Reversal effect of Ganoderma lucidum polysaccharide on multidrug resistance in K562/ADM cell line1
Introduction
Multidrug resistance (MDR), a major cause of cancer treatment failure, is a phenomenon whereby cancer cells develop resistance to a wide variety of chemotherapeutic drugs. MDR has been associated with the overexpression of P-glycoprotein (P-gp) or MDR-associated protein (MRP), 2 transmembrane transporters that act as pumps to remove toxic drugs from tumor cells. The MDR-1 overexpression has been reported in many tumors and in vitro-selected, drug-resistant cell lines[1–4]. Meanwhile, MRP1 has also been identified as having the ability to induce MDR. It has been found that the accumulation of chemotherapeutic agents in tumor cells with overexpressed P-gp170 or MRP1 was significantly reduced as compared to the MDR antagonist-treated tumor cells[5–7]. The other approach for MDR regulation is the modulation of the MDR-1 gene. Studies on the MDR-1 gene promoter suggest that the inhibition of the P-gp expression may occur at the gene level[8]. The MDR modulators may either block the induction of MDR-1 or MRP1 gene expression or downregulate P-gp expression. Up to now, there have been many drugs used to decrease the incidence of MDR clinically. Unfortunately, the drugs, such as verapamil (VER)[9], have distinct toxicity at MDR treatment concentrations, so their clinical treatments are restricted. It is necessary to find more effective and less toxic drugs that can be used for cancer therapy.
Ganoderma lucidum (Gl; Lingzhi) is a traditional Chinese medicine and has been widely used in China and other Oriental countries to prevent and treat various diseases for a long time. Gl polysaccharides (Gl-PS) are the main pharmacological ingredient extracted from the fruit body and mycelium of mushroom Gl (Fr) Karst. A lot of experimental evidence has been accumulated in the past several decades, and the data suggest that Gl-PS have wide activities, such as immune modulation, and antitumor[10,11], anti-atherosclerosis, antidiabetic, and anti-aging activities[12
Gl is possibly effective in cancer patients receiving conventional chemotherapy. Recently, investigators have focused attention on the effect of Gl-PS on antitumor and immunologic regulation. The water extract and the polysaccharide fraction of Gl showed significant antitumor effects in several tumor-bearing animals, mainly through its immunoenhancing activity. Recent studies also showed that the alcohol extract or the triterpene fraction of Gl also had antitumor effects, which may be directly related to the cytotoxic activity against tumor cells[13–20]. So far, it is not clear whether Gl-PS have effects on conversing MDR. The purpose of this study is to investigate the effects of Gl-PS on MDR and the influence on the gene expression of MDR-1 and MRP1 in human K562/adriamycin (ADM) cells.
Materials and methods
Reagents and drugs Gl-PS were extracted from the fruit body of Gl (Leyss ex Fr) Karst by boiling water, followed by ethanol precipitation, dialysis, and protein depletion. Gl contains polysaccharide peptides with a molecular weight of 584 900; the ratio of polysaccharides to peptides is 93.51%:6.49%. The polysaccharides consisted of D-hamnose, D-xylose, D-fructose, D-galactose, D-mannose, and D-glucose with a molar ratio of 0.793:0.964:2.944:0.167:0.389:7.94 and linked together by b-glycosidic linkages. The peptides contained 16 kinds of amino acids. RPMI-1640 medium was purchased from Gibco (Grand Island, NY, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and DMSO were purchased from Sigma (St Louis, MO, USA). ADM and VER were purchased from PRC Navy General Hospital (Beijing, China), and the P-gp monoclonal antibody was purchased from Santa Cruz (Santa Cruz, CA, USA). Total RNA isolation kits and one-step RT-PCR kits were purchased from SBS (Beijing, China).
Cell lines The K562 cell line (drug sensitive) was a generous gift from Prof Wen-jie WANG (Institute of Materia Medica, Chinese Academy of Medical Science, Beijing, China), and the multidrug cell line K562/ADM was purchased from the Chinese Medicine Academy (Beijing, China). These 2 cell lines were seeded in RPMI-1640 with 15% newborn bovine serum. K562/ADM was treated with ADM 2 weeks before the experiment. The cells in all of the experiments were gathered when they were in the exponential growth phrase.
Measurement of cytotoxicity by MTT method The cells were incubated in 96-well plates (1.0×105 /well), in 100 μL medium. ADM and Gl-PS were added at different concentrations (Gl-PS: 5, 10, 20, and 40 mg/L). VER was added as a positive control. After 44 h incubation at 37 ºC, 20 μL MTT (5 g/L in phosphate-buffered saline [PBS]) was added. The plates were incubated for 4 h, and the blue dye was dissolved in 100 μL DMSO. The absorbance at 570 nm was determined using an ELISA multiscan reader[21].
The survival rate of the cells was calculated as follows:
Survival rate (%)=(T–B)/(U–B)×100%,
where T represents treated: the absorbance of tumor cells exposed to drugs; U is untreated: the absorbance of untreated cells; B is blank: the absorbance of cells without drugs and MTT.
The IC50 for ADM in the Gl-PS-treated or untreated tumor cells was determined, and the reversing factor (RF) was calculated as follows:
RF=IC50 (control group)/IC50 (drug-treated group).
Analysis of intracellular accumulation of ADM by confocal laser scanning microscopy The reversing effect of Gl-PS was analyzed based on the inherent fluorescence of ADM, which accumulates in sensitive cells and is actively exported from cells expressing a functional P-gp and MRP1. The cells were maintained in medium for 48 h (K562/A cells were treated with 10 and 50 mg/L Gl-PS) prior to the ADM accumulation studies. The cells (100 000 cells/well) were seeded in chambered borosilicate cover glass slides (Nunc, Naperville, Ill) and incubated for 2 h (37 °C, humidified 5% CO2) to allow the cells to adhere to the chamber slides. Then ADM (10 mg/L) was added to the chamber slides. The chamber slides containing cells without drugs were used as negative controls. The cells were incubated with drugs for approximately 2 h, washed once with Dulbecco’s PBS, and examined immediately by confocal to quantitative intracellular fluorescence intensity. To determine the fluorescence intensities of ADM obtained by confocal microscopy from different treatments, excitation and detection parameters were kept constant. Correction for differences in optical thickness between the suspension analysis and intracellular ADM concentration was accomplished according to previously-described methods[22]. The laser cytometer was set at an excitation wavelength of 512 nm, and the emitted fluorescence was detected with a barrier filter (band pass 530/30). Ten microscopic fields, each containing aggregates of 10–15 cells, were analyzed for each treatment. At least 2 experiments were performed on different days.
Flow cytometric analysis of intracellular accumulation of ADM The K562 and K562/ADM cells were separately seeded in 5 mL of medium at a density of 1×106 cells/mL. In total, 100 μL drug solutions were added to obtain a final ADM concentration of 10 mg/L in the absence or presence of 10 mg/L Gl-PS. The cells were incubated at 37 ºC for 3 h, by which time ADM accumulation had reached a steady state. Aliquots of cellular suspension were collected. The cells were immediately centrifuged for 10 min at 1000 r/min (153×g), and washed 3 times with cold PBS, and then the cell pellets were resuspended in PBS. The intensity of ADM was determined by the auto-fluorescence of ADM using FACS analysis[23].
Flow cytometric analysis of P-gp The K562/ADM cells and K562 cells were plated at a density of 1×105 cell/mL in RPMI-1640. At 24 h after plating, the medium was replaced with fresh medium containing 10 and 50 mg/L Gl-PS alone. After 24 h, the cells were collected by trypsinization and adjusted to a density of 1×106 cells/mL and centrifuged at 1000 r/min (153×g) for 8 min at 20 ºC. The cell pellet was suspended in 20 μL anti-P-gp antibody (5 μL every 1×105 cells) and then incubated at 4 ºC for 30 min. After 2 washes with cold PBS containing l% bovine serum albumin, the cells were incubated under protection from light at 4 ºC for 30 min with fluorescein-isothiocyanate-conjugated goat antimouse immunoglobulin G (1:100). Then the cells were centrifuged at 1000 r/min (153×g) for 10 min at 20 ºC, and suspended in PBS. The fluorescence intensity in the cells was analyzed by the flow cytometer.
RT-PCR assay for checking the mRNA level of MDR and MRP
Isolation of total RNA The K562/ADM cells were seeded with 10 mg/L Gl-PS for 48 h. Then 5×106 cells were collected. The total RNA isolation kits from SBS were used. All operations followed the protocol in the kit.
Determination of RNA quality and concentration The concentration and purity of total RNA was determined by UV light absorption using a GeneQuant pro RNA/DNA calculator (Biochrom, Cambridge, England). Preparations were discarded if they had a ratio of optical densities at 260 nm/280 nm that was lower than 1.6[24]. To assess RNA quality, 5 μg of total RNA from each sample was loaded onto 1% agarose-formaldehyde gels and subjected to electrophoresis. Following ethidium bromide staining, RNA isolates were considered good quality if the UV fluorescence of the 28S rRNA band intensity was 2-fold of the 18S rRNA band, and no UV fluorescence was detected below the 18S rRNA band.
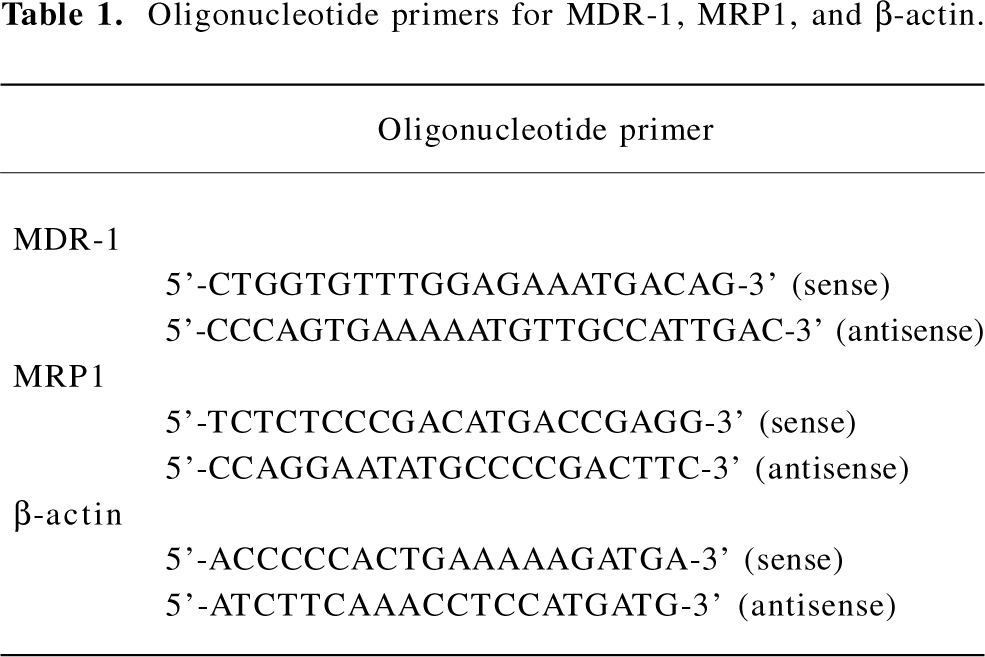
Oligonucleotide PCR primers Sense and antisense-specific primers were synthesized at SBS. All primers were designed to span intron-exon boundaries to distinguish between the amplification of mRNA and genomic DNA, and were based on published human cDNA sequences.
cDNA synthesis and PCR All RT-PCR processes were performed by the one-step RT-PCR kits purchased from SBS. All operations followed the RT-PCR kit protocol. In total, 5 µg total RNA from the K562/ADM cells treated with Gl-PS at 10 and 50 mg/mL and the control were treated by using the following parameters: a reverse transcription step of 1 h at 42 °C, an initial denaturing step of 10 min at 94 °C, denaturing at 94 °C for 1 min, and annealing at 58 °C for 50 s, extending at 72 °C for 1 min. The final polymerization step was extended for an additional 10 min. In total, 32 cycles of PCR were performed. PCR amplification reactions were evaluated through electrophoresis on 1% agarose gels containing ethidium bromide and visualized by UV transillumination on a GeneGenius imager (Frederick, MD, USA). The initial product identification was made by comparison to the myometrial control and the molecular weight ladder, photographed by GeneGenius, and quantified by BandScan software (Beijing, China). All RT–PCR experiments were carried out at least 3 times.
Statistical analysis
Data are showed as mean±SEM from triplicate samples of 3 independent experiments. Differences between the means were analyzed by ANOVA. Results were considered to be statistically significant when P<0.05.
Results
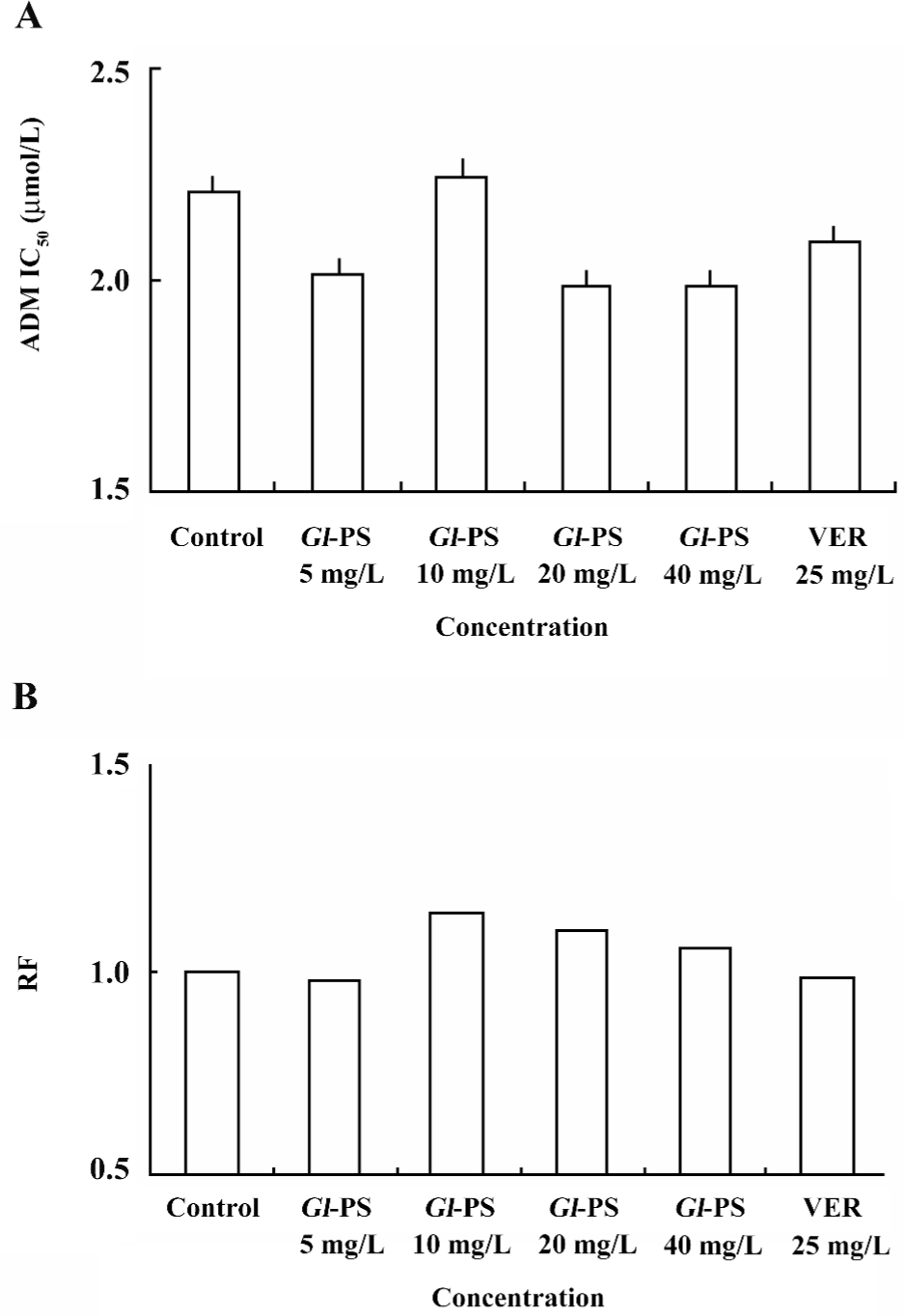
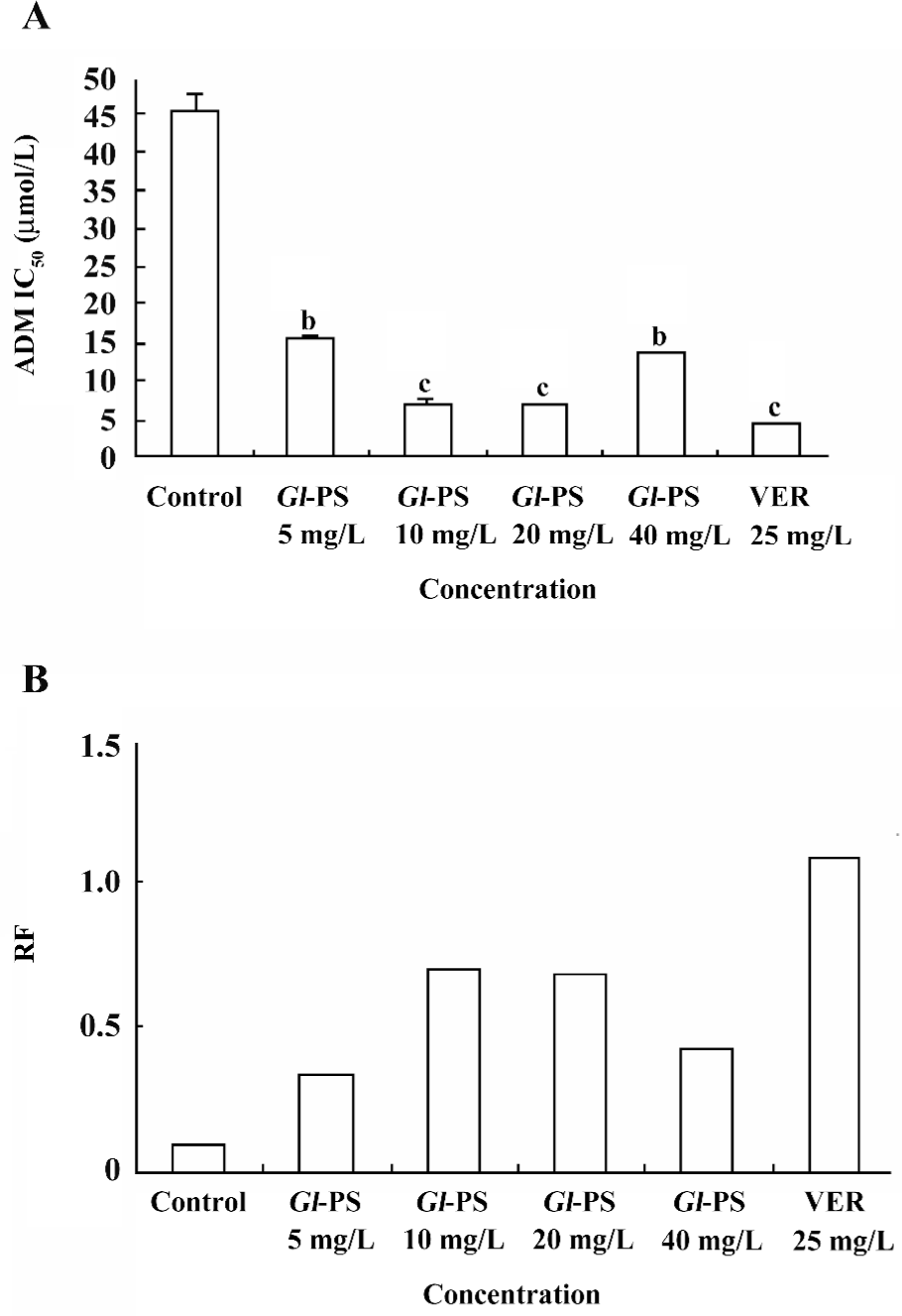
Reversal effect of Gl-PS on the multidrug K562/ADM cells To determine if Gl-PS are toxic to normal cells, the effects of Gl-PS on the K562 cells were assayed as described in Materials and methods. Figure 1 shows that Gl-PS did not have significant cytotoxity on normal cells, but VER and Gl-PS obviously reversed the resistance of K562/ADM to ADM. The RF were 2.96, 6.46, 6.80, and 3.35 in the 5, 10, 20, and 40 mg/L Gl-PS-treatment groups, respectively (Figure 2). In the same condition, the RF of VER was 10.76. Both VER and Gl-PS did not change the IC50 of sensitive K562 cells. These results indicated that Gl-PS can reverse the drug resistance of K562/ADM cells and enhance the cytotoxity of other antitumor drugs, such as ADM.
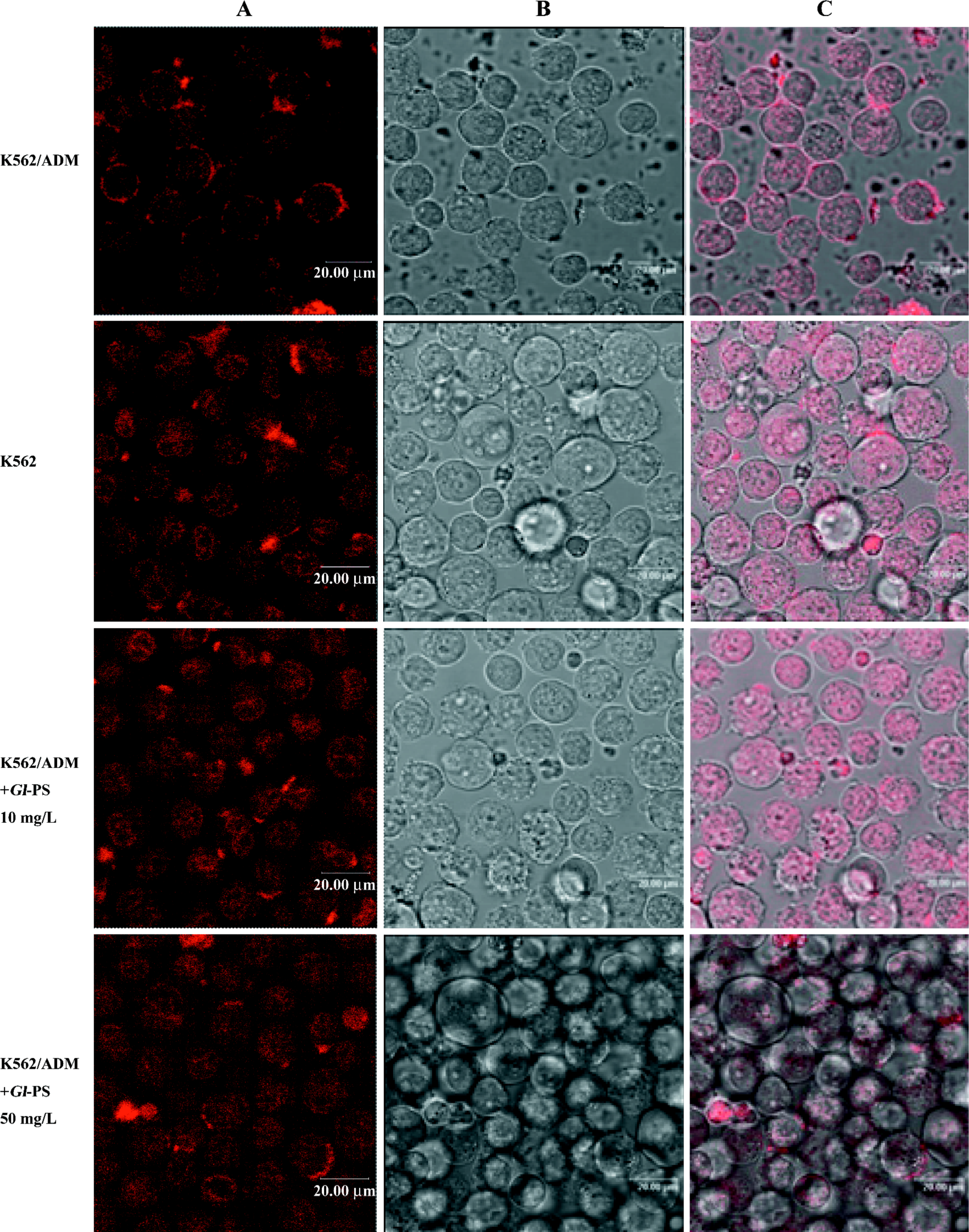
Effect of Gl-PS on ADM accumulation in K562/ADM cells assayed by confocal laser scanning microscopy In order to examine whether Gl-PS have a reversing effect on MDR, intracellular ADM accumulation was assessed by confocal laser scanning microscopy. Figure 3 shows the images of the fluorescence intensity representing intracellular ADM accumulation. Intracellular ADM accumulation in the K562/ADM cells was significantly less than that that in the K562 cells. The distribution of ADM accumulation was also different between the K562 cells and K562/ADM cells. After treatment with Gl-PS, ADM was distributed in the K562/ADM cells at higher concentrations than that in the K562 cells. We also found that in the K562 cells and K562/ADM plus Gl-PS, the ADM concentration was higher in nucleolus zones than in plasma, but not for K562/ADM cells. These results indicate that Gl-PS can also downregulate the expression of MRP1.
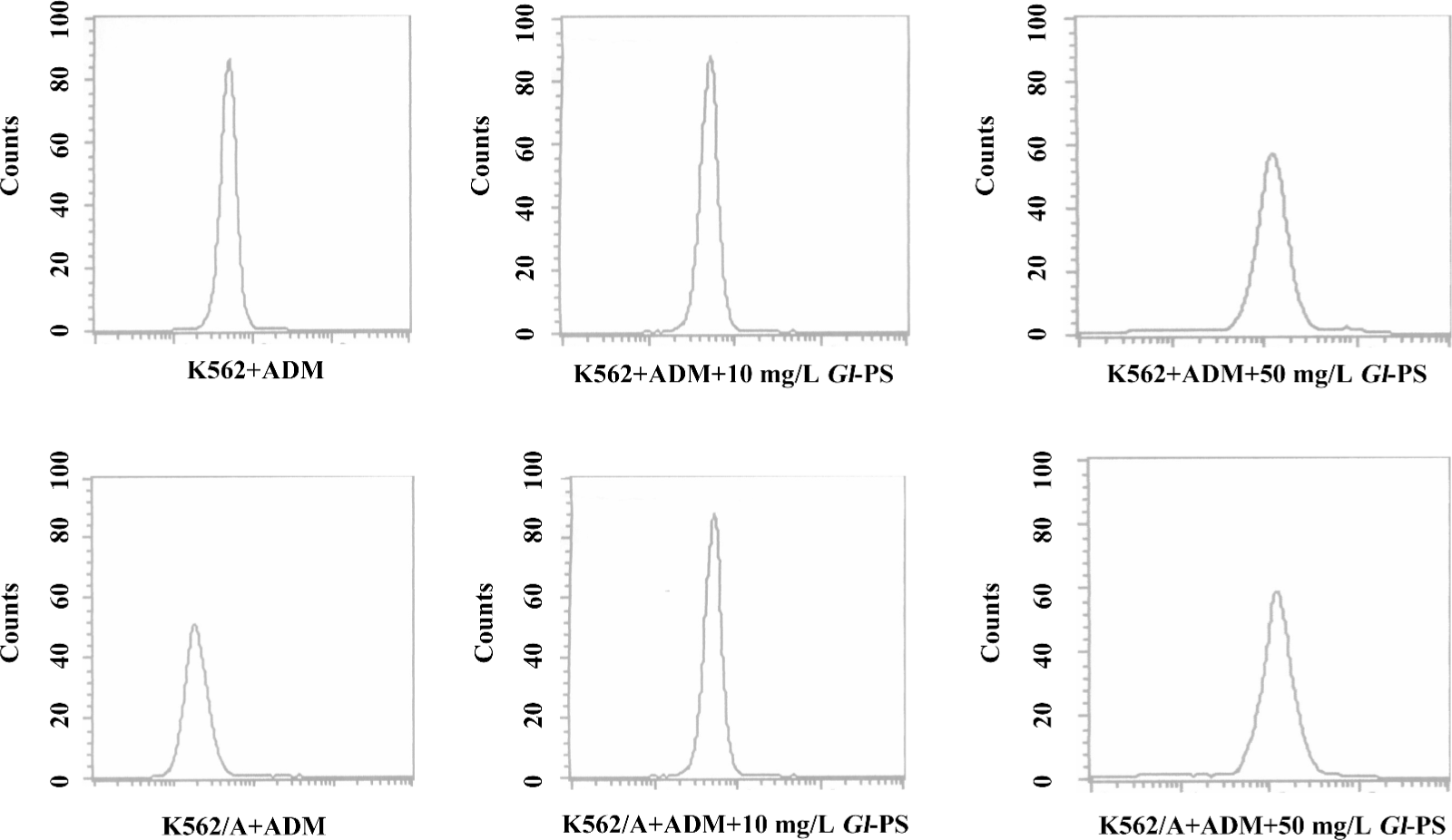
Influence of Gl-PS on ADM accumulation in K562/ADM cells determined by flow cytometric analysis The effect of Gl-PS modulation on ADM efflux was also studied in resistant cells overexpressing P-gp and MRP1. Gl-PS were initially tested at 10 and 50 mg/L. The Gl-PS did not have inherent fluorescence and did not modulate ADM in the non-MDR-overexpressing K562 cells. Following the uptake of ADM, the K562/ADM cells removed most of their intracellular ADM content during 90 min incubation in the medium alone; the intracellular ADM concentration was down to 2.39-folds that of the K562 cells. With the existence of Gl-PS, the efflux of ADM in the K562/ADM cells can be modulated. A total of 10 mg/L Gl-PS caused a 2.4-fold increase in ADM accumulation in the K562/ADM, cells and 50 mg/L Gl-PS caused a 2.2-fold increase (Figure 4).
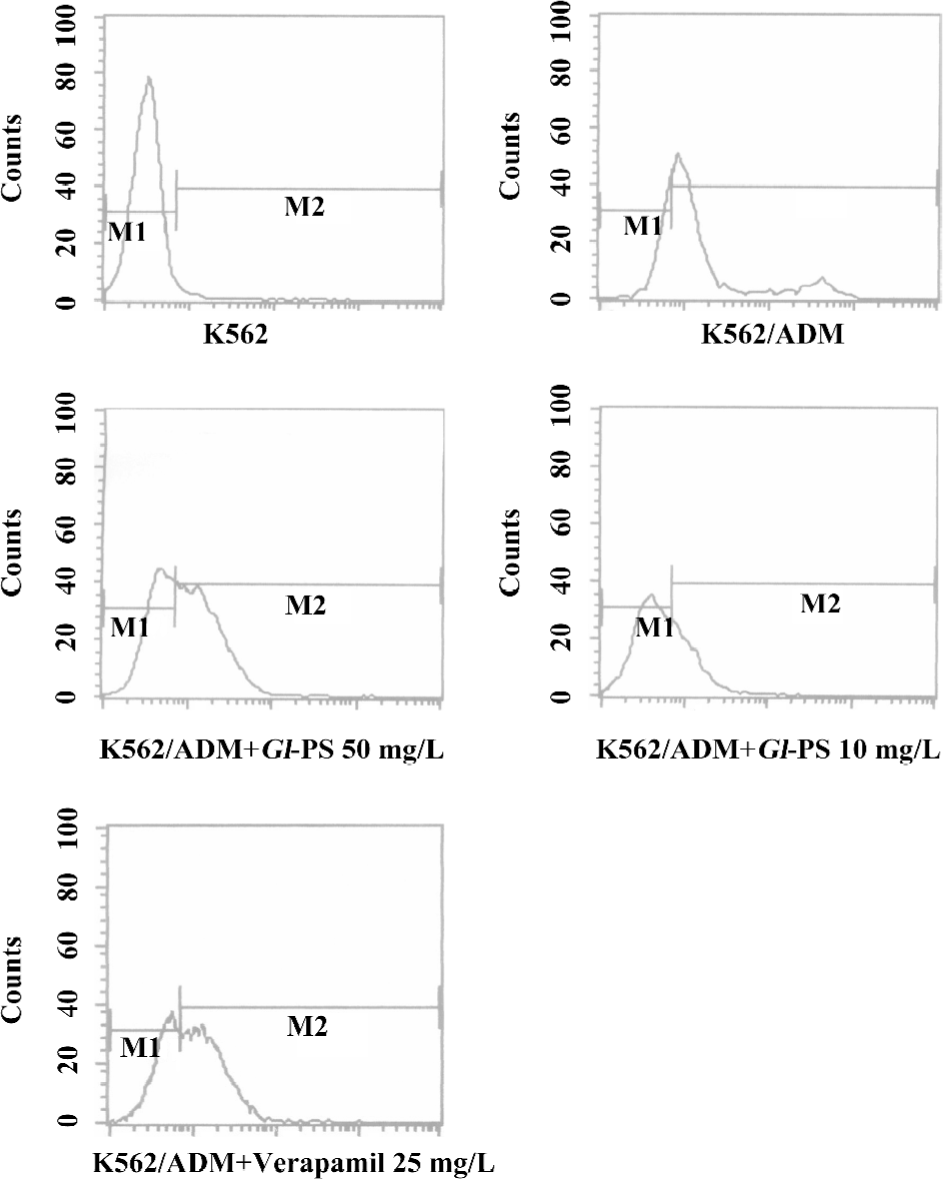
Effect of Gl-PS on expression of P-gp in K562 and K562/ADM cell lines To evaluate the P-gp expression level, the K562/ADM cells were analyzed with the anti-P-gp monoclonal antibody. VER was used as the positive control. As shown in Figure 5, the expression rates of P-gp were 78.54% in untreated K562/ADM cells, and 36.73% and 51.73% in 10 and 50 mg/L Gl-PS-treated K562/ADM cells, respectively. The VER group showed a level at 60.09%. So we can conclude that after treatment with Gl-PS, the expression rate of P-gp was reduced distinctly (Figure 5).
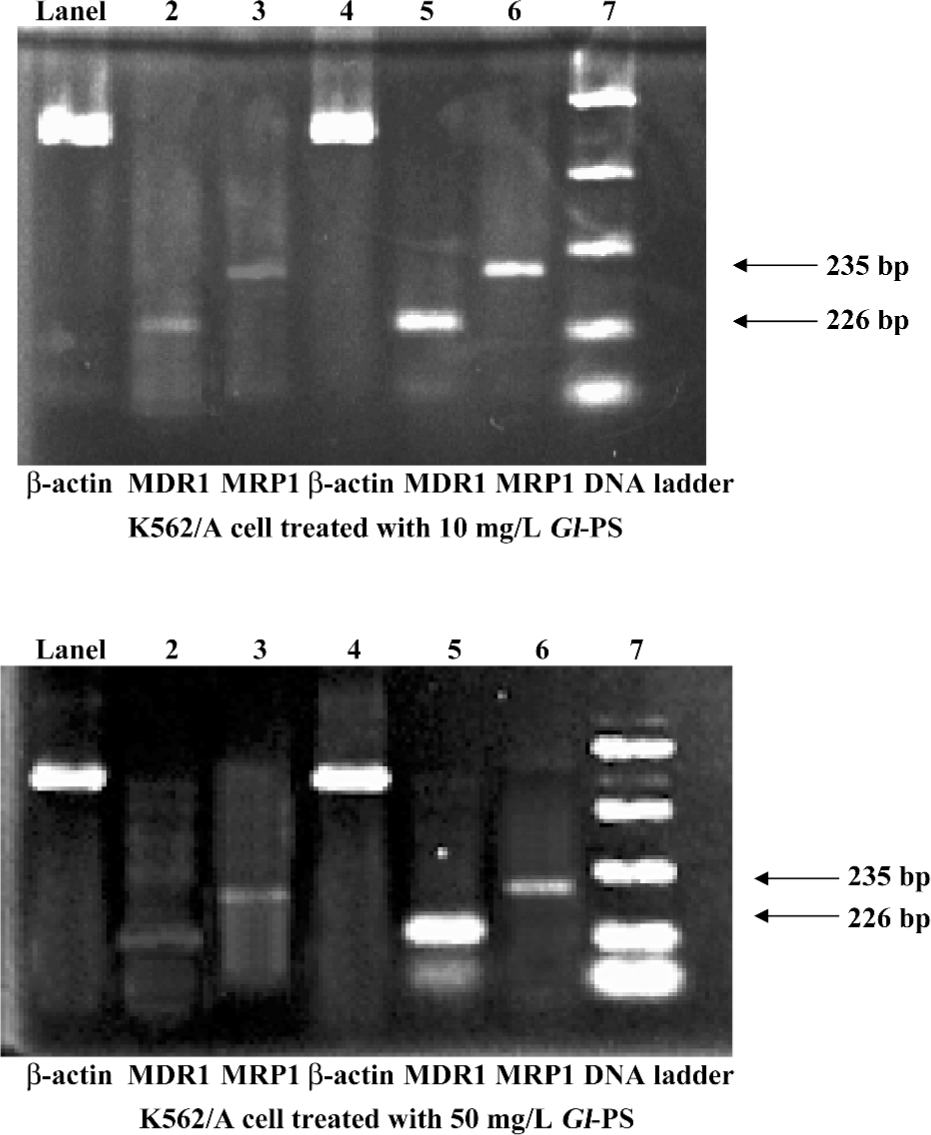
Gl-PS downregulating MDR-1 and MRP1 gene expression in MDR K562/ADM cells The K562/ADM cells were shown by RT-PCR to express MDR-1 and MRP genes at a rate of 56.58% and 37.59%, respectively. Neither of the genes was detectable in the K562 cells (data not shown). Treatment with Gl-PS (10 mg/L) for 24 h led to the downregulation of MDR-1 and MRP mRNA expression in MDR cells. The levels of MDR-1 and MRP1 mRNA nearly disappeared to a level of 8.89% and 7.95%, respectively; Gl-PS (50 mg/L) was also detected. The expression levels of MDR-1 and MRP-1 were also greatly reduced to approximately 11.12% and 10.05%, respectively (Figure 6).
Full table
Discussion
Nearly 50% of human cancers are either completely resistant to chemotherapy or respond to chemotherapy only transiently, after which they are no longer affected by common anticancer drugs. This phenomenon is referred to as MDR and is inherently expressed by some tumor types, while others acquire MDR after exposure to chemotherapy treatment. We are actively investigating the mechanisms involved in MDR, as well as developing therapeutic strategies to circumvent these resistance mechanisms. We evaluated tumor cells for MDR related to several resistance processes and have made significant breakthroughs in reversing MDR mediated by the drug efflux pump P-glycoprotein. Phenotypic and functional analyses of MDR mechanisms presented in human tumor specimens are also being conducted in order to relate specific MDR mechanisms with resistance to chemotherapy in specific tumor types.
MDR has been explained by the overexpression of a family of ATP-binding cassette (ABC) transporters called MDR proteins. ABC transporters are an ever-increasing family of proteins[25]. The human MDR-1 gene encoding the MDR transporter (P-gp-170) belongs to the ABC transporter superfamily, of which there are 9 members. Uptake and/or efflux of isotope-labeled drugs or rhodamine123 are frequently used for the functional assay of P-gp-170 in tumor cells. Several classes of MDR modulators that inhibit P-gp-mediated efflux have been identified. P-gp and MRP1 are constitutively expressed at many tumor cell lines and pump drugs out of cells. Such a spatial distribution of this efflux transporter has defined it as a functionally-important element in reducing the effects of antitumor drugs.
The Gl-PS, a traditional Chinese medicine, has been proven to be effective in tumor therapy. Our previous research proved that Gl-PS have antitumor activity both in vivo and in vitro[24]. There have been many studies on the antitumor activity of Gl-PS and their possible mechanisms, and many exciting results have been obtained. However, due to its complexity in structure, the mechanisms by which the Gl-PS act in the cells are unclear. Gl-PS have low toxicity in humans, which is in contrast to other drugs, such as VRPL, that leads to 40% lethality in mice at 150 mg/kg per day for 3 d. The remarkably low toxicity of Gl-PS coupled with their potent MDR-reversing activity as demonstrated earlier, renders them appropriate for further study and development.
In the present study, we sought to use several different, but complementary experimental approaches to examine whether Gl-PS can reverse MDR. K562 cells repelling ADM are the best models for MDR research and have been selected in many previous studies[26] We have validated that this cell line really has high-level expression of P-gp and MRP1 (unpublished data). Depending on P-gp and MRP1 activity, ADM was pumped out of the K562/ADM cells and formed a ring adhering to the cells. The condition in the K562 cells was different: ADM was distributed into the cells at a high concentration. After incubating the K562/ADM cells with both ADM and Gl-PS, the ADM-accumulating rings disappeared and the distribution conditions were greatly similar to that of the K562 cells. So we can conclude that Gl-PS has the ability to downregulate the effect of P-gp by an unknown mechanism. However, we chose ADM as the antitumor drug because of its auto-fluorescence that can be easily assayed in the experiment. It is interesting that Gl-PS has a prominent effect on reversing MDR. The cytotoxity assay results indicated that proper concentrations of Gl-PS can reduce the viability of K562/ADM cells in ADM. The results of the confocal assay supported this conclusion and showed that the rate of pumping drugs out of K562/ADM cells was also reduced. To determine whether Gl-PS could downregulate the P-gp and MRP1 functional expression in the K562/ADM cells, we tested the P-gp expression level on the cell membrane using the flow cytometric assay. Our results confirmed the hypothesis that the P-gp expression was downregulated under Gl-PS treatment. Furthermore, the results of the RT-PCR experiments confirmed that MRP1 and P-gp were significantly inhibited at the mRNA level. Our studies suggest that Gl-PS can reverse MDR by downregulating the expressions of P-gp and MRP1.
Acknowledgements
Gl-PS was kindly provided by Prof Shu-qian LIN and Sai-zhen WANG of the Fuzhou Institute of Green Valley Bio-Pharm Technology, Fuzhou, China.
References
- Goldstein LJ. MDR1 gene expression in solid tumors. Eur J Cancer 1996;32:1039-50.
- Jonker JW, Buitelaar M, Wagenaar E. The breast cancer resistance protein protects against a major chlorophy11-derived dietary phototoxin and protoporphyria. Proc Natl Acal Sci USA 2002;99:15649-54.
- Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a line between cancer genetics and chemotherapy. Cell 2002;108:153-64.
- Shen DW, Cardarelli C, Hwang J, Cornwell M, Richert N, Ishii S, et al. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicines, adriamycin, or vinblastine show changes in expression of specific proteins. J Biol Chem 1986;261:7762-70.
- Tan B, Piwnica WD, Rater L. Multidrug resistance transporters and modulation. Curr Opin Oncol 2000;12:450-8.
- Sharom FJ, Yu X, Lu P, Liu P, Chu JW, Szabo K, et al. Interaction of the P-glycoprotein multidrug transporter (MDR1) with high affinity peptide chemosensitizers in isolated membranes, reconstituted systems and intact cells. Biochem Pharmacol 2003;58:571-86.
- Sikic BI. Modulation of multidrug resistance: a paradigm for translational clinical research. Oncology 1999;13:183-7.
- Hu YP, Pourquier P, Doignon F, Crouzet M, Robert J. Effects of modulators of multidrug resistance on the expression of the MDR1 Gene on human KB cells in culture. Anticancer Drugs 1996;7:738-44.
- Tsuruo T, Iida H, Tsukagoshi S, Sakurai Y. Overcoming of vincristineresistance in P388 leukemia in vivo and in vitro through enhancedcytotoxicity of vincristine and vinblastine by verapamil. Cancer Res 1981;41:1967-72.
- Lei LS, Lin ZB. Effects of Ganoderma polysaccharides on the activity of DNA polymerase in spleenocytes and immune function in aged mice. Acta Pharm Sin 1993;28:577-82.
- Zhang QH, Lin ZB. The antitumor avtivity of Ganoderma lucidum (Curt:Fr.) P. Karst. (LingZhi) (Aphyllophoromycetideae) polysaccharides is related to tumor necrosis factor-α and interferon-γ. Int J Med Mushroom 1999;1:207-15.
- Lin ZB. Anti-aging activities of . In Lin ZB, editor. 2007. Modern research of .3rd ed. : Beijing Medical University Publishing House; 2007. p 241–2.
- Chen HS, Lin CH, Wong CH. Studies on the immuno-modulating and anti-tumor activities of Ganoderma lucidum polysaccharides. Bioorg Med Chem 2004;12:5595-601.
- Zhang QH, Lin ZB. Study on antitumor activity and mechanism of Ganoderma polysaccharides B. Chin J Integrated Tradit West Med 1999;19:544-7.
- Zhang QH, Yu D H, Lin ZB. Study on the antitumor mechanism of ganoderma lucidum extract (gle) by serologic pharmacological method. J Beijing Med Univ 2000;32:210-3.
- Hu YH, Lin ZB, He YQ, Zhao CJ. Polysaccharides isolated from mycelia of ganoderma lucidum induced on hl-60 cell apoptosis by enhancing macrophage activity. Chin Pharmacol Bull 1999;15:27-30.
- Sliva D, Labarrere C, Slivova V, Sedlak M, Lloyd FPJ, Ho NW. Ganoderma lucidum suppresses motility of highly invasive breast and prostate cancer cells. Biochem Biophy Res Commun 2002;298:603-12.
- Lei LS, Lin ZB, Chen Q, Li R Z, He YQ. Antagonistic effect of ganoderma lucidum polysaccharides on the immunosuppressive response induced by ciclosporin a, hydrocortisone and antitumor agents. Chin J Pharmacol Toxicol 1994;7:183-5.
- Li WD, Han Q, Lin ZB. Antagonization of ganoderma polysaccharides and lentinan on bearing sarcoma180 mice’s immunosuppressive effect induced by cyclophosphamide. Chin J Integt Trad West Med 2001;21 Suppl:101-4.
- Lin ZB, Zhang HN. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta Pharmacol Sin 2004;25:1387-95.
- Alley MC, Scudiero DA, Monks A. Feasibility of drug screening with panels of human tumour cell lines using a microculture tetrazolium assay. Cancer Res 1988;48:589-601.
- Barhoumi R, Bailey RH, Burghardt RC. Kinetic analysis of glutathione in anchored cells with monochlorobimane. Cytometry 1995;19:226-34.
- Minderman H, Suvannasankha A, O’Loughlin KL, Scheffer GL, Scheper RJ, Robey RW, et al. Flow cytometric analysis of breast cancer resistance protein expression and function. Cytometry 2002;48:59-65.
- Cao QZ, Lin ZB. Antitumor and anti-angiogenic activity of Ganoderma lucidum polysaccharides peptide. Acta Pharmacol Sin 2004;25:833-8.
- Jeanette W, Jan CD. Involvement of multidrug resistance proteins (P-gp) and in the modulation of glucocorticoid response. J Steroid Biochem Mol Biol 2002;82:277-88.
- Miao ZH, Ding J. Transcription factor c-Jun activation represses mdr-1 gene expression. Cancer Res 2003;63:4527-32.
