Curcumin inhibits cellular cholesterol accumulation by regulating SREBP-1/caveolin-1 signaling pathway in vascular smooth muscle cells1
Introduction
Curcumin is extracted from Curcumae longae, and has been demonstrated to have a variety of pharmacological effects, such as antitumoric, anti-inflammatory, as well as anti-oxidative effects[1-3]. Some studies have revealed that curcumin can decrease lipid peroxidation and cholesterol levels of sera and tissues in mice and human[4,5]. It is well known that atherosclerosis largely results from oxidative stress and massive lipid deposition in the aorta. Olszanecki et al[6] reported a preventive effect of curcumin on atherosclerotic development in apoE/LDL-Receptor double-knockout mice. A recent study showed that some genes, such as LDL-R, sterol response element-binding protein (SREBP), and CD36, involved in cholesterol homeostasis were influenced by curcumin[7]. However, the detailed anti-atherosclerotic mechanisms of curcumin are still to be elucidated.
Caveolin-1 is a 22-24 kDa constructive protein in caveolae[8]. Evidence demonstrates that the caveolin-1 is able to directly bind free cholesterol (FC) and form a cholesterol transport complex with other elements[9-11]. In addition, the upregulation of the caveolin-1 expression promotes intracellular cholesterol efflux and an improved lipid-loaded state[12]. SREBP-1 exists in the cytoplasm and nucleus, and is associated with cholesterol catabolism[13]. Its active form can translocate into the nucleus and regulate target gene transcription through binding with the sterol regulatory element (SRE) sequence within the promoter[14]. It was found that the SRE sequence also exists in the caveolin-1 promoter that is negatively regulated by SREBP-1[15]. A more recent report showed that SREBP expression could be regulated by curcumin and is associated with reverse cholesterol transport[7].
The present paper addresses whether curcumin prevents ox-LDL-induced cholesterol from accumulation in cultured vascular smooth muscle cells (VSMC) through inhibiting the nuclear translocation of SREBP-1 followed by raising the caveolin-1 expression.
Materials and methods
Reagents Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum were purchased from Gibco/BRL (Grand Island, NY, USA). Curcumin ([E,E]-1,7-bis[4-hydroxy-3-methoxyphenyl]-1,6-heptadiene-3,5-dione, purity =99%), N-acetyl-Leu-Leu-norleucinal (ALLN; purity =97%) and oil red O powder were purchased from Sigma–Aldrich (St Louis, MO, USA) and dissolved by DMSO (Sangon, Shanghai, China). All reagents were of analytical grade.
Animals and diets The apoE–/– mice were obtained from the barrier unit at the Laboratory Animal Center of Chongqing Medical University (Chongqing, China). The apoE–/– mice were randomized into 3 groups and had similar body weights (n=7 in the apoE–/– mice group; n=8 in the apoE–/– mice with curcumin group; and n=8 in the apoE–/– mice with lovastatin group). C57BL/6J mice (n=7) were used as the control. Three groups of apoE–/– mice were given a high-fat diet (21% lard and 0.15% cholesterol). The C57BL/6J mice were fed a normal diet. The animals were housed in single pens under controlled conditions (temperature between 18 °C and 22 °C, relative air humidity between 30%–70%, with 4 air changes per hour) and fed 3 times daily on a restricted schedule. All mice received the same amount of food (4% body weight). The dose of curcumin (purity =94%) administered (mixed with diet) for the curcumin group was 20 mg·kg–1·d–1 per mouse (4 months). The dose of lovastatin administered for the lovastatin group was 40 mg·kg–1·d–1. The total study period was 5 months. Morphological changes of atherosclerotic lesions in the aorta were measured by histological section and hematoxylin-eosin (HE) staining.
Morphological examination of atherosclerotic lesions At the end of the experimental period, the animals were euthanized by phlebotomy under light anesthesia with sodium pentobarbital (30 mg·kg–1 intravenously; Jilin Northern Medicine, Jilin, China). The atherosclerotic lesions were analyzed as described previously[16]. Briefly, the aorta was cut into transversal sections of 4 µm and fixed in 10% formalin for 24 h. The tissue slices were routinely processed and embedded in paraffin. Lipid deposition in the aorta was determined by the morphological assessment of the percentage of lesion-covered aortas as visualized by HE staining under a light microscope at 40× magnification.
Serum lipid and lipoprotein analysis Non-fasting mice were anesthetized with sodium pentobarbital, and blood was collected. Serum total cholesterol (TC), triglyceride (TG), High Density Lipiprotein-Cholesterol (HDL-C) and Lower Density Lipiprotein (LDL-C) were determined by commercial enzymatic methods (test kits, Shanghai Rongsheng Biotechnology, Shanghai, China).
Cell culture Rat VSMC were maintained in DMEM containing 10% fetal bovine serum in a humidified atmosphere of 5% CO2 and 95% O2. The cells were pre-incubated with 50 mg·L–1 ox-LDL for 48 h and then treated with various concentrations of curcumin (purity =94%; 12.5, 25, and 50 μmol·L–1) or ALLN (25 µmol·L–1) for 24 h. Untreated cells were used as controls.
Oil Red O staining The culture was washed 3 times by phosphate-buffered saline (PBS) to remove suspended cells. The VSMC were fixed with 10% formalin for 10 min. After washing with PBS, the cells were stained with oil red O solution (solubilized in isopropanol: water, 3:2) for 30 min. Then the cells were washed with 2-propanol for 10 s to remove background staining. The cells were then stained with HE for 5 min to stain the nuclei, and images were taken at 40× magnification.
Intracellular lipid level analysis by HPLC Cellular lipid (TC, FC, and cholesterol ester [CE]) contents were analyzed by our method described previously[17]. Briefly, the VSMC were scraped from the culture flasks into 0.9% NaCl (1 mL/50 cm2 flask) and homogenized on ice by sonication for 10 s. After the protein concentration was determined using a BCA kit (Pierce Biotechnology, Rockford, IL, USA), an equal volume of freshly-prepared, cold (–20 °C) potassium hydroxide in ethanol (150 g·L–1) was added to the cell lysates, and the mixture was repeatedly vortexed until clear. An equal volume of 3:2 hexane isopropanol (v/v) was then added. The mixture was vortexed for 5 min, followed by centrifugation at 800×g (15 °C) for 5 min. The extraction procedure was repeated twice. The combined organic phase was transferred to clean tapered glass tubes and thoroughly dried under nitrogen at 40 °C. The tubes were allowed to cool to room temperature. One hundred microliters of isopropanol-acetonitrile 20:80 (v/v) was added. The sample was solubilized in an ultrasound water bath at room temperature for 5 min. After centrifugation at 800×g for 5 min, the samples were introduced into the HPLC device (Agilent 1100, Agilent Technologies, Palo Alto, CA, USA). Cholesterol was eluted with 1 mL·min–1 of eluent consisting of 20:80 isopropanol-acetonitrile (v/v), and detected by UV absorption at 206 nm.
Protein isolation and Western blotting The proteins were isolated from flash-frozen apoE–/– mice aortas as previously described[18]. The total proteins (10-50 μg/lane) were electrophoresed, separated on 10% SDS-PAGE, and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA, USA), which was blocked in 5% non-fat dry milk in Tris-buffered saline Tween (TBST; pH 7.6). The membrane was incubated overnight with a rabbit polyclonal antibody to rat caveolin-1 or a monoclonal antibody to mouse caveolin-1 and SREBP-1p68 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:1000 on a rotating platform at 4 °C. Subsequently, the membrane was rinsed in TBST (pH 7.6) for 40 min and incubated with horseradish peroxidase (HRP)-conjugated antirabbit immunoglobulin G (IgG) antibodies (Boster, Wuhan, China) diluted in TBST (1:4000) for 1 h on a rotating platform at 37 °C. Bands were visualized using a HRP developer, and background-subtracted signals were quantified on a laser densitometer (Bio-Rad, Hercules, Califormia, USA). Blots were probed with a mouse anti-â-actin monoclonal antibody (Boster, China) to ensure equal protein loading. All protein levels were assessed by densitometry, with β-actin used as a control.
Indirect immunofluorescence with double staining For the immunofluorescence analysis, 2×105 cells were plated into each well of 6-well plate preplaced coverslips. The cells were pretreated with ox-LDL for 48 h and treated with or without curcumin. Then the cells were fixed with methanol acetic acid (3:1) for 15 min at room temperature. The samples were then permeabilized with 0.25% Triton X-100 in PBS (Amresco, Solon, OH, USA) and 5% DMSO for 20 min at –20 °C, then washed twice with PBS containing 0.25% Triton X-100. The cells were incubated with an anti-SREBP antibody (Santa Cruz Biotechnology, USA) overnight at 4 °C in a humidified chamber. After washing 3 times with PBS, the cells were incubated with Cy3-labeled goat antirabbit IgG (Sigma, USA) for 1 h and then incubated with 4’,6-diamidino-2-phenylindole (DAPI)(from 0.1 to 1.0 mg·mL–1) for 10 min to display the cell nucleus protected from light at room temperature. The cells were then visualized using a fluorescence microscope (Olympus, Tokyo, Japan), with red and blue representing the cytoplasm and nucleus, respectively. Data were acquired with a Pixera camera (Los Angeles, CA, USA).
Statistical analysis The results were expressed as mean±SD. The two-tailed Student’s t-test was used for statistical comparisons. P<0.05 was considered to be statistically significant. For non-quantitative data, results are representative of at least 3 independent experiments.
Results
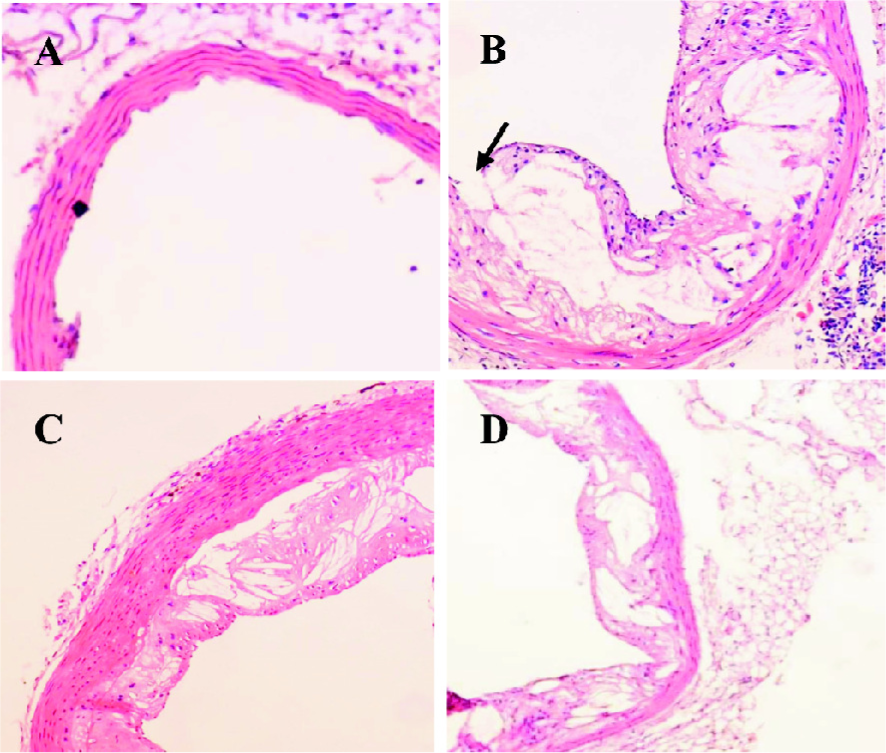
Curcumin improved atherosclerotic lesions and increased plaque stability in apoE–/– mice To identify the anti-atherosclerotic role of curcumin, we first observed its effects on plaque size and stability in apoE–/– mice. HE staining showed that the lesion area in apoE–/–mice seemed larger, the aorta lumen became narrower, and the structure fibrous cap in the lesions became brittle (Figure 1B). After administration of curcumin (20 mg·kg–1·d–1) and lovastatin (40 mg·kg–1·d–1) for 4 months, the lesion area was reduced by 50%, and the plaque size became more stable (Figure 1C, 1D).
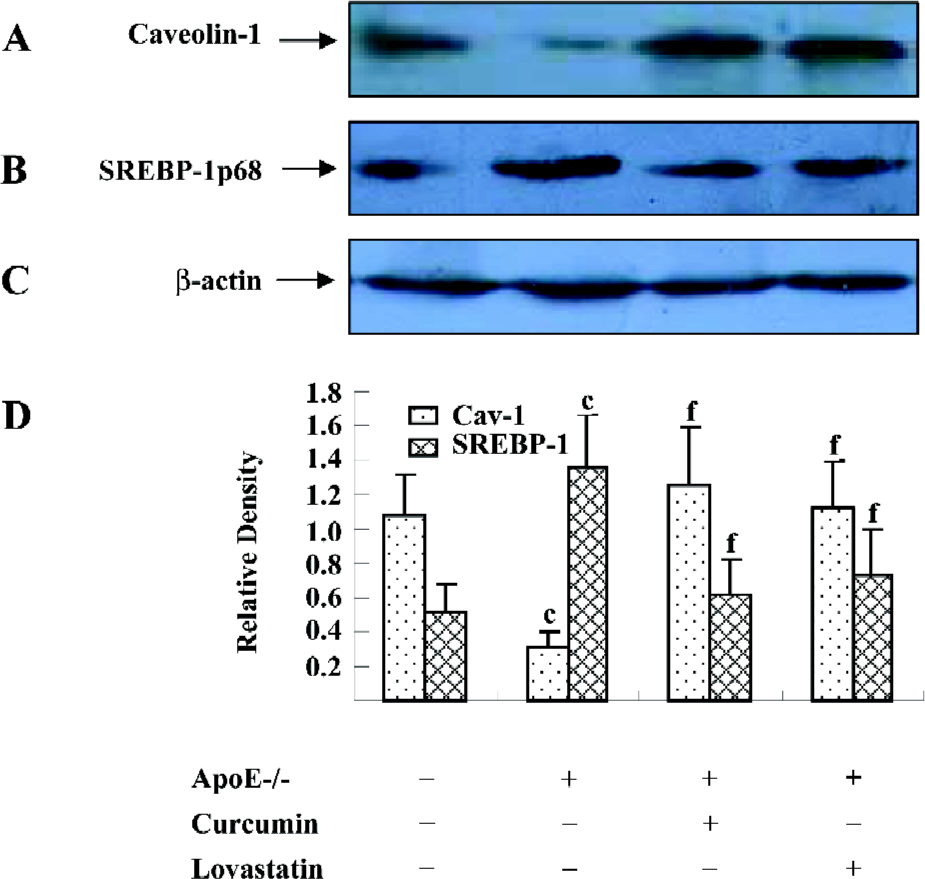
Curcumin increased the caveolin-1 expression and decreased the SREBP-1p68 expression of the aortic wall in apoE–/– mice Since caveolin-1 promotes intracellular cholesterol efflux and SREBP-1 regulates caveolin-1 expression, we were interested in examining whether the effect of curcumin in reducing atherosclerotic lesions in apoE–/– mice was related to the SREBP-1/caveolin-1 pathway. The Western blotting analysis showed an 80% lower caveolin-1 expression in apoE–/– mice as compared to C57BL/6J mice. However, the caveolin-1 level in the curcumin group showed a 5-fold elevation as compared with the models. In contrast, apoE–/– mice showed a 2-fold higher level of SREBP-1p68, an active form of SREBP-1, compared to C57BL/6J mice. Curcumin significantly inhibited high fat-induced the SREBP-1p68 expression in apoE–/– mice. The effects of curcumin were similar to lovastatin (Figure 2).
Curcumin improved plasma levels of lipids in apoE–/– mice TC, TG, and LDL-C were significantly increased in the apoE–/– mice fed a high-fat diet than those in the control group (C57BL/6J mice). The administration of curcumin markedly decreased plasma TC, TG, and LDL-C levels. Furthermore, a significant increase in HDL cholesterol was observed in curcumin-treated apoE–/– mice. The regulating effect of curcumin on blood lipids was similar to that of lovastatin (Table 1).
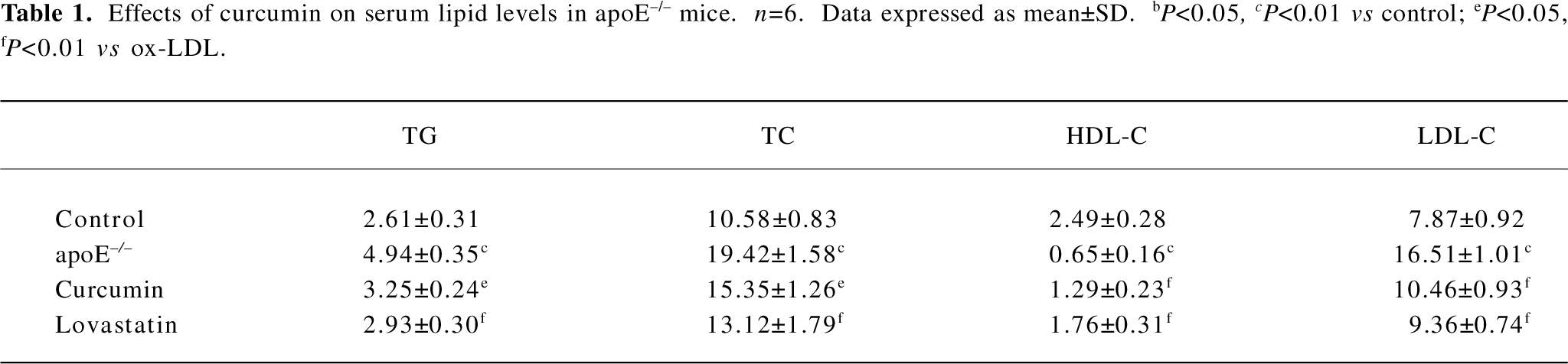
Full table
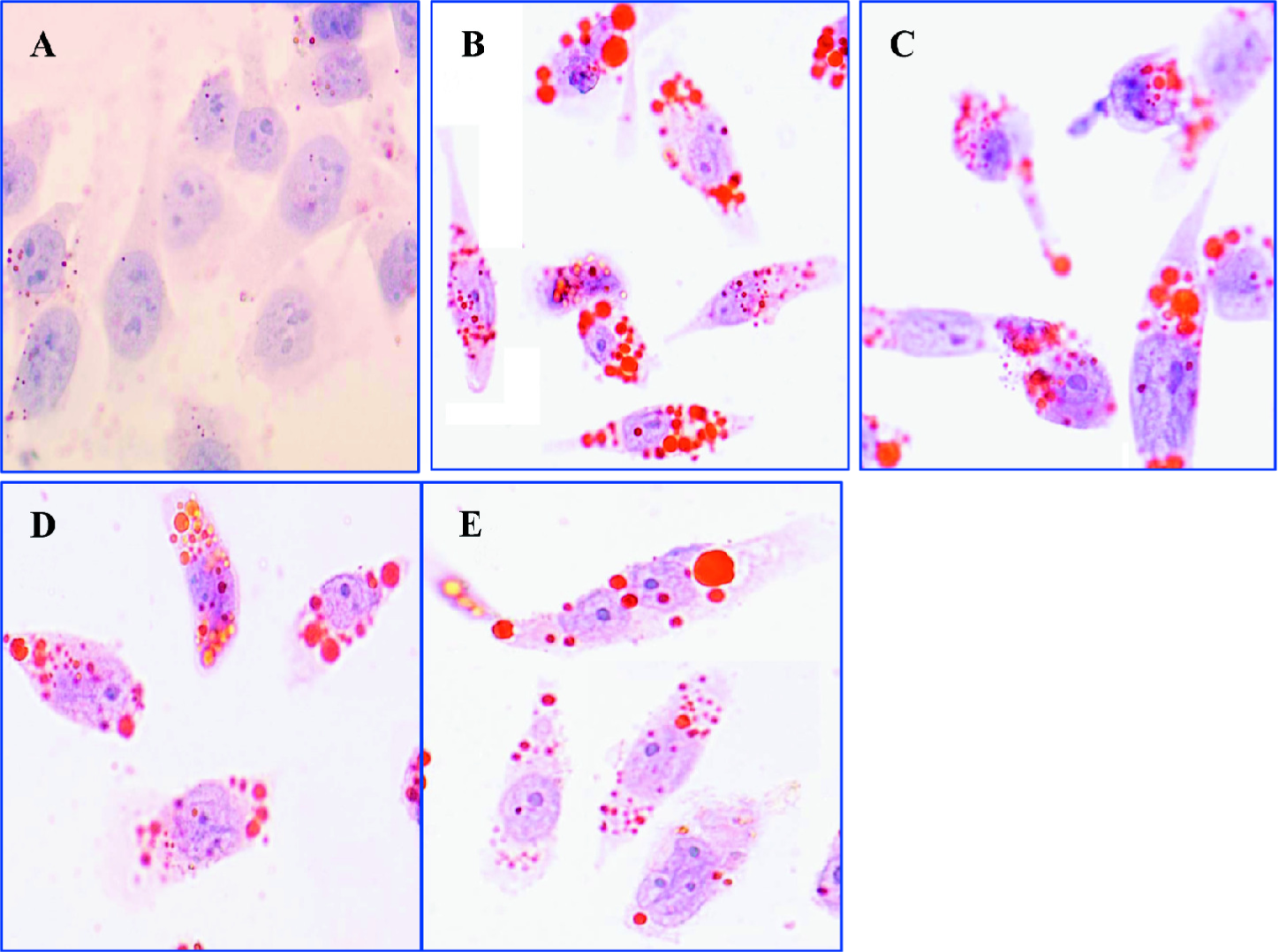
Curcumin inhibited cholesterol accumulation in rat VSMC and origin lipid-loaded cells To further investigate the mechanism of curcumin in inhibiting plaque size and stabilizing plaque, we observed the effects of curcumin on cholesterol accumulation in VSMC and origin lipid-loaded cells induced by ox-LDL. Oil red O staining demonstrated that many lipid droplets were full of lipid-loaded cells. Treatment with curcumin (12.5, 25, and 50 µmol·L–1) for 24 h markedly reduced the number of lipid droplets (Figure 3) without significant cellular toxicity (data not shown). The HPLC analysis showed that curcumin decreased intracellular lipid levels with a peak at 25 µmol·L–1 for 24 h; CE was reduced from 141±15 to 45±3.7 mg·g–1 protein (Table 2).
Full table
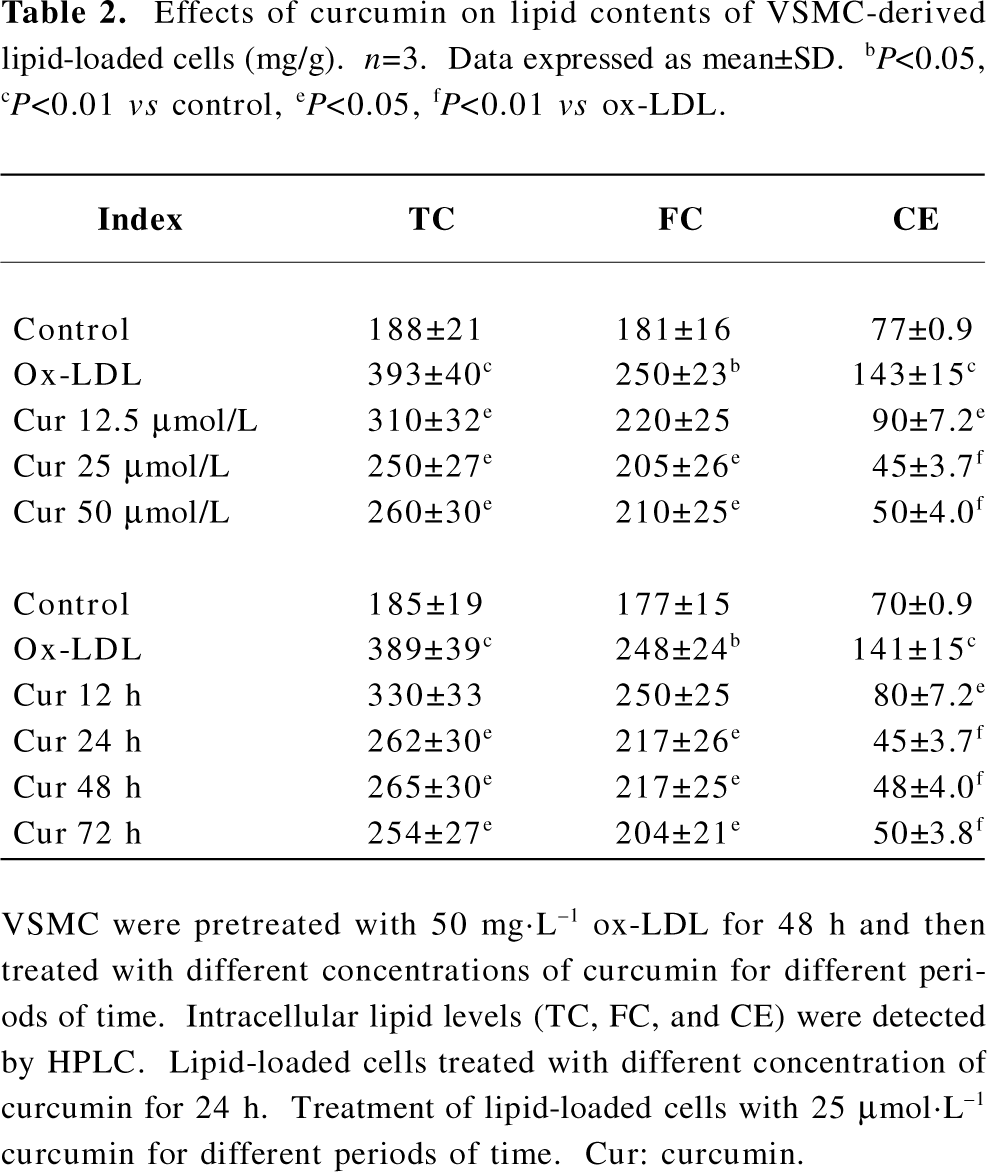
Curcumin facilitated caveolin-1 expression in rat VSMC and origin lipid-loaded cells As already stated, caveolin-1 plays a critical role in cholesterol flux[10,11]. We tried to determine whether the intracellular caveolin-1 expression could be influenced by curcumin during lipid-loaded state improvement. Lipid-loaded cells were exposed to curcumin at various concentrations (12.5, 25, and 50 µmol·L–1) for different durations (0, 6, 12, 24, and 48 h). Curcumin treatment for 24 h significantly increased the caveolin-1 expression in a concentration-dependent manner (Figure 4A) and a peak at 25 µmol·L–1 (Figure 4B).
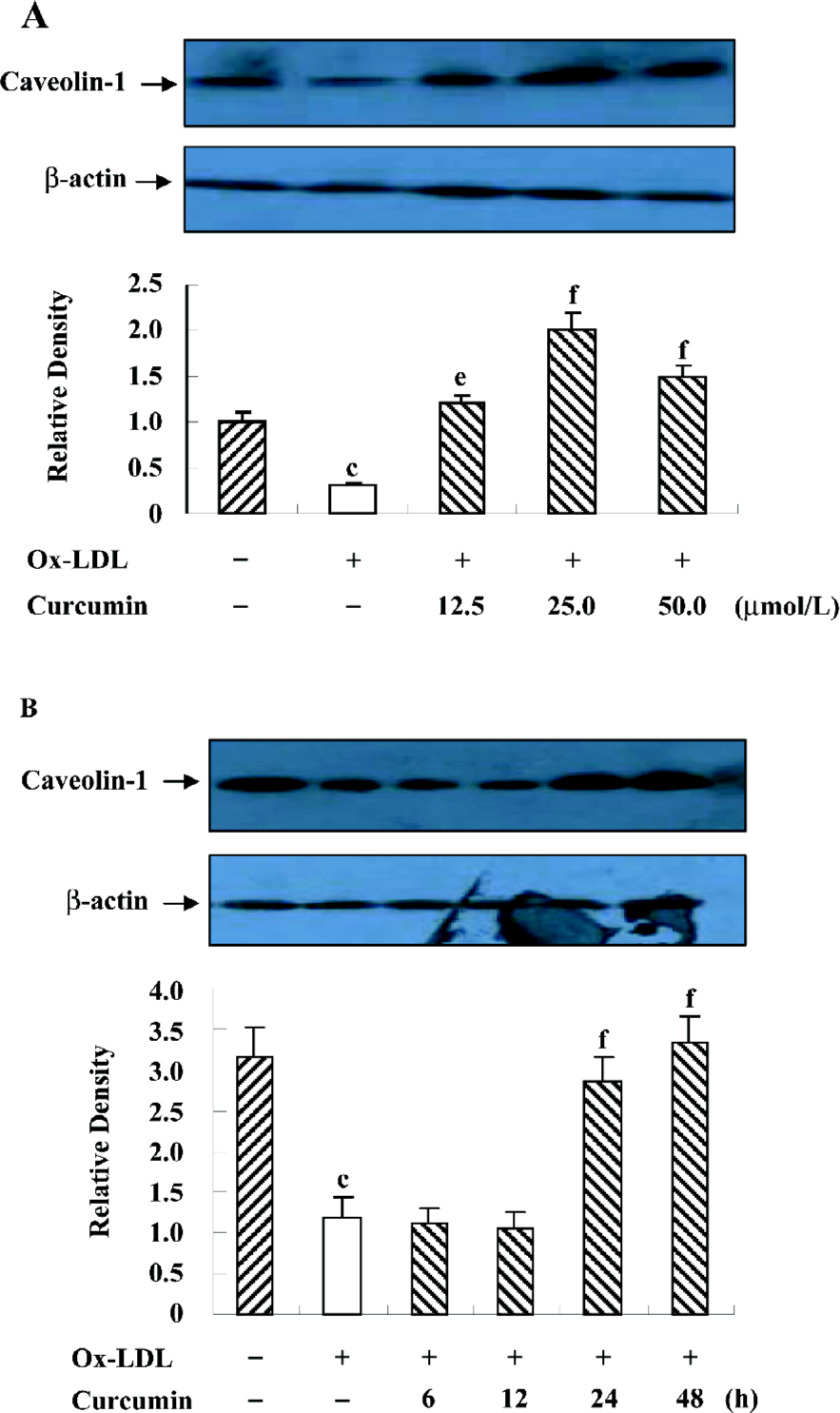
Curcumin inhibited the nuclear translocation of SREBP-1 in rat VSMC and origin lipid-loaded cells It has been reported that SREBP-1 regulates the caveolin-1 expression[22]. To examine the role of SREBP-1 under curcumin treatment, we observed SREBP-1 translocation by immunofluorescence with double staining. Compared with the controls, ox-LDL enhanced the SREBP-1 expression and stimulated SREBP-1 translocation from the cytoplasm into the nucleus (Figure 5). Yet there was a redistribution of SREBP-1 after the curcumin treatment. The SREBP-1 expression decreased gradually, and its substantial portion accumulated in the cytoplasm, and not in the nucleus (Figure 5).
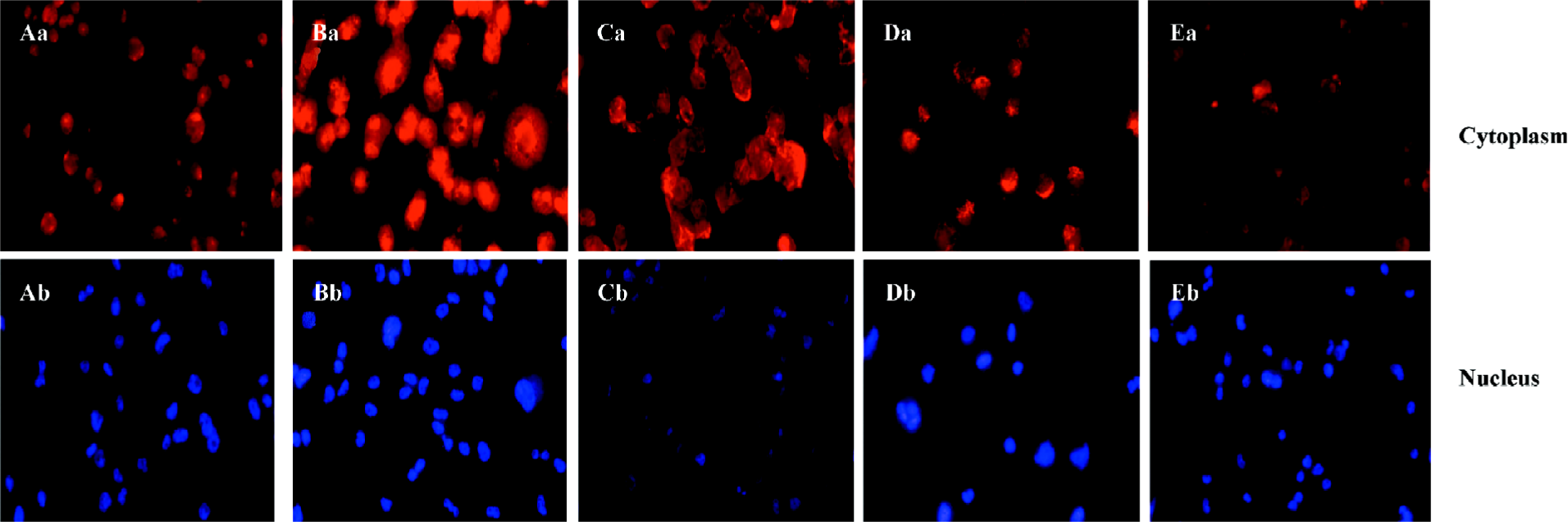
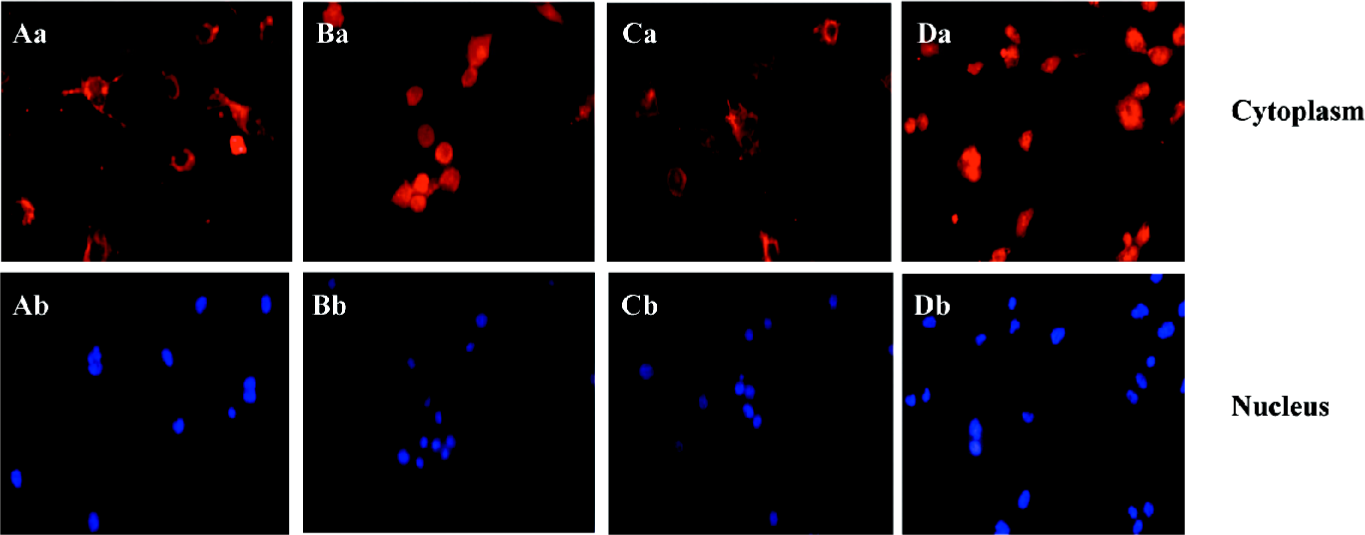
ALLN abolished the effect of curcumin on SREBP-1 translocation in rat VSMC and origin lipid-loaded cells To further confirm the relationship between SREBP-1 and curcumin, the lipid-loaded cells were treated with 25 µmol·L–1 curcumin for 24 h and then with or without ALLN, an inhibitor of SREBP-1 metabolism (or a promoter of SREBP-1). Compared with the controls, curcumin inhibited the SREBP-1 expression and its nuclear translocation. However, ALLN abolished the effect of curcumin and raised the SREBP-1 expression, as shown in Figure 6, with an increased density of red staining in the cell nuclei accompanying its nuclear translocation augmentation.
ALLN attenuated the effect of curcumin on the caveolin-1 expression in rat VSMC-derived lipid-loaded cells In an effort to understand the role of the SREBP-1/caveolin-1 pathway in the anti-atherosclerosis of curcumin, we observed the caveolin-1 expression in the presence or absence of ALLN. The Western blotting analysis indicated that ALLN could lower caveolin-1 expression in both conditions with or without ox-LDL (Figure 7A). Further experiments revealed that ALLN attenuated the curcumin-increased caveolin-1 expression in the presence of ox-LDL (Figure 7B), indicating that curcumin enhanced caveolin-1 levels by inhibiting the SREBP-1 expression.
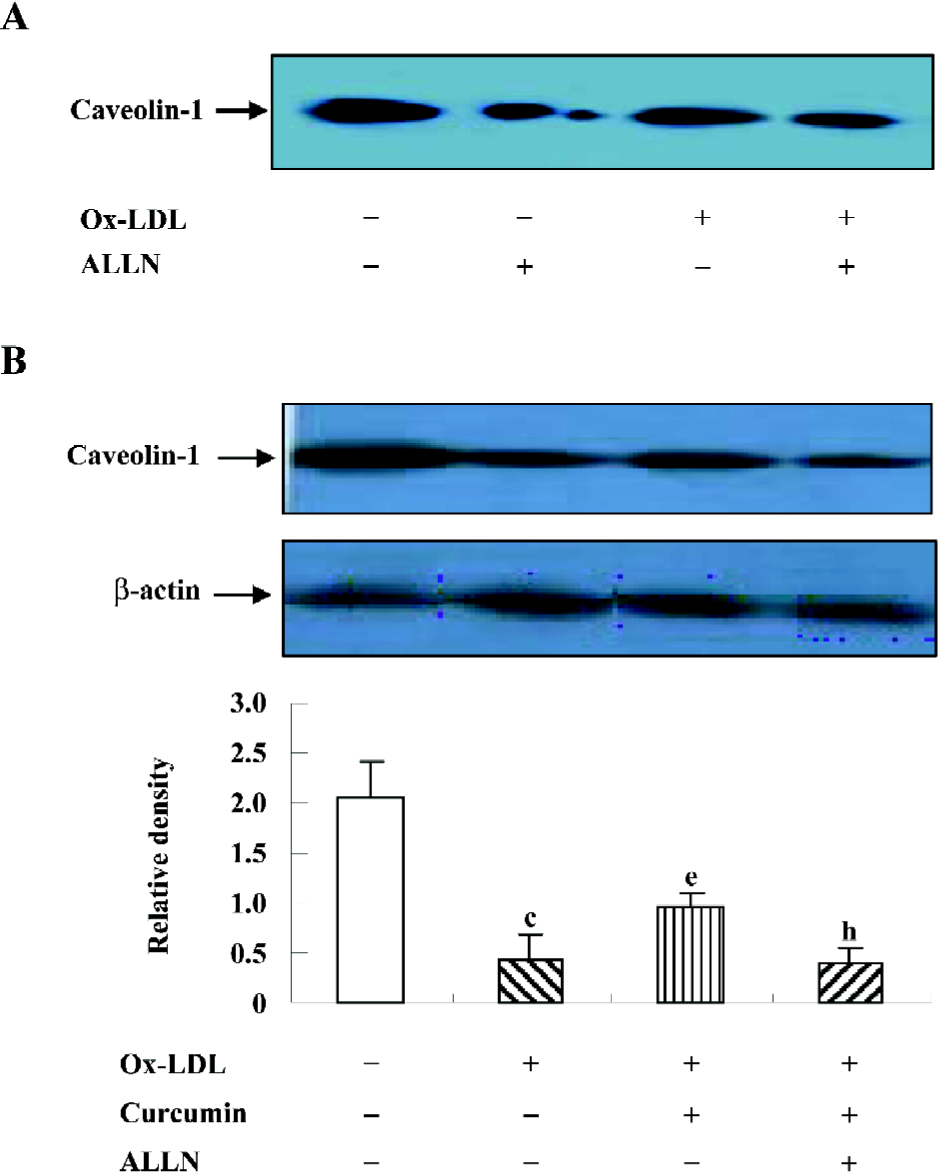
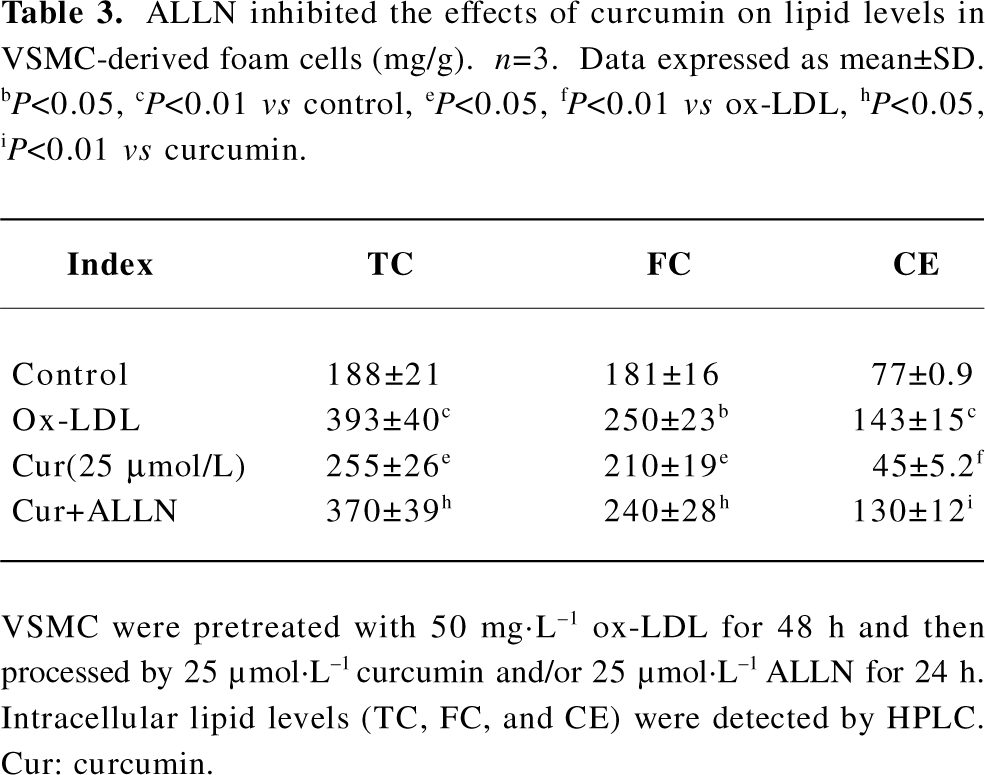
ALLN attenuated the effect of curcumin inhibiting cellular cholesterol accumulation in rat VSMC and origin lipid-loaded cells Because ALLN influenced the effects of curcumin on SREBP-1 translocation and caveolin-1 expression, whether intracellular lipid levels could be eventually affected by ALLN requires further study. We noticed that the pretreatment of ALLN for 24 h counteracted the effects of curcumin on decreasing cellular lipid levels (Table 3), indicating that curcumin inhibits cellular cholesterol accumulation through the regulation of the SREBP-1/caveolin-1 signaling pathway.
Full table
Discussion
To clarify the pharmacological mechanism of curcumin on anti-atherosclerosis, we observed its effects on apoE–/– mice and cultured rat VSMC and origin lipid-loaded cells. In the present study, we reproduced the anti-arthrosclerosis of curcumin at animal and cellular levels, and further documented that these effects were related to the inhibition of SREBP-1 nuclear translocation followed by the increment of the caveolin-1 expression.
As a constituent of the spice turmeric, the antitumoric and anti-inflammatory effects of curcumin have been studied[1-3]. Although several lines of evidence strongly suggest that curcumin could prevent atherosclerotic development by regulating some elements in cholesterol homeostasis[6,7], the potential mechanism was unclear. Our animal experiment showed that the administration of curcumin inhibited plaque formation and enhanced plaque stability in apoE–/– mice.
Caveolae, discovered approximately 50 years ago, was mainly formed by the caveolin family and is known as an important hub not only in signal transmission, but also in lipid transport across the plasma membrane[19,20]. Considering that plaque regression may be linked to intracellular lipid efflux, and caveolin-1 may play a key role, we subsequently studied the relationship between the caveolin-1 expression and anti-atherosclerosis of curcumin in apoE–/– mice and cholesterol accumulation in cultured VSMC. Our results demonstrated that curcumin promoted the caveolin-1 expression in apoE–/– mice. In rat VSMC and origin lipid-loaded cells induced by ox-LDL, curcumin promoted the caveolin-1 expression and downregulated lipid levels in a dose-dependent manner. The data demonstrated that curcumin anti-atherosclerosis and this effect are perhaps linked to caveolin-1 expression enhancement.
The SREBP-1 precursor is located on membrane of the endoplasmic reticulum. When intracellular FC changes, its active fragment (SREBP-1p68) transfers into the nucleus to regulate caveolin-1 transcription[13,15], which implies that SREBP-1 probably participates in the effects of curcumin on the caveolin-1 expression and lipid levels. To identify this possibility, we observed the expression and translocation of SREBP-1 in cultured VSMC treated with ox-LDL or curcumin. The results showed that ox-LDL induced the high expression of SREBP-1, but curcumin counteracted this effect and inhibited its nuclear translocation. Meanwhile, curcumin upregulated the caveolin-1 expression and reduced the intracellular lipid levels correspondingly. The relationship between SREBP-1 and caveolin-1 in our experiment was consistent with a previous study[15].
It has been reported that ALLN could enhance the SREBP-1 level by inhibiting the catabolism of SREBP-1, and the latter could negatively regulate the caveolin-1 expression[15,21]. However, we were interested in whether the effects of curcumin on SREBP-1 translocation and subsequent caveolin-1 expression, as well as cellular lipid levels, could be decreased by ALLN. Interestingly, in the present of ALLN at a concentration of 25 µmol·L–1, when the lipid-loaded cells were incubated with curcumin, the curcumin-induced blockage of SREBP-1 translocation was obviously eliminated, curcumin-induced caveolin-1 expression was reduced, and intracellular lipid levels were upregulated again. These findings strongly suggest the effects of curcumin on increasing the caveolin-1 expression and lowering intracellular lipid levels, which were mediated by the inhibition of the nuclear translocation of SREBP-1.
In conclusion, our data demonstrate that curcumin inhibits ox-LDL-induced cholesterol accumulation in cultured VSMC by regulating the SREBP-1/caveolin-1 pathway. This new mechanism may be beneficial for anti-atherosclerotic research. Together with previous studies, our results indicate the potential of curcumin or its chemically-modified derivatives as ideal anti-atherosclerotic drugs.
Acknowledgements
The authors are indebted to Dr Xue-feng XIA (Department of Internal Medicine, University of Texas, Houston Health Science Center, USA) for his valuable help in the preparation of the manuscript.
References
- Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE, . Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim Biophys Acta 2007; 1773: 1116–23.
- Davis JM, Mruphy AE, Carmichael MD, Zielinski MR, Groschwitz CM, Brown AS, et al. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am J Physiol Regul Integr Comp Physiol 2007;292:R2168-73.
- Manjunatha H, Srinivasan K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rat. FEBS J 2006;273:4528-37.
- Soudamini KK, Unnikrishnan MC, Soni KB, Kuttan R. Inhibition of lipid peroxidation and cholesterol levels in mice by curcumin. Indian J Physiol Pharmacol 1992;36:239-43.
- Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol 1992;36:273-5.
- Olszanecki R, Jawien J, Gajda M, Mateuszuk L, Gebska A, Korabiowska M, et al. Effect of curcumin on atherosclerosis in apoE/LDLR-double knockout mice. J Physiol Pharmacol 2005;56:627-35.
- Peschel D, Koerting R, Nass N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J Nutr Biochem 2007;18:113-9.
- Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell 1992;68:673-82.
- Epand RM, Sayer BG, Epand RF. Caveolin scaffolding region and cholesterol-rich domains in membranes. J Mol Biol 2005;345:339-50.
- Uittenbogaard A, Ying YS, Smart EJ. Characterization of a cytosolic heat-shock protein-caveolin chaperone complex. J Biol Chem 1998;273:6525-32.
- Uittenbogaard A, Smart EJ. Palmitoylation of caveolin-1 is required for cholesterol binding chaperone complex formation and rapid transport of cholesterol to caveolae. J Biol Chem 2000; 75: 25 595–9.
- Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D. Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem 2004; 279: 14 140–6.
- Brown AJ, Sun L, Feramisco JD, Brown MS, Goldstein JL. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol Cell 2002;10:237-45.
- Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter I. Identification of the protein and delineation of its target nucleotide sequence. J Biol Chem 1993;268:14490-6.
- Bist A, Fielding PE, Fielding CJ. Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc Natl Acad Sci USA 1997;94:10693-8.
- Staprans I, Rapp JH, Pan XM, Hardman DA, Feingold KR. Oxidized lipids in the diet accelerate the development of fatty streaks in cholesterol-fed rabbits. Arterioscler Thromb Vasc Biol 1996;16:533-8.
- Jiang P, Yan PK, Zhu BY, Lei XY, Yin WD, Liao DF. HDL3 inhibits Ox-LDL-induced apoptosis by promoting cholesterol efflux in RAW264.7 cells. Acta Pharmacol Sin 2006;27:151-7.
- Singaraja RR, Bocher V, James ER, Clee SM, Zhang LH, Leavitt BR, et al. Human ABCA1 BAC transgenic mice show increased high density lipoprotein cholesterol and apoAI-dependent efflux stimulated by an internal promoter containing liver X receptor response elements in intron 1. J Biol Chem 2001;276:33969-79.
- Palade GE. Fine structure of blood capillaries. J Appl Phys 1953;24:1424.
- Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev 2002;54:431-67.
- Wang X, Sato R, Brown MS, Hua X, Goldstein JL. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell 1994;77:53-62.
