Assay for malondialdehyde level The malondialdehyde (MDA) level, an index of lipid peroxidation, was measured by using commercially available kits according to the manufacturer’s protocol (Jiancheng Bioengineering Institute, Nanjing, China). The MDA level was expressed as nmol/mg protein.
Assay for catalase activity Catalase (CAT) activity was measured by the decrease in the concentration of hydrogen peroxide after incubation with various volumes of the homogenates, according to a previously described method[12]. The presence of hydrogen peroxide was assessed using horseradish-peroxidase-dependent oxidation of phenol red to a blue derivative. After 1 h incubation at room temperature (25 °C), horseradish peroxidase and phenol red were added to react with the remaining hydrogen peroxide. The absorbance was read at 630 nm. The protein concentration was measured using Bradford assay. CAT-like activity was presented as micrograms of protein required to scavenge 50% hydrogen peroxide.
Assay for superoxide dismutase activity Superoxide dismutase (SOD) activity was measured by the inhibition of tetrazolium salt reduction due to the superoxide anion generated by a combination of xanthine and xanthine oxidase, according to a previously described method[12]. The reaction was started by adding xanthine oxidase and monitored by the increase in absorbance at 545 nm. The measurements were taken at 3–5 min. SOD-like activity was presented as micrograms of protein that mediates 50% inhibition of tetrazolium salt reduction by super oxide anion.
Measurement of nerve growth factor by enzyme-linked immunosorbent assay The bladder body tissue (100 mg) was lysed in 1 mL of pH 7.4 Tris/EDTA buffer at 4 °C and homogenized for 15 s. The homogenate was centrifuged at 10 000×g for 4 min, and the supernatant was diluted with 4 volumes of phosphate-buffered saline. The samples were acidified with 10 mol/L HCl to pH 2−3 for 15 min at room temperature and then neutralized with 10 mol/L NaOH to pH 7.5–8. After acidification, the samples were stored at –80 °C until assayed. A commercial enzyme-linked immunosorbent assay kit (Promega, Madison, WI, USA) was used to determine nerve growth factor (NGF) protein content according to the manufacturer’s instruction. All tissue NGF values were standardized by tissue protein levels and expressed as pg/µg protein.
Drugs and chemicals α-LA was purchased from Stada Arzneimittel AG (Bad Vibel, Germany). STZ was obtained from Sigma (St Louis, MO, USA). All other chemicals were available commercially and of reagent grade.
Statistical analysis All data were expressed as mean±SEM. The comparison between groups was performed by one-way ANOVA, followed by Student’s t-test to compare the mean values between two groups. P<0.05 was considered statistically significant.
Results
General characteristics of the rats Twelve weeks after the STZ administration, the vehicle-treated diabetic rats showed 2%−15% weight loss from their initial body weight and a significant increase in the blood glucose level compared with the controls. They also displayed significantly greater bladder weight than the controls. The administration of α-LA showed no influence on decreased body weight or elevated blood glucose level in the diabetic rats. In addition, α-LA treatment did not have any effect on lowering the bladder weight of diabetic rats (Table 1). Voided urine volume for 24 h was significantly greater in vehicle-treated diabetic rats than in normal rats (108.6±13.1 vs 11.8±1.4 mL, P<0.01). Treatment with α-LA did not influence the urine production per 24 h (97.0±12.9 vs 108.6±13.1 mL, P>0.05).
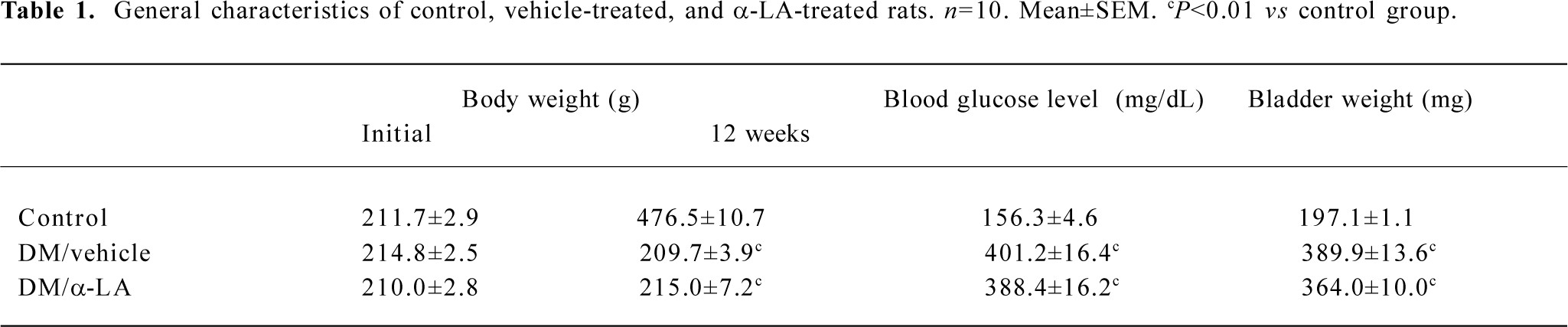
Full table
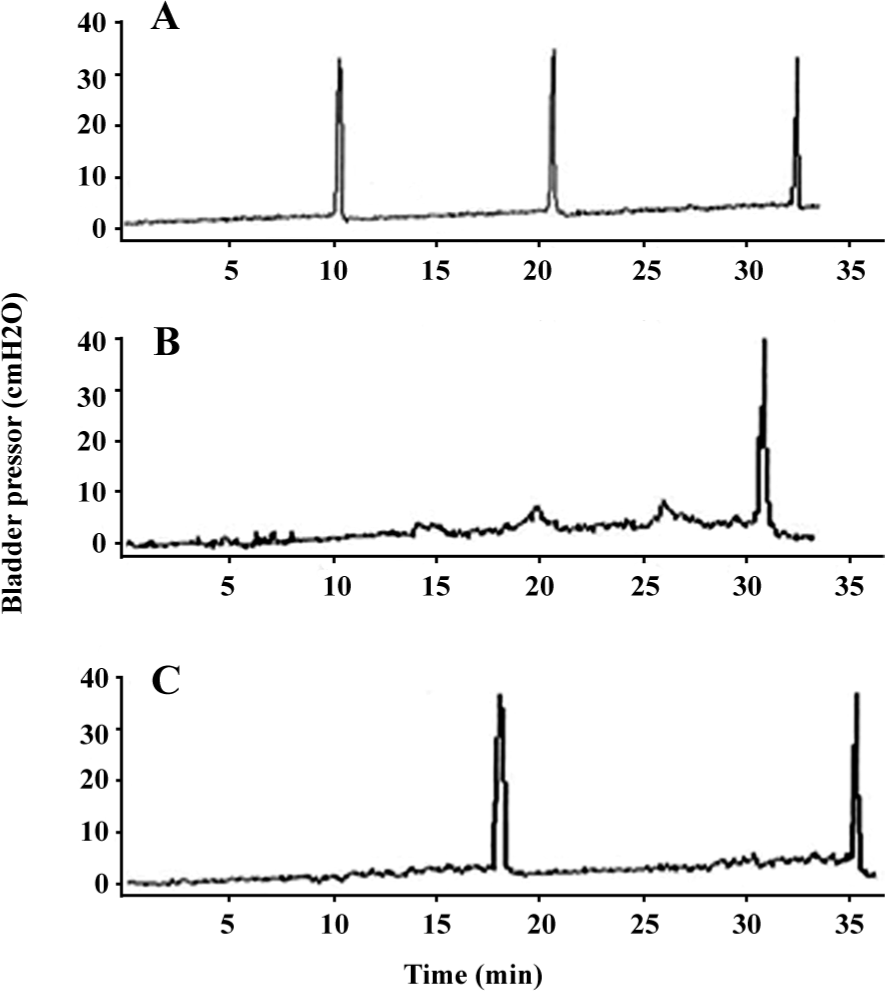
Cystometry findings DC was confirmed on cystometry in every selected diabetic rat. Cystometry performed under urethane anesthesia showed that bladder capacity, single-voided volume, and post-void residual volume were significantly increased in vehicle-treated diabetic rats compared with the controls. Bladder capacity, single-voided volume, and post-void residual volume in the diabetic rats treated with α-LA were significantly lower than those that were vehicle treated, although they were still greater compared with the controls. The estimated VE in the vehicle-treated rats was significantly decreased compared with that of the controls; however, after treatment with α-LA, it increased to normal levels. The maximal intravesical presser was not significantly different among the 3 groups (Table 2). In addition, the DM/vehicle rats showed frequent unstable spontaneous contraction in the filling phase, which was less apparent in the DM/α-LA rats and did not occur in the control rats, indicating bladder hypersensitivity induced by diabetes (Figure 1).
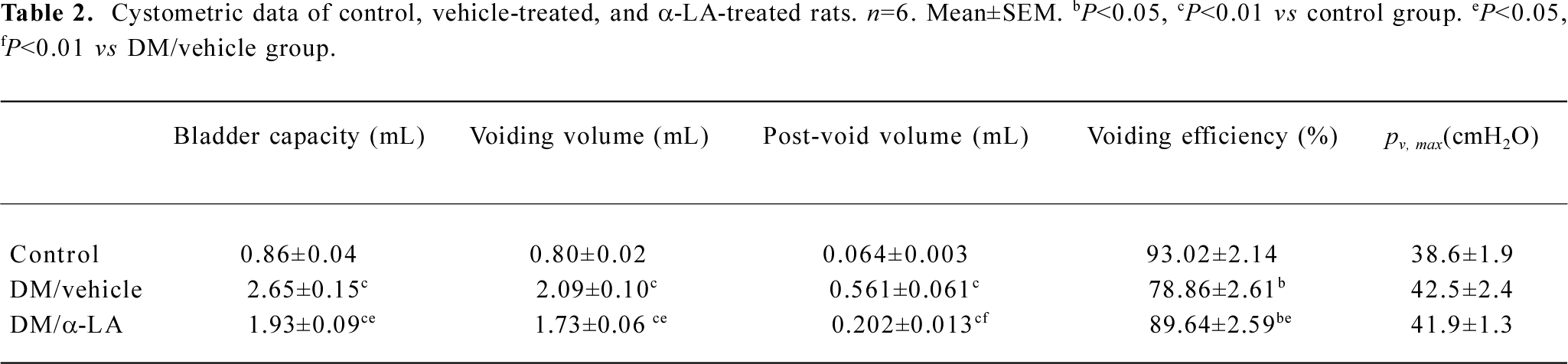
Full table
Effect of α-LA on bladder oxidant status In most cases, α-LA treatment reversed the effect of the elevated oxidative stress system and impaired antioxidant defenses. The level of MDA in the bladders of the diabetic rats with or without α-LA treatment was significantly increased compared with the rats in the control group. However, the MDA level of diabetic rats with α-LA treatment was significantly lower than that of the vehicle-treated diabetic rats.
CAT activity and SOD activity in the bladders of the vehicle-treated diabetic rats decreased significantly compared with the controls, because more protein was required to scavenge 50% of hydrogen peroxide or to mediate a 50% inhibition of tetrazolium reduction, respectively. The administration of α-LA significantly increased the activities of SOD and CAT, even though they were still lower than those of the controls (Table 3).
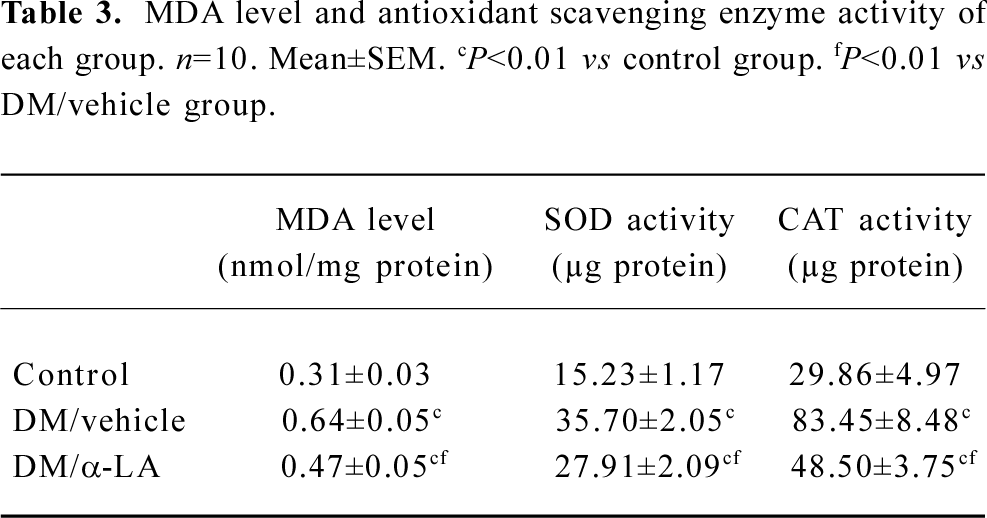
Full table
Bladder NGF protein level The mean NGF protein level in the bladder at 12 weeks of DM was significantly reduced compared with the normal rats (48.39±8.03 vs 86.76±10.82 pg/µg protein, P<0.01). However, 6 weeks of treatment with α-LA (12 weeks after the administration of STZ) significantly reversed the decreased level of the bladder NGF protein (76.49±10.66 pg/µg protein, P<0.05).
Discussion
DM in rats, which has similar pathological and functional changes as humans, is a reliable and useful model for rapidly observing the protective effects of investigated agents on DC[5,21,23-25]. In the present study, we have shown that in STZ-induced diabetic rats, the voiding function was severely impaired, as evidenced by increased bladder capacity, single-voided volume, post-void residual volume, and decreased voiding efficiency. These results are consistent with other studies[5,21,23-25], suggesting that the STZ-induced diabetic rat model is reliable for DC research. Recently, some authors[34,35] reported that diabetes induced an increase in maximum detrusor pressure during voiding, and inferred that this increase occurred by urethral dysfunction associated with diabetic neuropathy. We and other researchers found no significant alteration in maximum detrusor pressure. The discrepancy may be due to the different end-point when the cystometry was performed, as the duration of diabetes may influence the function of the lower urinary tract. The specific methods used in determining the urethral function may also influence the results.
A number of studies have demonstrated the beneficial effects of lipoic acid in the treatment of diabetic complications[8-11], but the present study is the first indication that α-LA treatment improves impaired bladder function. The mechanism underlying this effect may be partly due to its antioxidant activity. Varies studies have shown that DM is accompanied by oxidative stress caused by the misbalanced oxidant and antioxidant system, leading to oxidative damage of cell components, such as proteins, lipids, and nucleic acids[13]. Growing evidence suggests that oxidative stress is important in the development and progression of diabetic complications[14,15]. Antioxidant treatments have been demonstrated to be effective for preventing or reversing diabetic complications. In STZ-induced diabetic rats, it has been demonstrated that oxidative stress occurs in the bladder[16]. In the present study, we observed a significant increase in the MDA level, as well as reduced activities of SOD and CAT in the diabetic rat bladders, and confirmed the pathogenic role of oxidative stress in diabetic cystopathy.
MDA levels have been found to be increased in the brain, liver, and kidney in STZ-induced diabetic rats[17], suggesting that hyperglycemia induces peroxidative reactions in lipids. Under diabetic conditions, the level of lipid peroxi-dation in the bladder was enormously higher than that in control; however, treatment with α-LA significantly decreased the MDA level, which may be partly due to the ability of α-LA to scavenge free radicals. In a diabetic Goto–Kakizaki rat model, treatment with α-LA completely reversed the increased level of plasma MDA and partially improved the impaired endothelial function[18], which is a fundamental pathophysiological alteration for most diabetic com-plications.
SOD and CAT can decompose superoxide and hydrogen peroxide in the cells, respectively. The decreased activities of these enzymes can lead to an excess availability of superoxide and hydrogen peroxide in biological systems, which in turn generates hydroxyl radicals involved in the initiation and propagation of lipid peroxidation[19]. We observed significantly reduced activities of both SOD and CAT in the rat bladder 12 weeks after the induction of diabetes. However, the activities of SOD and CAT have been previously reported to be increased or unchanged in STZ-diabetic rats[16,20]. The discrepancy may be partly explained by the fact that different tissues have varied responses to oxidative stress. More-over, in our study, the biochemical tests were performed 12 weeks after STZ injection, which may represent a relatively late stage of diabetes with a severely impaired antioxidant system. Therefore, it can be inferred that the ability of α-LA to increase or decrease the activity of SOD is dependent on the actual status of SOD in the targeted organ.
Diabetic cystopathy is induced by polyneuropathy, which predominantly affects sensory and autonomic nerve fibers[4]. It is generally accepted that an alteration in the availability of neurotrophic factors, such as NGF, produced in the targeted organ is a major mechanism inducing diabetic neuropathy. The results of our study agree with those of previous studies[5,21] in which a gradual decrease in the NGF level in the bladder of diabetic rat models was reported. Long-term progressive decline of the bladder NGF level can lead to decreased retrograde axonal transport of the growth factor to the dorsal root ganglia and sensory neurons, leading to sensory neuropathy in diabetic cystopathy[21]. An increase in the bladder NGF level has also been reported; however, it may be an early and acute reaction to STZ-induced diabetes and decreases gradually[22]. Therapies based on altered NGF levels represent an intriguing avenue of investigation for the management of diabetic cystopathy[23]. Gene therapy using the replication-defective herpes simplex virus vector expressing NGF was effective in increasing the NGF level in the diabetic bladder and improving voiding function[24,25]. However, in the present study, the reduced NGF level in the diabetic bladder was remarkably reversed indirectly by exogenous antioxidant α-LA. In experimental animal models of diabetes and in type 2 diabetic patients, α-LA treatment improves neural blood flow, endoneural glucose uptake, and metabolism and nerve conduction[26]. The results of the present study thus indicate that α-LA can also exert its neurotrophic support role by increasing the NGF concentration.
Both oxidative stress and decreases of the NGF level in the diabetic rat bladder in our study were partially reversed by exogenous α-LA. Thus it can be inferred that oxidative stress plays an important role in impaired neurotrophic support in diabetic cystopathy, as previously dissected in diabetic peripheral nerves[27]. However, the relationship between oxidative stress and NGF is complex because NGF contributes to the neutralization of superoxide anion radicals and hydrogen peroxide by inducing the expressions of the SOD and CAT genes[28]. Oxidative stress-induced deficiency of NGF in diabetes may in turn further disrupt antioxidative defense. These findings, together with our results, lead to a reasonable interpretation for the potent function of α-LA in reversing bladder dysfunction. It has been reported that α-LA boosts neurotrophic support in diabetic rats, with effects beyond those related to NGF[27]. Recent studies have demonstrated that hyperglycemia directly promotes an endothelial dysfunction by inducing the process of overproduction of superoxide at the mitochondrial level[29], and LA can work as an intracellular superoxide scavenger to improve mitochondrial function and reduce DNA damage[30]. Therefore, α-LA can exert beneficial effects on diabetic complications where classical antioxidants fail.
The current study provides the first evidence of the efficiency of treatment with exogenous α-LA for diabetic cystopathy in a STZ-induced diabetic rat model. After ip administration of α-LA, voiding function was significantly improved along with the restoration of the decreased tissue NGF level and alleviation of oxidative stress in the bladder. Therefore, α-LA administration may represent a useful approach to treat diabetic cystopathy.
Author contribution
Chui-ze KONG, Yuan-jun JIANG, Da-xin GONG designed the research; Yuan-jun JIANG, Da-xin GONG, Hai-bo LIU performed the research; Da-xin GONG contributed new reagents and analytical tools; Da-xin GONG, Chun-ming YANG, Yuan-jun JIANG analyzed the data; Yuan-jun JIANG and Chui-ze KONG wrote the paper.
Acknowledgment
We thank the Departments of Biochemistry and Physiology, China Medical University (Shenyang, China) for their technique assistance.
References
- Brown JS, Wessells H, Chancellor MB, Howards SS, Stamm WE, Stapleton AE, et al. Urologic complications of diabetes. Diabetes Care 2005;28:177-85.
- Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int 2005;95:733-8.
- Bradley WE. Diagnosis of urinary bladder dysfunction in diabetes mellitus. Ann Intern Med 1980;92:323-6.
- Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol 1997;157:580-4.
- Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S. Diabetic cystopathy correlates with long-term decrease in nerve growth factor (NGF) levels in the bladder and lumbosacral dorsal root ganglia. J Urol 2002;168:1259-64.
- Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr 2000;72:S653-69.
- Packer L, Kraemer K, Rimbach G. Molecular aspects of lipoic acid in the prevention of diabetes complications. Nutrition 2001;17:888-95.
- Bhatti F, Mankhey RW, Asico L, Quinn MT, Welch WJ, Maric C. Mechanisms of antioxidant and pro-oxidant effects of alpha-lipoic acid in the diabetic and nondiabetic kidney. Kideny Int 2005;67:1371-80.
- Shotton HR, Broadbent S, Lincoln J. Prevention and partial reversal of diabetes-induced changes in enteric nerves of the rat ileum by combined treatment with alpha-lipoid acid and evening primrose oil. Auton Neurosci 2004;111:57-65.
- Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care 2006;29:2365-70.
- Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, et al. The sensory symptoms of diabetic polyneuropathy are improved with α-lipoid acid: the SYDNEY trial. Diabetes Care 2003;26:770-6.
- Zini A, de Lamirande E, Gagnon C. Reactive oxygen species in semen of infertile patients: level of superoxide dismutase- and catalase-like activities in seminal plasma and spermatozoa. Int J Androl 1993;16:183-8.
- Maritim AC, Sanders RA, Watkins JB III. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 2003;17:24-38.
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54:1615-25.
- Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys 2005;43:289-330.
- Beshay E, Carrier S. Oxidative stress plays a role in diabetes-induced bladder dysfunction in a rat model. Urology 2004;64:1062-7.
- Baydas G, Canatan H, Turkoglu A. Comparative analysis of the protective effects of melatonin and vitamin E on streptozocin-induced diabetes mellitus. J Pineal Res 2002;32:225-30.
- Sena CM, Nunes E, Louro T, Proenca T, Seica RM. Endothelial dysfunction in type 2 diabetes: effect of antioxidants. Rev Port Cardiol 2007;26:609-19.
- Halliwell B, Chiricos S. Lipid peroxidation: its mechanism, measurements and significance. Am J Clin Nut 1993;57:S715-25.
- Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem 1995;151:113-9.
- Tong YC, Cheng JT. Changes in bladder nerve-growth factor and p75 genetic expression in streptozotocin-induced diabetic rats. BJU Int 2005;96:1392-6.
- Steinbacher BC, Nadelhaft I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res 1998;782:255-60.
- Steers WD, Tuttle JB. Mechanisms of disease: the role of nerve growth factor in the pathophysiology of bladder disorders. Nat Clin Pract Urol 2006;3:101-10.
- Goins WF, Yoshimura N, Phelan MW, Yokoyama T, Fraser MO, Ozawa H, et al. Herpes simplex virus mediated nerve growth factor expression in bladder and afferent neurons: potential treatment for diabetic bladder dysfunction. J Urol 2001;165:1748-54.
- Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, et al. Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes 2004;53:2723-30.
- Smith AR, Shenvi SV, Widlansky M, Suh JH, Hagen TM. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr Med Chem 2004;11:1135-46.
- Garrett NE, Malcangio M, Dewhurst M, Tomlinson DR. alpha-Lipoic acid corrects neuropeptide deficits in diabetic rats via induction of trophic support. Neurosci Lett 1997;222:191-4.
- Li XM, Juorio AV, Qi J, Boulton AA. L-Deprenyl potentiates NGF-induced changes in superoxide dismutase mRNA in PC12 cell. J Neurosci Res 1998;53:235-38.
- Ceriello A. Controlling oxidative stress as a novel molecular approach to protecting the vascular wall in diabetes. Curr Opin Lipidol 2006;17:510-8.
- Da Ros R, Assaloni R, Ceriello A. Molecular targets of diabetic vascular complications and potential new drugs. Curr Drug Targets 2005;6:503-9.
- Saito M, Wada Y, Ikeda K, Wang Z, Smith SD, Foster HE, et al. Gene expression, localization, and pharmacological characterization of endothelin receptors in diabetic rat bladder dome. Eur J Pharmacol 2000;387:253-63.
- Poladia DP, Bauer JA. Early cell-specific changes in nitric oxide synthases, reactive nitrogen species formation, and ubiquitinylation during diabetes-related bladder remodeling. Diabetes Metab Res Rev 2003;19:313-9.
- Gouty S, Regalia J, Cai F, Helke CJ. Alpha-lipoic acid treatment prevents the diabetes-induced attenuation of the afferent limb of the baroreceptor reflex in rats. Auton Neurosci 2003;108:32-4.
- Saito M, Kinoshita Y, Satoh I, Shinbori C, Suzuki H, Yamada M, et al. Ability of cyclohexenonic long-chain fatty alcohol to reverse diabetes-induced cystopathy in the rat. Eur Urol 2007;51:479-87.
- Torimoto K, Hirao Y, Matsuyoshi H, de Groat WC, Chancellor MB, Yoshimura N. alpha1-Adrenergic mechanism in diabetic urethral dysfunction in rats. J Urol 2005;173:1027-32.
