In vitro and in vivo induction of bone formation based on adeno-associated virus-mediated BMP-7 gene therapy using human adipose-derived mesenchymal stem cells1
Introduction
Enhanced bone formation is often required to treat bone loss associated with trauma, revision joint arthroplasty, and tumor resection. Autograft is currently the gold standard for inducing bone repair. However, only a limited amount of autogenous bone graft is available, and bone graft harvest can involve substantial donor site morbidity[1,2]. Therefore, there is great interest in identifying bone graft substitutes that can stimulate bone repair. Bone morphogenetic proteins (BMP) are known to possess strong osteo-inductive properties and BMP gene therapy plays an important role in modulating bone regeneration[3]. BMP are members of the TGF-β (transforming growth factor-beta) superfamily of growth factors[4,5]. Recombinant BMP stimulate bone formation and repair in a variety of preclinical animal models[6–8]. Recently, BMP have received FDA (Food and Drug Administration, USA)approval to treat recalcitrant tibial non-unions [OP-1 (osteogenic protein-1)or BMP-7]. However, since very large doses (ie milligrams) of BMP are necessary to induce a biological effect in humans, more efficient and safer delivery vectors must be obtained before clinical trials can be carried out successfully. To date, viruses are the most efficient vectors for gene delivery. Previous studies have demonstrated that the adenoviral-mediated delivery of BMP can stimulate new bone formation and healing in a critical-sized femoral defect [9–11]. However, the application of adenoviral vectors in clinical situations is limited by the lack of persistent target gene delivery and the pronounced immune response in immunocompetent animals and humans[12,13].
Recombinant adeno-associated virus (AAV) may be an ideal vector for delivering therapeutic factors. The vector is non-pathogenic and elicits no inflammatory response[14,15]. Furthermore, AAV often leads to efficient long-term expression of secreted proteins both in vivo and in vitro[16]. These advantages have been manifest in the considered use of AAV in clinical trials[17]. However, only a few studies have addressed the use of AAV vectors carrying BMP to induce bone healing[18–20]. Thus, it is not known whether the transfer of the BMP-7 gene into human adipose-derived mesenchymal stem cells (ADMS) cells using a BMP-7-harboring AAV could mirror the consistent success of these earlier studies. This avenue is worth exploring, since committed osteoblastic differentiation of ADMS cells would be important prerequisite for AAV2–BMP-7 in vivo gene therapy for bone healing.
Currently, there are 2 general types of pluripotent stem cells that are potentially useful for gene therapy and tissue engineering: embryonic mesenchymal and autologous mesenchymal stem cells. Autologous mesenchymal stem cells are more promising because they are immunocompatible and there are fewer ethical issues to consider. Recent studies have indicated that ADMS cells are capable of differentiating along multiple mesenchymal cell lineages (osteoblasts, adipocytes, chondrocytes, and myoblasts)[21–25]. Under osteogenic culture conditions, these cells can differentiate into osteoblasts[24]. Large numbers of autologous ADMS cells can easily be obtained from fat[22]. Hence, these cells are a promising substrate for the clinical application of bone tissue engineering. However, whether ADMS cells could be effectively used in an ex vivo system (requiring harvesting, manipulation, and re-implantation) for osteo-inductive regional gene therapy requires further investigation.
The purpose of this study was to: (i) determine whether human ADMS cells could be successfully transduced with an AAV vector carrying the BMP-7 gene and express the BMP-7 protein; (ii) observe whether these BMP-7-expressing cells could display the differentiated osteoblastic phenotype in vitro; and (iii) investigate whether implantation of collagen-wrapped BMP-7-expressing cells could induce new bone formation in vivo.
Materials and methods
Cells BHK-21 cells (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS, Gibco BRL, USA) and penicillin (100 U/mL)/streptomycin (100 mg/mL) (Sigma, St Louis, MO, USA) at 37 oC in humidified atmosphere with 5% CO2.
Animals Eight- to 12-week-old male severe combined immune-deficient (SCID) mice were purchased from the Animal Administration Center of Sun Yat-sen University (Guangzhou, China). All animal experimental protocols were approved by the Animal Care and Use Committee of Sun Yat-sen University (China).
Plasmids The pcDNA1.1/AMP(ampicillin)-hBMP-7 plasmid was provided by Pu-yi SHENG of the First Affiliated Hospital, Sun Yat-sen University (China). The 1.3 kb BMP-7 coding sequence was amplified by a PCR system (Invitrogen, Carlsbad, CA, USA) from the pcDNA1.1(+) plasmid containing the human BMP-7 cDNA. The primer sequence for the PCR was as follows: upstream primer: 5'-GT
Packaging, purification, and titration of the AAV vector BHK-21cells were transfected with the purified pSNAV-BMP-7 plasmid according to a standard calcium phosphate precipitation method. The cells were then cultured in selection medium containing 800 µg/mL G418 (Gibco BRL, USA). G418-resistant BHK-21 cell clones were isolated and the integrity of the hBMP-7 gene was determined by PCR using the primers detailed previously. To package the virus, stably-transfected BHK-21 cells were infected with recombinant herpes simplex virus type 1 (rHSV-1), which can express the AAV2 rep and cap genes of wild-type AAV. For large-scale AAV production and purification, BHK-21 cells were cultured in 6 roller bottles (110 mm×480 mm, Wheaton, Millville, NJ, USA) at 37 oC at 1 roll/min. Confluent cells in 10 mL medium were infected with helper virus rHSV-1 at a MOI (multiplicities of infection) of 0.1 and incubated for 2 h. The collected cells were treated with chloroform, PEG8000 (Polyethylene Glycol 8000)/NaCl for precipitation, and chloroform extraction for purification, sequentially. The viral titer was quantitatively determined using a DNA dot blot[26], and the purity was examined by SDS-PAGE. The titers averaged approximately 4×1011 vector genomes (vg) per mL and the purity was >95%. AAV-enhanced green fluorescence protein (EGFP) was also constructed using the same procedure. The pEGFP-C1 plasmid (TaKaRa, Shiga, Japan) was used for the amplification of the EGFP-sequence.
Preparation of human ADMS cells from adult human fat Human raw lipoaspirates from patients undergoing selective suction-assisted lipectomy were collected after obtaining informed consent from the patients according to procedures approved by the Ethics Committee at the First Affiliated Hospital of Sun Yat-Sen University (China). The procedure described by Zuk et al[27] was used with some modifications. Briefly, the raw liposuctioned aspirate was extensively washed with D-Hanks’ solution to remove contaminating blood cells and local anesthetics. The extracellular matrix was digested with 0.2% collagenase II (Sigma, USA) at 37 oC for 30 min to release the cellular fractions. The cells were washed twice, then plated in T-75 tissue culture flasks at a density of 2×106/mL and cultured in DMEM/F-12 medium (Gibco BRL, USA) containing 10% FBS (Gibco BRL, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco BRL, USA) at 37 oC in a humidified atmosphere with 5% CO2. Once the adherent cells were more than 80%, they were detached with 0.125% trypsin and 0.01% EDTA (Life Technologies, Gaithersburg, MD, USA), and replated at a 1:3 dilution under the same culture conditions. All the experiments were done with cells at the fifth and tenth passages, and the results presented here are all based on the fifth passage cell clones.
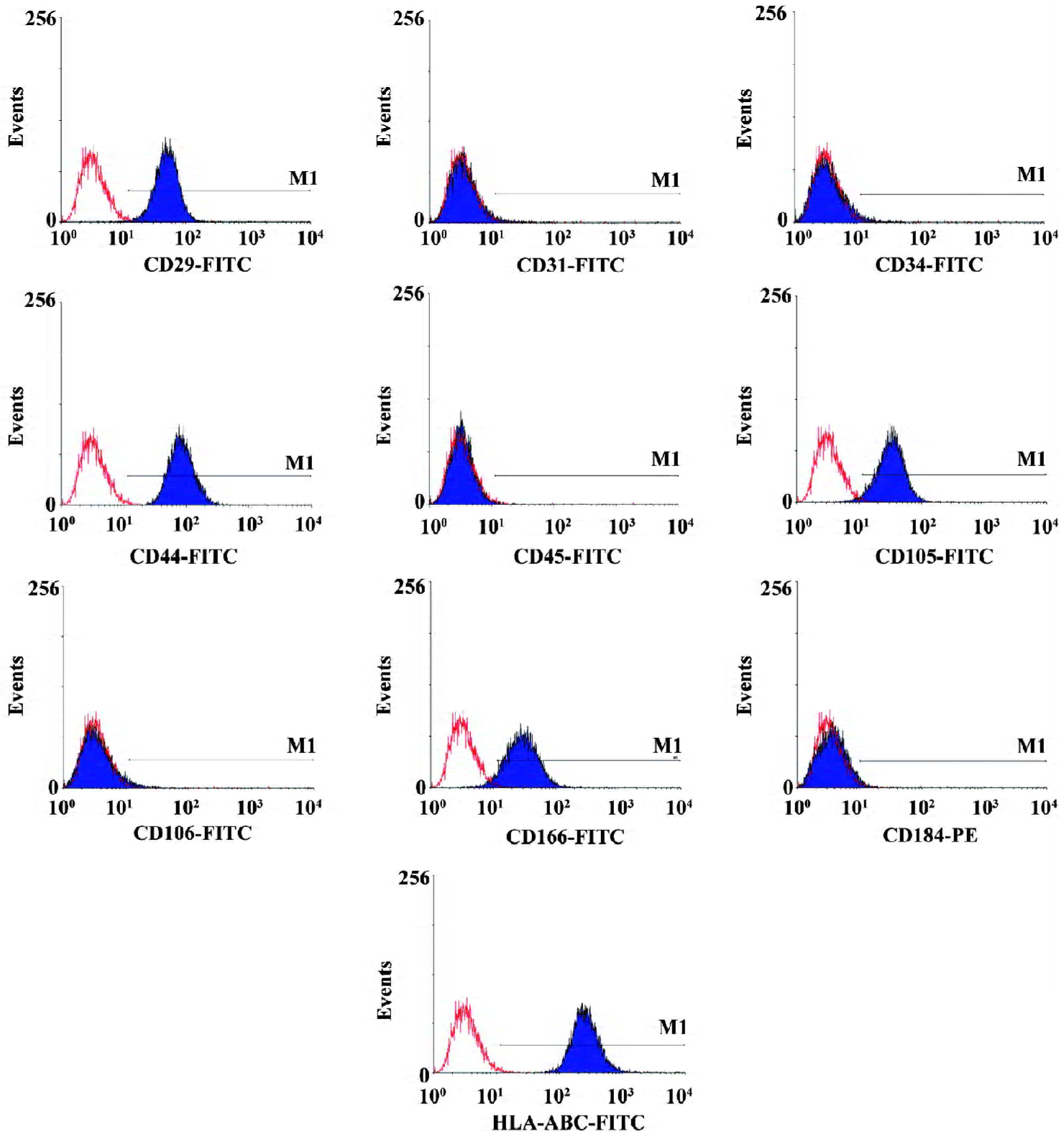
Immunophenotype analysis The medium was removed from the flasks, and the cell layers were detached and washed with phosphate-buffered saline (PBS, Gibco BRL, USA) containing 0.5% bovine serum albumin (BSA, Sigma, USA), and incubated with primary antibodies for 30 min at 4 oC. To detect intracellular antigens, we fixed the cells in 2% paraformaldehyde for 15 min at 4 oC and then permeabilized them with 0.1% saponin (Sigma, USA) for 1 h at room tem-perature. Working concentrations for primary antibodies against human CD29, CD31, CD34, CD44, CD45, CD105, CD106, CD166, CD184, and HLA-ABC (BD Biosciences, San Jose, CA, USA) were 10–20 ng/mL, respectively. We used same-species, same-isotype-irrelevant antibody as the negative control. After washing with PBS containing 0.5% BSA, the cells were incubated with fluorescein isothiocyanate and phycoerythrin-conjugated secondary antibodies for 30 min at 4 oC. After three washes, the cells were resuspended in PBS and analyzed by flow cytometry using a FACSCalibur flow cytometer and CellQuest Pro software (BD Biosciences, CA USA).
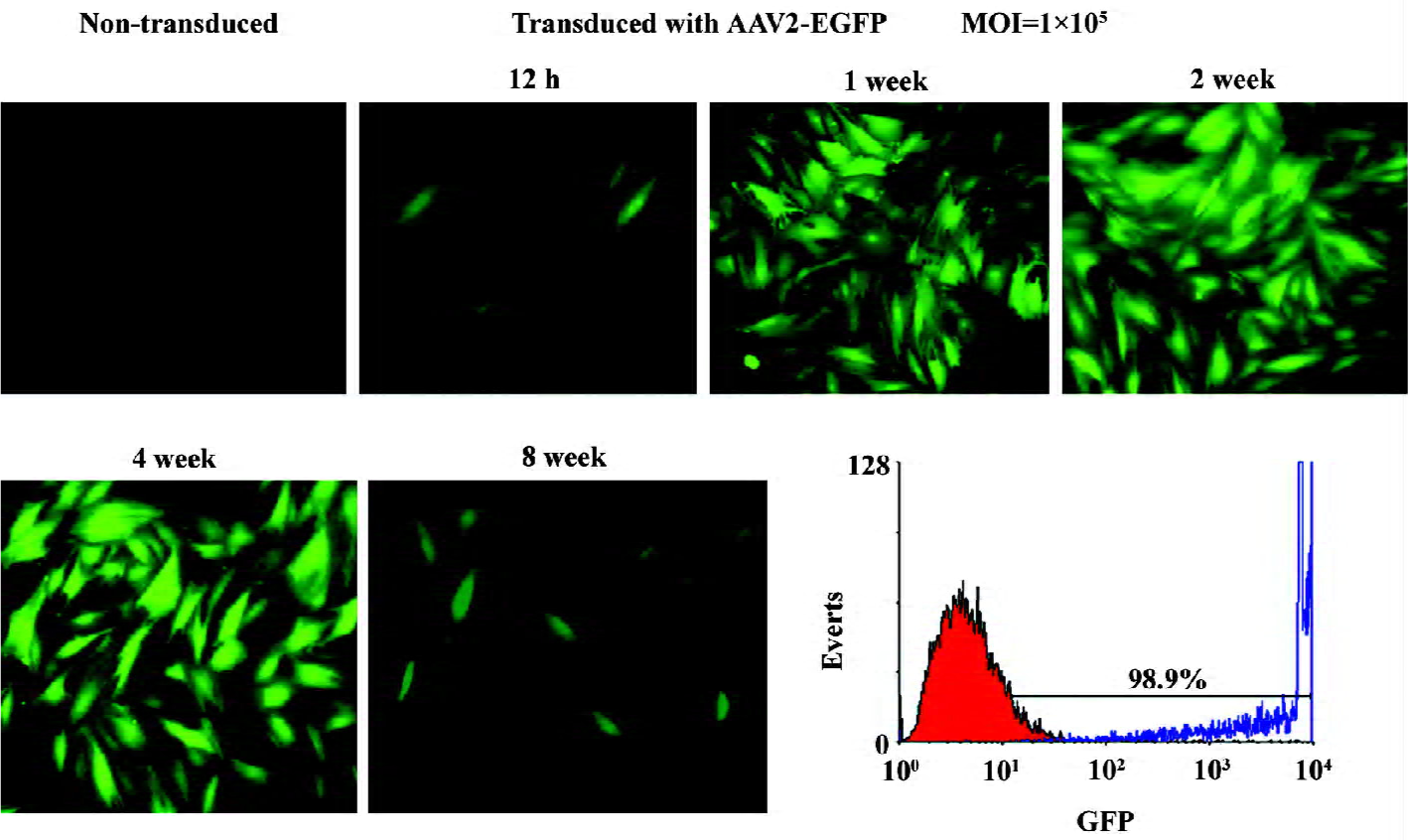
AAV vector transduction The cells recovered after 2 passages were cultured as a monolayer in 6-well plates at a density of 2×105 cells per well in DMEM/F-12 containing 10% FBS. Subconfluent cells were incubated with either AAV2-BMP-7 or AAV2-EGFP at a MOI of 1×105 in a total volume of 500 µL serum-free medium for 1 h at 37 ℃. The medium was then aspirated and 1 mL fresh growth medium (FBS-DMEM/F-12 containing 30 mmol/L sodium butyrate) was added. A MOI of 1×105 was chosen as a result of pilot studies demonstrating that the MOI of 1×105 would produce the highest level of transgenic expression. Morphological changes were monitored with a phase contrast microscope. All experiments were carried out in triplicate. EGFP expression was detected by fluorescent microscope or flow cytometry.
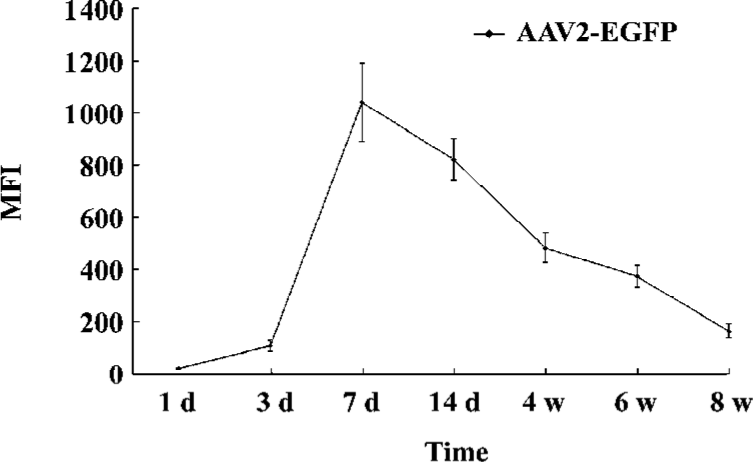
Flow cytometry EGFP expression was detected in AAV2-EGFP-transduced ADMS cells 1 week after transduction by FACScan flow cytometry. One million harvested cells were analyzed by flow cytometry, and the rest of the cells were maintained in culture again for a subsequent analysis. The percentage of EGFP-positive cells and the expression of the mean fluorescence intensity (MFI) and EGFP were analyzed using CellQuest Pro software (BD Biosciences, USA). The EGFP expression of the cells was also detected by fluorescence microscopy.
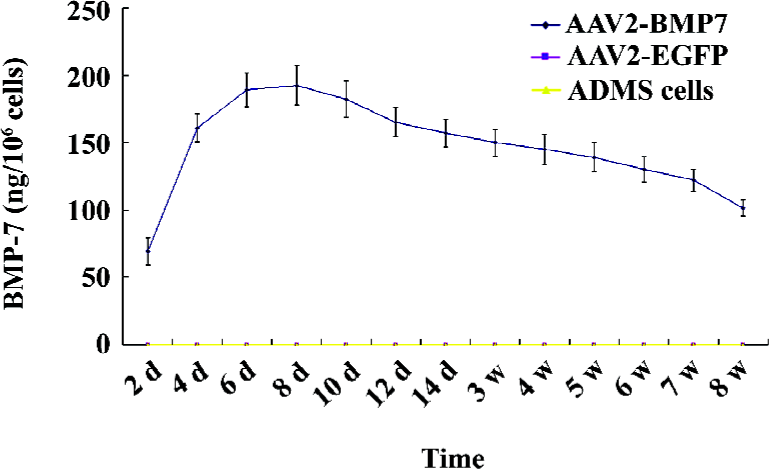
Determination of BMP-7 production To quantify the expression level of BMP-7, ADMS cells culture medium was collected from d 2 to d 56 after transduction for ELISA analysis (ADL, San Antonio, TX, USA). ELISA was carried out according to the manufacturer’s recommendations. Briefly, standards and culture medium were incubated at room temperature with sample buffer in 96-well plates for 90 min and then with biotin-labeled anti-human BMP-7 detection antibody for 60 min. Finally, a streptavidin-horseradish peroxidase conjugated antibody was added at room temperature and incubated for 30 min. Bound BMP-7 was detected by adding tetramethylbenzidine substrate solution for 15 min and the plates were read at 450 nm.
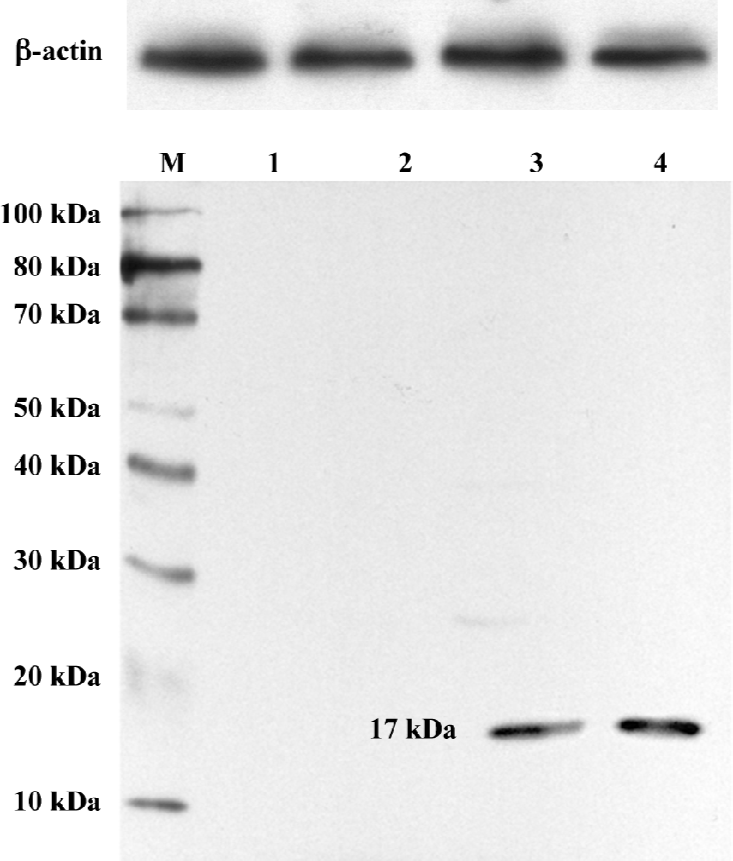
For the Western blot analysis, human ADMS cells were transduced with either AAV2-BMP-7 or AAV2-EGFP, or left non-transduced. The harvested medium was collected on d 28 and d 56 post transduction. Proteins were separated by SDS-PAGE and transferred to the nitrocellulose membrane. Following incubation with a goat polyclonal antibody against BMP-7 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:250, the membrane was incubated with a rat anti-goat IgG-horseradish peroxidase (Zhongshan Bio-chemical, Beijing, China) at a dilution of 1:1000. Immunoreactivity was determined using the ECL chemiluminescence reaction (Amersham, Arlington Heights, IL, USA).
ALP staining (Ca-Co technique) Cells that began to grow on the cover glass 14 d after transduction were extensively washed with PBS, fixed with cool acetone for 10 min, flushed with distilled water, dipped into 5 mL buffer [consisting of 20 g/L β-phosphoric acid glycerin natrium (10 mL), 20 g/L barbital sodium (20 mL), 20 g/L addex-magnesium (1 mL), and 5 mL distilled water] (pH =9.2, Zhongshan Biochemical, China), and incubated at 37 oC for 6 h. The samples were then washed with distilled water and dipped into 20 g/L aqueous solution of cobaltous nitrate for 5 min. After another wash with distilled water, the samples were dipped into aqueous solution of sulphon ammonium for 3 min. Following a final wash, each sample was dehydrated routinely, mounted, and examined microscopically.
Chinalizarin staining Fourteen days after transduction, Chinalizarin staining was performed. The culture medium in the petri dish was withdrawn, and the cells were washed with distilled water and fixed with 40 g/L formaldehyde. The samples were stained with Chinalizarin S liquid for 5 min. After washing with acetone, a mixture of acetone, dimethyl-benzene, and distilled water, the samples were microscopically examined.
Scanning electron microscopy Fourteen days after transfection, the cells grown on the cover glass were fixed with 25 g/L glutaral for 24 h. After washing with PBS, the cells were dehydrated with gradient ethanol, permutated with isoamyl acetate, dehydrated at the critical point, and vacuumized with spurted metal. Treated samples were observed with JSM-T300 scanning electron microscopy (SEM, JEOL, Tokyo, Japan).
Transmission electron microscopy analysis Fourteen days after transfection, digestive juices, including pancreatin-ethylene dinitrilotetraacetic acid, was fallen after digestion, and a little DMEM culture medium including 10% FBS was added to stop digestion. The suspension was put in a centrifuge tube and centrifuged at 1000×g for 8 min. After being fixed with 25 g/L glutaral at room temperature for 12 h, the samples were put into 10 g/L osmium tetroxide and treated for 24 h at room temperature. After being dehydrated with gradient ethanol and permutated with acetone, the samples were embedded with epoxy resin and cut into ultra-thin sections. The cut sections were stained with acetic acid double uranium and lead acetate, and its ultrastructure was observed with JEM21200EX TEM (JEOL Company, Japan).
Osteocalcin production Osteocalcin secreted into the culture medium was determined by a radioimmunoassay using a human osteocalcin assay kit following the manufacturer’s recommendations (Dongya Immune Technique Institute, Beijing, China).
Implantation of BMP-2-transduced cells into SCID mice Eight- to twelve-week-old male SCID mice were used in this study. Animals were anesthetized with an intramuscular injection of ketamine (1.5 mg) and xylazine (0.3 mg) and were prepared for aseptic surgery. A 1 cm incision was made on the lateral aspect of the left thigh. The quadriceps musculature was identified in which a 1 cm slit was made. Two and half million human ADMS cells transduced with AAV2-BMP-7 at a MOI of 1×105 were harvested and resuspended with 50 µL PBS, and then placed onto a collagen type I matrix (Sangon Biochemical, Shanghai, China) that was cut in the shape of a 5×3×2 mm3 rectangular block. Finally, these carriers were implanted into a muscle pouch in the quadriceps portion of the hind limb and the wound was sutured immediately. After surgery, the animals were allowed ad libitum activity. Human ADMS cells were transduced by infection with the AAV2-BMP-7 vector at a MOI of 1×105 for 24 h, 7 d prior to implantation. Non-transduced and AAV2–EGFP-transduced (at a MOI of 1×105) human ADAS cells served as the controls. Six animals were allocated to each of the treatment groups.
Evaluations for bone formation Eighteen SCID mice were sacrificed 3 weeks after implantation in a muscle pouch, and radiographic examination was performed. The newly-formed bone tissues were harvested from the hind limbs of the SCID mice and fixed in buffered 10% formalin, and then decalcified with 10% EDTA. The specimens were then dehydrated and embedded in paraffin. The tissues were cut into 5 μm sections and stained with HE.
Statistical analysis For each experiment, multiple samples (n=3) were taken, and data were reported as mean± SD. Data were analyzed using the two-tailed Student’s t-test with a level of significance of P<0.05. The SPSS 11.0 software package (Chicago, IL, USA) was used for the statistical analysis.
Results
Morphology and phenotypes of cultured human ADMS cells The adherent-cultured ADMS cells assumed a fibroblast-like morphology when observed under a light microscope. The morphology was maintained through repeated subcultures under non-stimulating conditions. To characterize the phenotypes of adherent adipose-derived cells at the fifth passage, flow cytometry was performed. The results showed that these cultured cells were positive for CD29, CD44, CD105, CD166, and HLA-ABC. In addition, no expression of the hematopoietic and endothelial lineage markers (CD31, CD34, CD45, CD106, and CD184) was observed (Figure 1). The phenotype was similar to those we isolated from the bone marrow, except that the latter was positive for CD106. It was also similar to that reported in previous studies[27–29].
EGFP expression in vitro The expression of EGFP in human ADMS cells, initiated 12 h after transduction with AAV2-EGFP as the MFI increased gradually. AAV2-EGFP-transduced ADMS cells demonstrated the highest MFI (values were 1040) at d 7, and lasted for 8 weeks [P<0.05 at each time point (different sample at each time point); Figure 2]. The flow cytometry analysis revealed that the expression of EGFP in ADMS cells occurred in approximately 98.9% of the cell population when infected at a MOI of 1×105. However, more than 60% of the cell population died when the MOI was 1×106. More than 95% of the cell population died when the MOI reached1×107 (Figure 3).
Phenotypic changes of rAAV2-BMP-7 transduced cells Seven days after infection of human ADMS cells with AAV2-BMP-7 or AAV2-EGFP at a MOI of 1×105, obvious cellular phenotypic changes were observed. Uninfected cells or cells transduced with AAV2-EGFP displayed significant fibroblast-like differentiation. However, ADMS cells transduced with AAV-BMP-7 did not fuse and displayed mononuclear ellipse-like or polygonal cell morphology, similar to osteoblastic cells.
Expression of BMP-7 in ADMS cells The in vitro release kinetics of BMP-7 from AAV2-BMP-7-infected ADMS cells was evaluated over the course of 56 d using ELISA. No detectable BMP-7 was produced by the uninfected and AAV2-EGFP-treated cells (t=14.34, P<0.05). However, cells transduced with AAV2-BMP-7 produced low levels of BMP-7 by d 2 (69.14±3.21 ng/106 cells), followed by an increase in production with a mean of 146.45±10.60 ng/106 cells from d 6 to d 56 (Figure 4). Furthermore, the secreted BMP-7 protein in the harvested medium of AAV2-BMP-7-transduced human ADMS cells was confirmed by Western blot analysis (Figure 5).
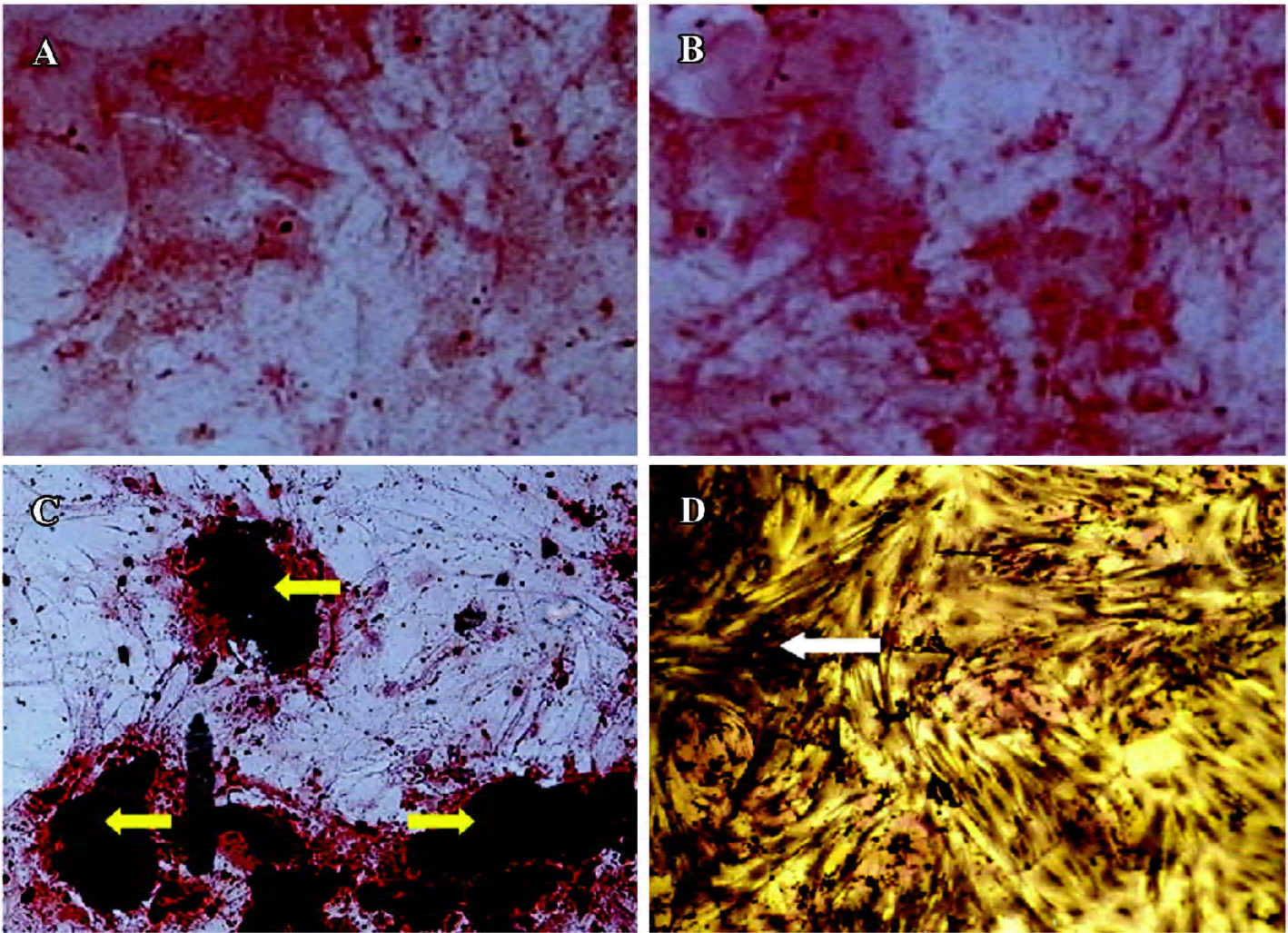
ALP and Chinalizarin staining ALP staining revealed a small number of tiny, brown-black granules in the plasma. The positive stain rate in the 500 cells evaluated was 85% in the hBMP-7 group (Figure 6D), while no such granules were evident in the non-treated and AAV2-EGFP-treated ADMS cells. Fourteen days after transduction, the aggregation of ADMS cells was obvious, and grew to form a calcium nod. Chinalizarin staining produced a red color (Figure 6C), indicative of Chinalizarin and calcium salts. No red compound was found in the non-treated and AAV2-EGFP-treated cells (Figure 6A,6B).
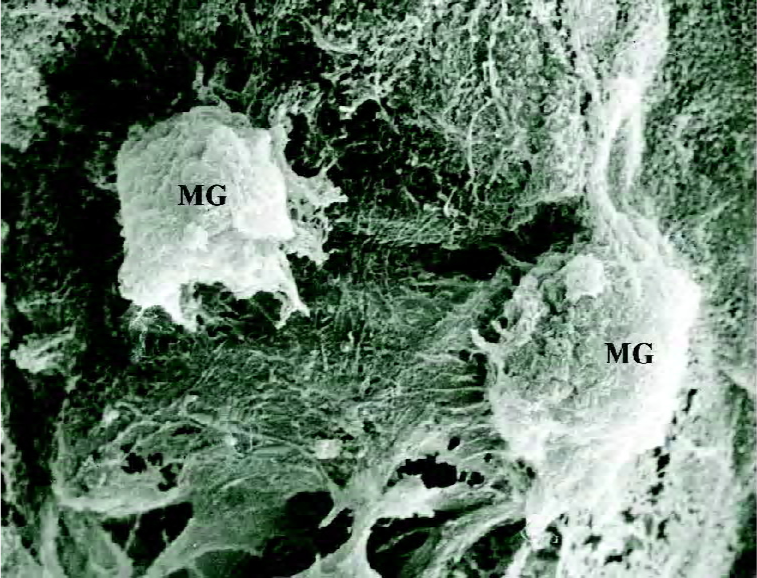
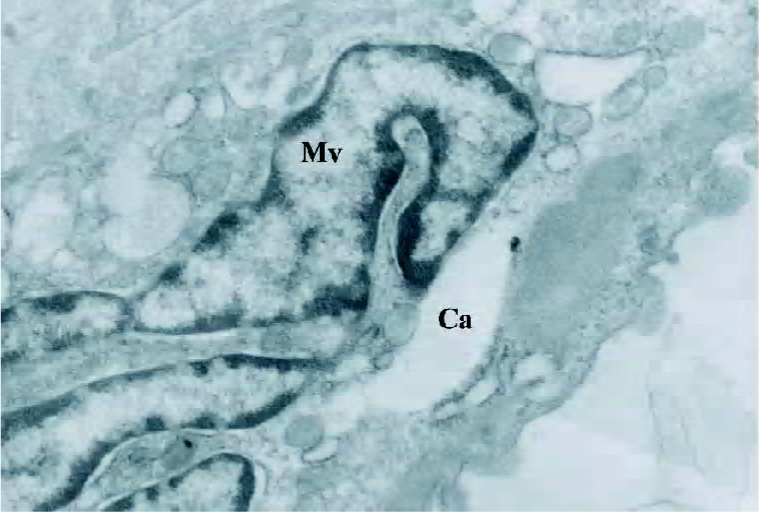
Observation under SEM and transmission electron microscopy Calcium nod of cells were visualized as high-density, irregularly-shaped, dense granules, the central density of which was highest, decreasing gradually towards the circumference (Figure 7). Collagen fibers were observed in the stroma and were lined as a band shape with bubbles of dense corpuscle, which was similar to matrix vesicles; part of matrix vesicle started having deposition of calcium salts (Figure 8).
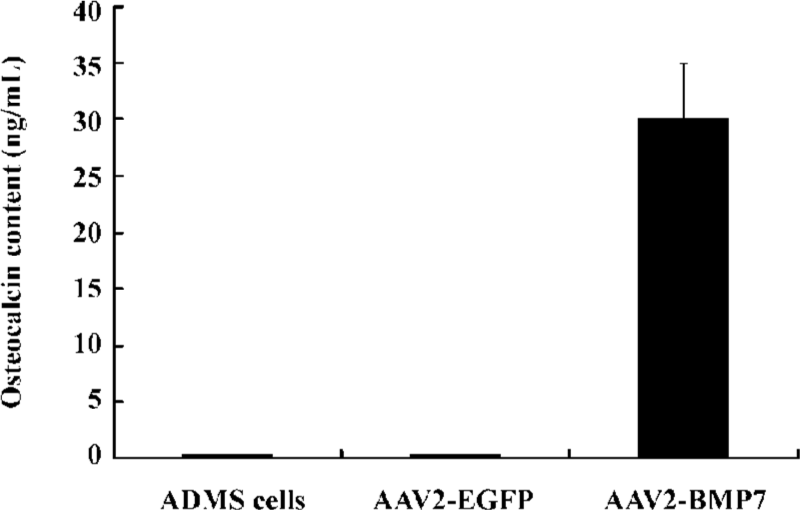
Osteocalcin assay Osteocalcin production was detected at d 14 (30.0±5.0 ng/mL) in the AAV-BMP-7-treated human ADMS cells (Figure 9), but was undetectable in the untreated and AAV-EGFP-treated human ADMS cells (t=23.97, P<0.05).
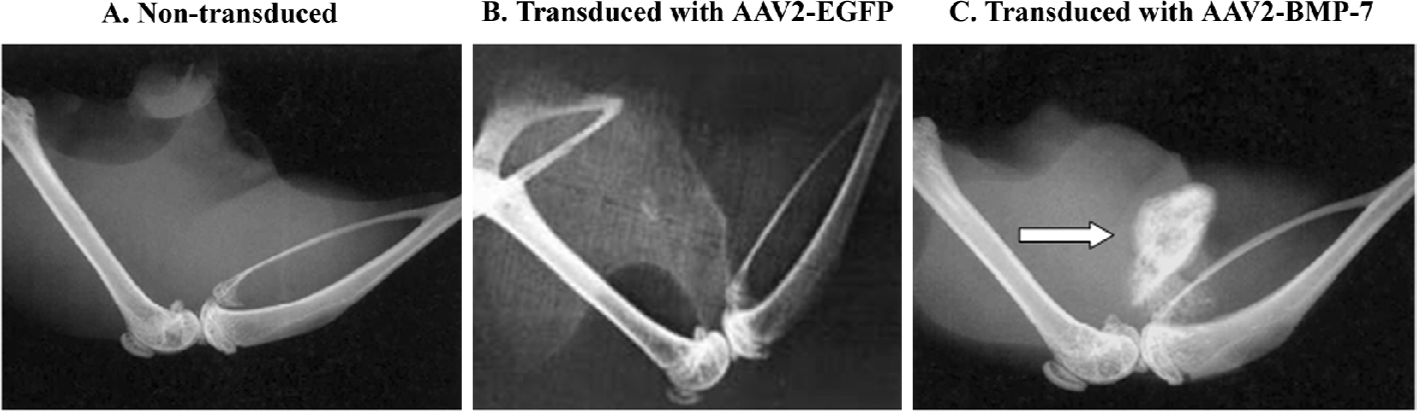
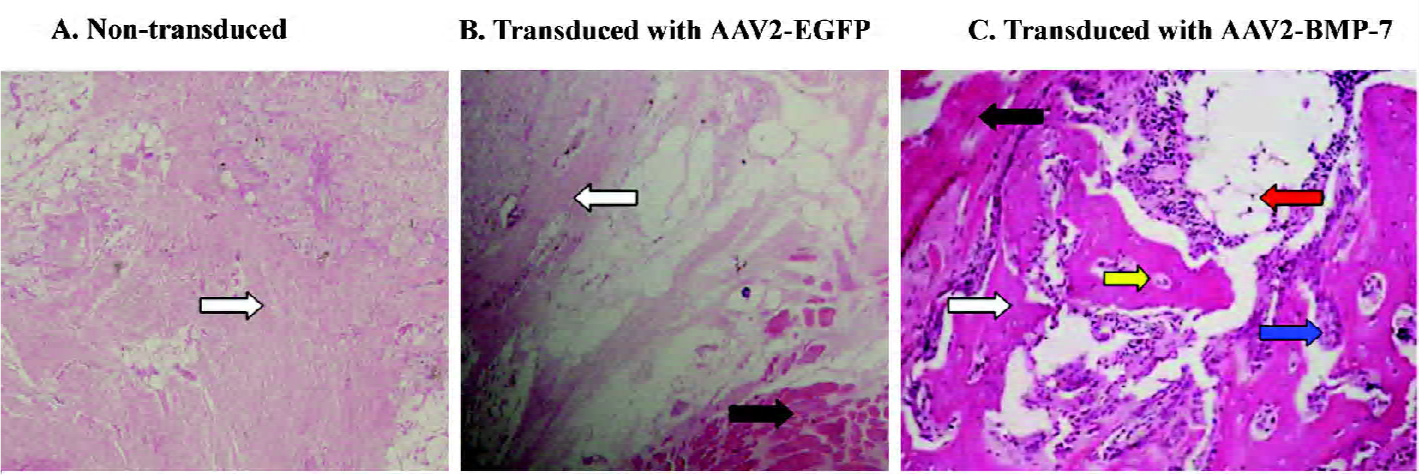
Osteoinductive activity of transduced human ADMS cells We transduced human ADMS cells with AAV2-BMP-7 at a MOI of 1×105, and implanted them into the hind limb of SCID mice to determine the biological activity of genetically-modified cells in terms of ectopic bone formation. Radiographic examination revealed ectopic bone formation in all mice implanted with AAV2-BMP-7-transduced human ADMS cells at 3 weeks after implantation (Figure 10C). A histological examination revealed woven bone with reconstitution of the bone marrow cavity in the muscle pouch 3 weeks after implantation (Figure 11C). No ectopic bone formation was seen in the control mice implanted with the naive ADMS cells (Figures 10A,11A) or AAV2-EGFP-transduced cells (Figures 10B, 11B).
Discussion
The present study demonstrates that the abundant and easily obtained human adipose tissue is an ideal source of autologous mesenchymal stem cells for gene therapy application. Furthermore, AAV2-BMP-7 infects and efficiently induces human ADMS cells to display the differentiated osteoblast phenotype and ectopic bone formation in SCID mice. To the best of our knowledge, this is the first report in the field of AAV2-based BMP-7 gene transfer using human ADMS cells. Ex vivo transduction of human ADMS cells with AAV2-BMP-7 was associated with long-term transgene expression in vitro and the induction of new bone formation in vivo.
Protein therapies are hampered by high manufacturing costs, unpredictable side effects, and the lack of an ideal matrix to deliver proteins in a continuous manner over time[30]. Therefore, both viral- and non-viral-based gene therapies are currently being developed to enhance bone repair by both in vivo and ex vivo strategies[8,11,31–34]. Our goal has been to develop regional gene therapy as 1 aspect of a comprehensive tissue engineering strategy to enhance bone repair. Previous studies have demonstrated that adenoviral gene therapy is an attractive vector for regional gene therapy[35–37], but there are several potential limitations of adenoviral vectors in clinical situations. Although adenoviral vectors infect dividing and non-dividing cells, there is no integration into the host genome, and protein production is limited to 2 weeks in vitro[38]. In addition, there is a marked immune response to the adenovirus by immunocompetent animals, which makes its clinical utility somewhat limited. Recent studies have demonstrated that the AAV vector is ideal for the delivery of therapeutic factors[6,13]. The vector is non-pathogenic, elicits no inflammatory response, can infect dividing and non-dividing cells, and often leads to the efficient, long-term expression of secreted proteins in vivo and in vitro[9,16]. However, whether rAAV2-BMP-7 induces human ADMS cells to display the differentiated osteoblast phenotype in vitro and bone formation in vivo requires further study.
The present results confirm that the AAV2-BMP-7-infected human ADMS cells display the osteoblast differentiated phenotype and can induce new bone formation in vivo. It has been reported that human ADMS cells are pluripotent mesenchymal precursor cells, which are capable of differentiating into myoblasts, adipocytes, and osteoblasts under appropriate stimulation conditions[25]. In this study, we observed that human ADMS cells infected with AAV2-BMP-7 gave rose to 1 terminally-differentiated cell type expressing the markers of osteoblasts and new bone formation in vivo.
We report that the production of BMP-7 protein reaches a peak as late as 1 week after infection, compared with adenovirus-mediated gene delivery in human ADMS cells, in which the desired BMP-7 protein is produced as early as 24 h after infection[39]. This may be due to the fact that the AAV is a single-stranded DNA virus, and there is a rate-limiting step of second-strand DNA synthesis in the nucleus of infected cells. Some in vivo examinations have also demonstrated the same delayed transgene expression[40]. Further-more, we observed that the delayed BMP-7 protein expression did not affect the osteogenic biological function of BMP-7. From a clinical standpoint, it seems that the short period of delayed osteoinductive protein production does not hamper the treatment of relative longer-term cases of fracture healing or spinal fusion. Delayed transgene expression might protect secreted therapeutic proteins from immunologic attack induced by destruction of the vascular barrier at the time of virus injection[41].
Different types of clinical strategies will be necessary to manage bone repair problems based on the extent of bone loss and soft tissue injury. Recombinant proteins may be suitable for small bone defects or primary bone repair scenarios such as spinal fusion. Since AAV are associated with transient BMP production, they may be more successful in the treatment of small to medium-sized defects associated with more adverse biological environments. Finally, the potential for long-term protein expression associated with the AAV vector may be better suited to use in more sophisticated tissue engineering strategies that will be necessary to treat massive bone defects often associated with tumor resection, fracture nonunion, and revision total joint arthroplasty. Others have noted that transduced cells can produce EGFP for several months. Since we do not have satisfactory solutions for severe bone loss problems at this time, the use of AAV gene therapy may be an effective strategy. In addition, for some systemic and metabolic bone diseases such as osteoporosis, the efficient long-term secretion of BMP-7 proteins mediated by AAV is another outstanding advantage. The advantages of direct gene therapy strategy also include relatively simple technique requirements, minimized invasion, and the potential for lower costs.
Direct in vivo application of the recombinant BMP-7 protein can induce the endochondral ossification cascade[42], but its clinical use is severely limited by the lack of an appropriate delivery system to achieve a sustained and localized effect in vivo[30]. Our results demonstrate that a genetically-engineered, cell-based protein delivery system using ADMS cells can generate BMP-7 protein continuously until bone nodules are formed in vitro, and that this sustained BMP-7 delivery platform allows in vivo bone formation. Although we found that heterotopic new bone was induced after transplanting BMP-7-producing ADMS cells, we have no direct evidence to demonstrate whether the implanted cells underwent endochondral bone formation or just exerted their effect as a vehicle delivering BMP-7, inducing new bone formation in primitive pluripotent mesenchymal cells attracted to the sites of implantation. In previous studies, Lee et al[43] used Y-chromosome-specific FISH (fluorescence in situ hybridization)to follow the fate of transplanted cells in vivo. They demonstrated that only 5% of genetically-engineered, original, muscle-derived cells differentiated into osteogenic cells, while a large number of implanted cells enhanced bone healing primarily by delivering BMP-2. Whether the ADMS cells underwent the same fate in our study remains unknown. Further investigation is being performed to determine the early cellular events after transplantation.
One potential disadvantage of AAV vector use in this setting is that prolonged BMP-7 production could lead to heterotopic bone formation. This potential problem will have to be evaluated when the AAV vector is tested in bone repair models. One strategy to avoid continued protein expression is to use a gene-regulated system such as a tetracycline-regulated expression vector[44]. A doxycycline-regulated system has been used to induce bone repair by regulated the BMP expression in 2 different critical-sized defect models[45,46]. In clinical situations when protein expression needs to be tightly regulated, this type of regulated gene expression system would be quite useful.
The mechanism of differentiation of human ADMS cells into osteoblasts remains unclear. Nonetheless, we propose that AAV2–BMP-7 gene therapy using human adipose-derived mesenchymal stem cells represents a novel and feasible approach for treating a variety of orthopedic problems.
Acknowledgement
We thank the AGTC Gene Technology Company for their technical assistance in packaging the AAV vector.
References
- Colterjohn NR, Bednar DA. Procurement of bone graft from the iliac crest: an operative approach with decreased morbidity. J Bone Joint Surg Am 1997;79:756-9.
- Gamradt SC, Lieberman JR. Bone graft for revision hip arthro-plasty: biology and future applications. Clin Orthop Relat Res 2003.183-94.
- Hannallah D, Peterson B, Lieberman JR, Fu FH, Huard J. Gene therapy in orthopaedic surgery. J Bone Joint Surg Am 2002;84A:1046-61.
- Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res 1989;1:267-80.
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science 1988;242:1528-34.
- Philips FM, Turner AS, Seim HB 3rd, MacLeay J, Toth CA, Pierce AR, et al. In vivo BMP-7 (OP-1) enhacement of osteoporotic vertebral bodies in an ovine model. Spine J 2006;6:500-6.
- Sugiyama O, An DS, Kung SPK, Feeley BT, Gamradt S, Liu NQ, et al. Lentivirus-mediated gene transfer induces long-term transgene expression of BMP-2 in vitro and new bone formation in vivo. Mol Ther 2005;11:390-8.
- Pluhar GE, Turner AS, Pierce AR, Toth CA, Wheeler DL. A comparison of two biomaterial carriers for osteogenic protein-1 (BMP-7) in an ovine critical defect model. J Bone Surg Br 2006;88:960-6.
- Abe N, Lee YP, Sato M, Zhang X, Wu J, Mitani K, et al. Enhancement of bone repair with a helper-dependent adenoviral transfer of bone morphogenetic protein-2. Biochem Biophys Res Commun 2002;297:523-7.
- Lee JY, Peng H, Usas A, Musgrave D, Cummins J, Pelinkovic D, et al. Enhancement of bone healing based on ex vivo gene therapy using human muscle-derived cells expressing bone morphogenetic protein 2. Hum Gene Ther 2002;13:1201-11.
- Sugiyama O, Orimo H, Suzuki S, Yamashita K, Ito H, Shimada T, et al. Bone formation following transplantation of genetically modified primary bone marrow stromal cells. J Orthop Res 2003;21:630-7.
- Wilson JM. Adenoviruses as gene-delivery vehicles. N Engl J Med 1996;334:1185-7.
- Anderson WF. Human gene therapy. Nature 1998;392:25-30.
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol 1992;158:97-129.
- Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol 1989;63:3822-8.
- Stiehler M, Duch M, Mygind T, Li H, Ulrich-Vinther M, Modin C, et al. Optimizing viral and non-viral gene transfer methods for genetic modification of porcine mesenchymal stem cells. Adv Exp Med Biol 2006;585:31-48.
- Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther 2000;7:24-30.
- Chen Y, Luk KD, Cheung KM, Xu R, Lin MC, Lu WW, et al. Gene therapy for new bone formation using adeno-associated viral bone morphogenetic protein-2 vectors. Gene Ther 2003;10:1345-53.
- Luk KD, Chen Y, Cheung KM, Kung HF, Lu WW, Leong JC. Adeno-associated virus-mediated bone morphogenetic protein-4 gene therapy for in vivo bone formation. Biochem Biophys Res Commun 2003;308:636-45.
- Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther 2004;9:587-95.
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7:211-28.
- De Ugarte DA, Ashjian PH, Elbarbary A, Hedrick MH. Future of fat as raw material for tissue regeneration. Ann Plast Surg 2003;50:215-9.
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 2001;189:54-63.
- Lee JA, Parrett BM, Conejero JA, Laser J, Chen J, Kogon AJ, et al. Biological alchemy: engineering bone and fat from fat-derived mesenchymal stem cells. Ann Plast Surg 2003;50:610-7.
- Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, et al. Yield of human adipose-derived mesenchymal stem cells from liposuction aspirates. Cytotherapy 2004;6:7-10.
- Ponnazhagan S, Yoder MC, Srivastava A. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J Virol 1997;71:3098-104.
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent mesenchymal stem cells. Mol Biol Cell 2002;13:4279-95.
- Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 2001;189:54-63.
- Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived mesenchymal stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 2005;332:370-9.
- Li R, Wozney J. Delivering on the promise of bone morphogenetic proteins. Trends Biotechnol 2001;19:255-65.
- Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, et al. Bone induction by BMP-2 transduced mesenchymal stem cells derived from human fat. J Orthop Res 2003;21:622-9.
- Kawai M, Bessho K, Kaihara S, Sonobe J, Oda K, Iizuka T, et al. Ectopic bone formation by human bone morphogenetic protein-2 gene transfer to skeletal muscle using transcutaneous electroporation. Hum Gene Ther 2003;14:1547-56.
- Park J, Ries J, Gelse K, Kloss F, von der Mark K, Wiltfang J, et al. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: a comparison of adenoviral vectors and liposomes. Gene Ther 2003;10:1089-98.
- Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, et al. Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther 2007. [Epub ahead of print].
- Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 1999;81:905-17.
- Hidaka C, Goshi K, Rawlins B, Boachie-Adjei O, Crystal RG. Enhancement of spine fusion using combined gene therapy and tissue engineering BMP-7-expressing bone marrow cells and allograft bone. Spine 2003;28:2049-57.
- Wang JC, Kanim LE, Yoo S, Campbell PA, Berk AJ, Lieberman JR. Effect of regional gene therapy with bone morphogenetic protein-2-producing bone marrow cells on spinal fusion in rats. J Bone Joint Surg Am 2003;85:905-11.
- Amalfitano A, Parks RJ. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr Gene Ther 2002;2:111-33.
- Yang M, Ma QJ, Dang GT, Ma K, Chen P, Zhou CY. In vitro and in vivo induction of bone formation based on ex vivo gene therapy using rat adipose-derived mesenchymal stem cells expressing BMP-7. Cytotherapy 2005;7:273-81.
- Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet 2000;24:257-61.
- Aubert D, Pichard V, Durand S, Moullier P, Ferry N. Cytotoxic immune response after retroviral-mediated hepatic gene transfer in rat does not preclude expression from adeno-associated virus 1 transduced muscles. Hum Gene Ther 2003;14:473-81.
- Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res 1996;222:38-47.
- Lee JY, Musgrave D, Pelinkovic D, Fukushima K, Cummins J, Usas A, et al. Effect of bone morphogenetic protein-2-expressing muscle-derived cells on healing of critical-sized bone defects in mice. J Bone Joint Surg Am 2001;83:1032-9.
- Noel D, Gazit D, Bouquet C, Apparailly F, Bony C, Plence P, Millet V, et al. Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal mesenchymal stem cells. Mesenchymal Stem Cells 2004;22:74-85.
- Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, et al. Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther 2001;3:449-61.
- Peng H, Usas A, Gearhart B, Young B, Olshanski A, Huard J, et al. Development of a self-inactivating Tet-On retroviral vector expressing bone morphogenetic protein 4 to achieve regulated bone formation. Mol Ther 2004;9:885-94.