Association of KCNJ11 and ABCC8 genetic polymorphisms with response to repaglinide in Chinese diabetic patients1
Introduction
It is well known that there is a large variation in patients’ response to a given therapy. Factors, such as age, sex, and genetic composition, can play an important role[1]. The effect of genetic polymorphism on drug response has been described in a recent study on sulfonylurea glibenclamide[2]. Patients with the KCNJ11 E23K variant in the K allele were more susceptible to secondary failure to sulphonylurea, which leads to higher fasting plasma glucose and glycosylated hemoglobin concentrations[2]. However, a similar study on non-sulfonylurea has not been reported.
As a novel, short-acting insulin secretagogue, non-sulfonylurea, such as repaglinide, lowers the plasma glucose concentrations in patients with type 2 diabetes by enhancing glucose-stimulated insulin release from the pancreatic beta cells[3–5]. ATP-sensitive potassium (KATP) channels are required by both non-sulfonylurea and sulfonylurea in the stimulation of insulin secretion in pancreatic beta cells, although they act on different binding sites in the channel protein[6]. In vitro studies have shown that repaglinide action is dependent on pancreatic beta cell KATP channels[7,8]. The inward rectifier potassium channel Kir6.2 (encoded by KCNJ11) and sulfonylurea receptor SUR1 (encoded by ABCC8) are 2 subunits in the channel protein[9]. SUR1 carries the high-affinity binding sites for repaglinide[7]. Variants in the E23K of KCNJ11 gene (rs5219) and exon16–3T/C of ABCC8 gene (rs1799854) are associated with type 2 diabetes in populations with distinct ethnic backgrounds[10,11]. Whether these 2 variants influence the therapeutic efficacy of repaglinide has not been reported. Therefore, we addressed this question in this study.
In our study, we analyzed gene polymorphisms in 100 patients with type 2 diabetes who were treated with repaglinide. The aim of this study was to investigate the association of KCNJ11 E23K and ABCC8 exon16–3T/C with therapeutic effects of repaglinide, a representative of non-sulfonylurea.
Materials and methods
Patients and study design A total of 104 newly diagnosed type 2 diabetic patients were recruited from the outpatient clinics at 10 hospitals in Shanghai, China. The study was approved by the institutional review board of Shanghai Jiaotong University Affiliated Sixth People’s Hospital, Shanghai, China. Written informed consent was obtained from each patient. Type 2 diabetes mellitus was diagnosed according to the World Health Organization criteria in 1999[12]. One hundred patients (66 men and 34 women, average age 52 years) completed the 24 week repaglinide treatment. Four patients withdrew from the study due to high hemoglobin A1c (HbA1c) values (>8%). Patients were between 30 and 70 years old. No prior antihyperglycemic therapies were allowed before the study. The entry HbA1c value was >6.5%. The exclusion criteria were as follows: (1) allergic to repaglinide; (2) type 1 diabetes mellitus; gestational diabetes mellitus or other specific type of diabetes mellitus; (3) clinical hepatic or renal impairment; (4) cardiac abnormalities(unstable angina pectoris, myocardial infarction or heart failure of New York Heart Association (NYHA) stage III–V; (5) any history of diabetic ketoacidosis or non-ketotic hyperosmolar coma or chronic diabetic com-plication; (6) fasting plasma glucose (FPG) >13 mmol/L (234 mg/dL) and/or 2 h postprandial plasma glucose (2hPG) >18 mmol/L (324 mg/dL); (7) uncontrolled hypertension (systolic blood pressure >180 mmHg and/or diastolic blood pressure >110 mmHg); (8) use of medications that could influence glucose metabolism; (9) infected with hepatitis virus; and (10) malignant diseases, hematological diseases, psychosis, autoimmune diseases, significant digestion and resorption disturbances, acute cerebrovascular accidents within the last 6 months.
After a 2 week run-in period (diet and exercise therapy only), all patients were treated with repaglinide for 24 weeks at an initial mealtime dosage of 0.5 mg. The dosage was increased stepwise to 1, 1.5, and 2 mg until it reached the target of FPG <=7 mmol/L (126 mg/dL) and/or 2hPG <=11 mmol/L (200 mg/dL). Patients with FPG >13 mmol/L (234 mg/dL) or 2hPG >18 mmol/L (324 mg/dL) or HbA1c>=8% at 2 consecutive times (a maximal interval of 6 d) were excluded from the study. Arginine stimulation tests were performed on all the patients at the beginning of the run-in period (–2 week) and 24 weeks after repaglinide treatment in overnight-fasted states (12 h fast). At time 0, arginine hydrochloride (10% arginine hydrochloride of 50 mL, 5 g) was injected intravenously within 30–60 s. Blood samples were drawn at times 2, 4, and 6 min after injection[13]. The acute insulin response to arginine (AIR-Arg, in mU/L) was calculated as the mean insulin value of 2, 4, and 6 min samples minus fasting insulin concentration. Height (in m) and weight (in kg) were measured, and body mass index (BMI; in kg/m2) was calculated.
Clinical laboratory tests Serum insulin concentrations were measured by a radioimmunoassay kit (Diagnostic Systems Laboratories, Upper Heyford, UK). Plasma glucose concentrations were measured by a glucose oxidase–peroxidase assay kit (Shanghai Biological Products Institution, Shanghai, China). HbA1c values were measured by high-performance liquid chromatography method (Bio-Rad Laboratories, Hercules, CA, USA). Insulin resistance and beta cell function were calculated with the following formulas: homeostasis model assessment of insulin resistance (HOMA-IR)=fasting insulin concentrations (FIN; in mU/L)×FPG (in mmol/L)/22.5; homeostasis model assessment beta cell function (HOMA-β)=20×FIN/(FPG-3.5)[14]. The change of each parameter (Δ value) was calculated as: Δ value=baseline (T0) value–T24weeks value.
Genotyping Genomic DNA was extracted from peripheral blood and amplified by PCR. The DNA segment containing the exon16–3 T/C variant (rs1799854) in ABCC8 was amplified with the forward primer: 5'-CTT TCT GGG TAA TGG TTG TTC AGA C-3' and reverse primer: 5'- AAG GAG ATT TCC CCT CCA CTG G-3'. The PCR products were digested with PstI (MBI Fermentas, Glen Burnie, MD, USA) at 37 °C for 3 h. The DNA segment containing the E23K variant (rs5229) in KCNJ11 was amplified with the forward primer: 5'-GAC TCT GCA GTG AGG CCC TA-3'; and reverse primer: 5'-ACG TTG CAG TTG CCT TTC TT-3'. The PCR products were digested with BanII (New England Biolabs, Beverly, MA, USA) at 37 °C for 4 h. Twenty percent of the samples were duplicated to confirm the genotyping accuracy. No discrepancy was detected in the current study.
Definition of response to repaglinide As there were no satisfactory criteria for assessing the response rate to repaglinide, we summarized the current study and all the other similar clinical trials on repaglinide treatment in antidiabetic, drug-naive patients to find a proper definition of the response to repaglinide. FPG levels and HbA1c values were reduced by 28% and 23%, respectively, in our study. Other similar studies reported a decline of FPG by 18%−30% and HbA1c by 15%−30%, respectively, with the mean value of 22% for FPG and 19% for HbA1c[15-19]. As a result, responders were defined by a greater than 25% decrease in FPG or a greater than 20% decrease in HbA1c values (or both) after the 24 week repaglinide treatment in our study.
Statistical analysis A Hardy–Weinberg equilibrium test was performed. Normal distributed data were shown as mean±SD. For non-normal distributed variables, data were shown in median (25% percentile and 75% percentile). Comparisons between baseline and 24 weeks after repaglinide treatment were conducted with paired t-test or signed rank–sum test. Differences between genotypes were analyzed using ANOVA or Kruskal–Wallis test for 3 genotypes when appropriate. Student–Newman–Keuls or Nemenyi test multiple comparisons were made to check which of the 2 groups were significant. χ2-test or Fisher’s exact test were applied to detect the genotype distribution difference between responders and non-responders. Logistic regression and multiple stepwise regression analyses were used respectively to test the major determinants (age, sex, BMI at baseline, FIN at baseline, drug dosage, and the 2 variants) of repaglinide response rate and the decrease of HbA1c (ΔHbA1c). The odds ratio (OR) value was presented with 95% confidence interval (CI). Statistical significance was considered when P<0.05 (two tailed). All calculations and statistics were performed using SAS for Windows (version 6.12; SAS Institute, Cary, NC, USA).
Results
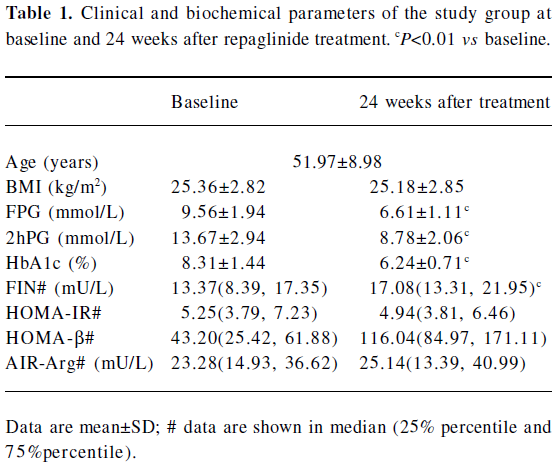
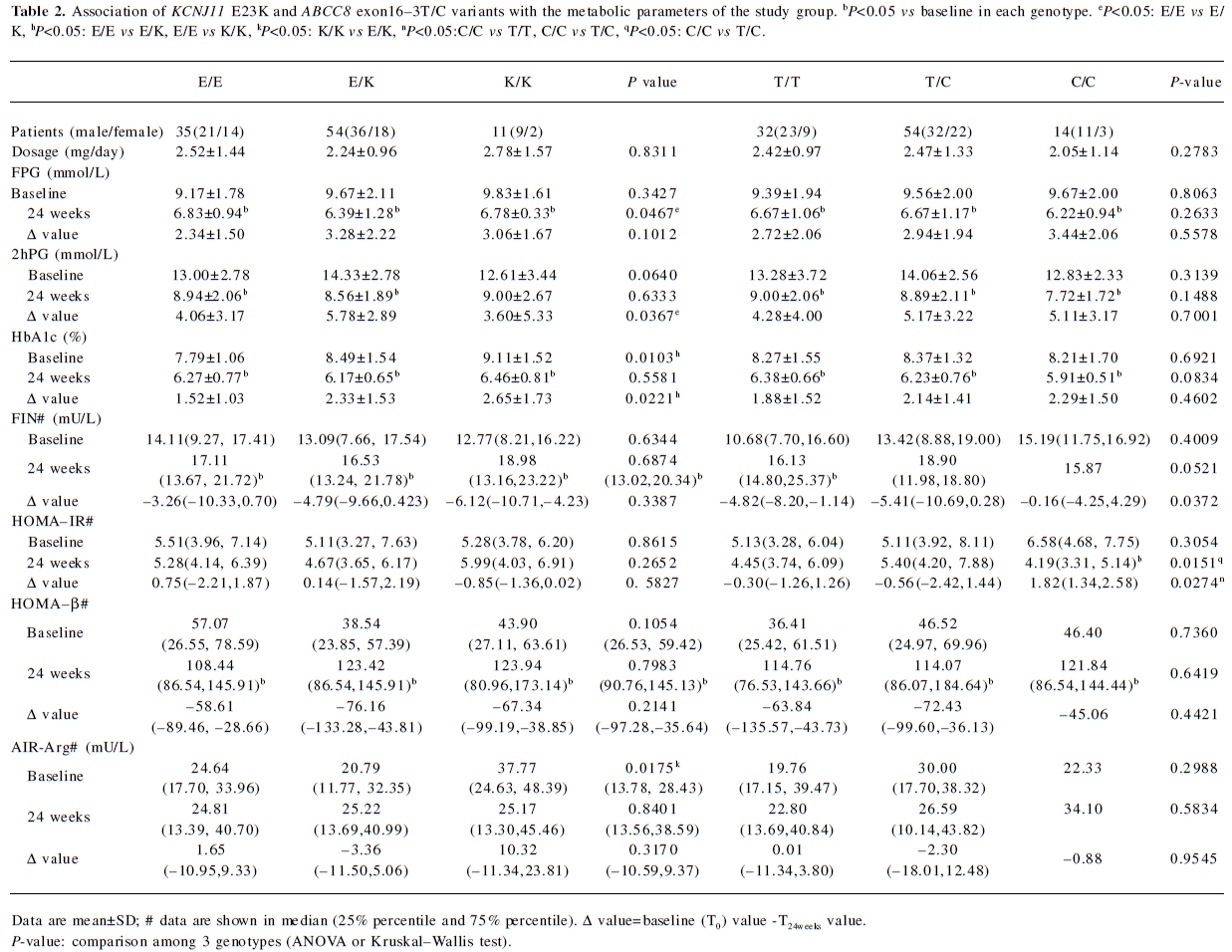
Clinical character of the patients before and after repaglinide treatment The baseline (T0) and end-point (T24week) characteristics of the 100 patients who finished the study are shown in Table 1. The genotype distributions of KCNJ11 E23K and ABCC8 exon16–3T/C variants in the 100 patients are shown in Table 2. All observed genotypes were in the Hardy–Weinberg equilibrium and similar to what was found in other studies[10,20–22]. The 4 patients who withdrew the study had the ABCC8 exon16–3T/C genotype. As for KCNJ11 E23K variant, 1 had the KCNJ11 E/E genotype, 1 had the E/K genotype, and 2 had the K/K genotype.
Full table
Full table
After 24 weeks of treatment with repaglinide, the FPG concentration and HbA1c value were reduced by 28% and 23%, respectively, in the 100 patients (data not shown). HbA1c, FPG, and 2hPG decreased, while FIN and HOMA-β increased significantly compared with baseline for the 100 patients and every genotype of the 2 variants, except for 2hPG in the KCNJ11 K/K homozygotes and FIN in the ABCC8 exon16–3C/C homozygotes. HOMA-IR decreased only in the ABCC8 exon16–3C/C homozygotes (Tables 1,2).
Effect of KCNJ11 and ABCC8 genetic polymorphisms on clinical characters For the KCNJ11 E23K variant, both baseline HbA1c and ΔHbA1c were significantly higher in patients with E/K and K/K genotypes when compared with E/E homozygotes (P=0.0103 and 0.0221, respectively; Table 2). The KCNJ11 E23K variant is the major confounding factors for ΔHbA1c (P=0.0086) as that was found in multiple stepwise regression. Meanwhile, the decrease in 2hPG (Δ2hPG) was significantly greater in E/K carriers than those with E/E homozygotes (P=0.0367). FPG was lower in patients with E/E homozygotes than E/K heterozygotes after the 24 week treatment with repaglinide. No significant difference of change in FPG was found among 3 genotypes until E/K heterozygotes and K/K homozygotes were combined as 1 group (E/E vs E/K+K/K, P=0.0340, data not shown). The baseline AIR-Arg was significantly higher in patients with K/K homozygotes than those with E/K heterozygotes (P=0.0175).
For the ABCC8 exon16–3 T/C variant, the increase of fasting insulin secretion was greater in T/T homozygotes and T/C heterozygotes than patients with C/C homozygotes (P=0.0372). HOMA-IR decreased in C/C homozygotes, while it increased in T/C and T/T genotypes, with a significant change between patients carrying C/C homozygotes and those with T/T and T/C genotypes. (P=0.0274; Table 2).
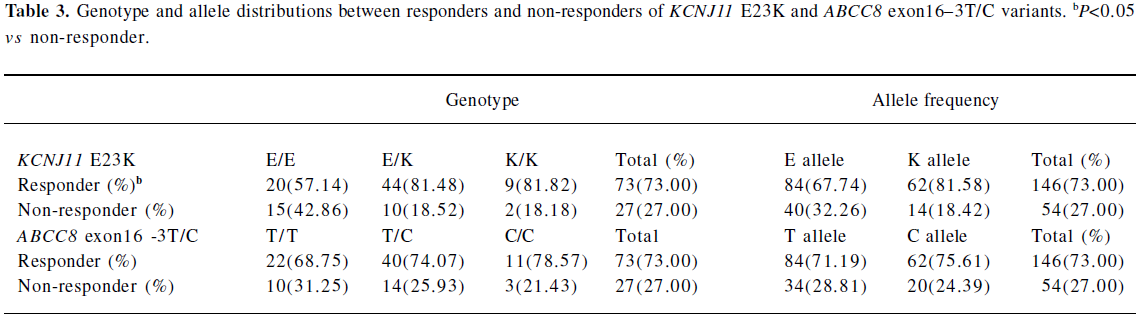
Association of KCNJ11 and ABCC8 genetic polymorphisms with response rate to repaglinide treatment According to predetermined criteria, the total response rate to repaglinide in our study was 73%. There was a significant difference in the response rate to repaglinide treatment in the KCNJ11 E23K variant (E allele 68% vs K allele 82%, P=0.0324). Approximately 43% of the E/E homozygotes failed compared with 19% in E/K heterozygotes and 18% in K/K homozygotes (P=0.0364; Table 3). No significant effect was detected for the ABCC8 exon16–3T/C variant on repaglinide treatment. Age, sex, BMI at baseline, FIN at baseline, drug dosage, and the 2 variants were identified in logistic regression as predominant confounding factors for the response rate to repaglinide treatment. The KCNJ11 E23K variant was the only confounding factor. Compared with E/E homozygotes, the OR for treatment failure was 0.232 for E/K and K/K genotypes carriers (EK+KK, 95% CI: 0.080–0.669, P=0.0143).
Full table
Discussion
This study represents the first investigation of KCNJ11 E23K and ABCC8 exon16–3T/C in pharmacogenomics of non-sulfonylurea repaglinide in patients with type 2 diabetes. Variants at KCNJ11 E23K and ABCC8 exon16–3T/C are common polymorphisms associated with type 2 diabetes[11,23,24]. The E23K variant is the most extensively studied KCNJ11 polymorphism in type 2 diabetes[23,25]. The association of other polymorphisms with type 2 diabetes has been unclear until now. In a study in Chinese Han populations, 6 single nucleotide polymorphisms were analyzed, and it was found that the E23K polymorphism was related to type 2 diabetes[21]. An in vitro study showed that the K allele of the KCNJ11 E23K variant caused a reduction in KATP channel sensitivity to ATP[26] and has been shown to be related to the secondary failure to sulfonylurea in a recent pharmacogenomics study[2]. In addition, 3 variants of the ABCC8 gene were studied in a Japanese population of 2834 patients[10]. Among the 3 variants, only exon16–3T/C was related to type 2 diabetes. The exon16–3T/C variant was associated with beta cell dysfunction, and tested in a pharmacogenomics study of sulfonylurea[27,28]. The association of KCNJ11 E23K, ABCC8 exon16–3T/C variants with response to sulfonylurea has been reported in type 2 diabetic patients. This type of study has not been reported for repaglinide. Therefore, we chose KCNJ11 E23K and ABCC8 exon16–3T/C as the candidate variants in the current study.
KCNJ11 E23K variant This study not only supports the association of the KCNJ11 E23K variant with the baseline HbA1c concentration[2], but also provides evidence for the association of the KCNJ11 E23K variant with the therapeutic efficacy of repaglinide. Previous studies demonstrated that the K allele of the KCNJ11 E23K variant was associated with the impairment of beta cell function[24,26]. This association was also observed in the current study. In addition, diabetic patients with K/K and E/K genotypes responded better to repaglinide therapy. In these patients, the HbA1c concentration and 2hPG level were reduced significantly compared with E/E homozygotes. This point is supported by results of logistic and multiple regression analyses. Our result suggests that patients with E/K and K/K genotypes might have a better response to repaglinide in glucose-stimulated insulin secretion. This conclusion is supported by beta cell function in the K/K homozygote as indicated by a high level of AIR-Arg. Further study is required to understand the potential mechanism.
ABCC8 exon16 -3T/C variant Our study suggests that insulin sensitivity was improved by repaglinide in type 2 diabetic patients with the C/C homozygote. This effect was not observed in the other 2 genotypes, C/T and T/T. This possibility is supported by other studies in which repaglinide was shown to improve insulin sensitivity in patients and reduce fasting free fatty acids[29,30]. In our study, HOMA-IR was reduced in patients with the C/C homozygote after repaglinide treatment for 24 weeks, suggesting improvement of insulin sensitivity. This change was associated with less increase in the FIN level in C/C homozygotes. A previous study elucidated the association of the ABCC8 exon16–3T/C variant with insulin sensitivity and insulin secretion[28,31]. However, the effect of ABCC8 exon16–3T/C variants on the therapeutic efficacy of repaglinide has not been reported. This calls for functional research on the polymorphisms to explain it theoretically in addition to the population study.
There were several limitations in our study. First, the sample size was relatively small. In this study, only 104 patients were recruited based on our criteria for newly diagnosed and untreated type 2 diabetic patients, with FPG <=13 mmol/L (234 mg/dL) or 2hPG <=18 mmol/L (324 mg/dL) or HbA1c <8%, thus we did not have enough statistical power to detect the effect of genetic variants on some of the parameters. For example, the significant difference of ΔFPG was not detected until E/K heterozygotes and K/K homozygotes of the KCNJ11 E23K variant were combined together as 1 group. We can not exclude the influence of the sample size. Second, due to the limitation of the oral antidiabetic therapy, our study was conducted in patients with FPG <=13 mmol/L (234 mg/dL) or 2hPG <=18 mmol/L (324 mg/dL) or HbA1c <8%. Whether this result reflects the efficacy of repaglinide in patients with higher blood glucose remains to be investigated.
In conclusion, the present study demonstrates that the KCNJ11 E23K variant was associated with the therapeutic effect of repaglinide treatment. Patients with E/K and K/K genotypes of the KCNJ11 E23K variant showed a higher baseline HbA1c, but exhibited a better response to repaglinide. The C/C homozygotes of the ABCC8 exon16–3T/C variant responded better to repaglinide in insulin sensitivity than the T/C and T/T genotypes. Our study is at an early stage of pharmacogenomics investigation on repaglinide. Further studies with large samples are needed to confirm our findings.
Acknowledgement
We thank all the patients who participated in this study and are appreciative of the doctors and nurses from Shanghai Jiaotong University Affiliated Sixth People’s Hospital, First People’s Hospital, Renji Hospital, Ruijin Hospital, Xinhua Hospital, Fudan University Affiliated Zhongshan Hospital, Huashan Hospital, Second Military Medical University Affiliated Changzheng Hospital, Changhai Hospital, and Tongji University Affiliated East Hospital, We thank Dr Jian-ping YE from Louisiana State University for his help in revision of this manuscript.
References
- Hatorp V. Clinical pharmacokinetics and pharmacodynamics of repaglinide. Clin Pharmacokinet 2002;41:471-83.
- Sesti G, Laratta E, Cardellini M, Andreozzi F, Del Guerra S, Irace C, et al. The E23K variant of KCNJ11 encoding the pancreatic beta-cell adenosine 5'-triphosphate-sensitive potassium channel subunit Kir6.2 is associated with an increased risk of secondary failure to sulfonylurea in patients with type 2 diabetes. J Clin Endocrinol Metab 2006;91:2334-9.
- Grell W, Hurnaus R, Griss G, Sauter R, Rupprecht E, Mark M, et al. Repaglinide and related hypoglycemic benzoic acid derivatives. J Med Chem 1998;41:5219-46.
- Rizzo MR, Barbieri M, Grella R, Passariello N, Barone M, Paolisso G. Repaglinide is more efficient than glimepiride on insulin secretion and post-prandial glucose excursions in patients with type 2 diabetes. A short term study. Diabetes Metab 2004;30:81-9.
- Owens DR, Luzio SD, Ismail I, Bayer T. Increased prandial insulin secretion after administration of a single preprandial oral dose of repaglinide in patients with type 2 diabetes. Diabetes Care 2000;23:518-23.
- Gromada J, Dissing S, Kofod H, Frokjaer-Jensen J. Effects of the hypoglycaemic drugs repaglinide and glibenclamide on ATP-sensitive potassium-channels and cytosolic calcium levels in beta TC3 cells and rat pancreatic beta cells. Diabetologia 1995;38:1025-32.
- Hansen AM, Hansen JB, Carr RD, Ashcroft FM, Wahl P. Kir6.2-dependent high-affinity repaglinide binding to beta-cell K(ATP) channels. Br J Pharmacol 2005;144:551-7.
- Dabrowski M, Wahl P, Holmes WE, Ashcroft FM. Effect of repaglinide on cloned beta cell, cardiac and smooth muscle types of ATP-sensitive potassium channels. Diabetologia 2001;44:747-56.
- Seino S. ATP-sensitive potassium channels: a model of heteromul-timeric potassium channel/receptor assemblies. Annu Rev Physiol 1999;61:337-62.
- Yokoi N, Kanamori M, Horikawa Y, Takeda J, Sanke T, Furuta H, et al. Association studies of variants in the genes involved in pancreatic beta-cell function in type 2 diabetes in Japanese subjects. Diabetes 2006;55:2379-86.
- Laukkanen O, Pihlajamaki J, Lindstrom J, Eriksson J, Valle T, Hamalainen H, et al. Polymorphisms of the SUR1 (ABCC8) and Kir6.2 (KCNJ11) genes predict the conversion from impaired glucose tolerance to type 2 diabetes. The Finnish Diabetes Prevention Study. J Clin Endocrinol Metab 2004;89:6286-90.
- Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 1999;16:442-3.
- Larsson H, Ahren B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 1998;41:772-7.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9.
- Marbury T, Huang WC, Strange P, Lebovitz H. Repaglinide versus glyburide: a one-year comparison trial. Diabetes Res Clin Pract 1999;43:155-66.
- Jovanovic L, Dailey G 3rd, Huang WC, Strange P, Goldstein BJ. Repaglinide in type 2 diabetes: a 24-week, fixed-dose efficacy and safety study. J Clin Pharmacol 2000;40:49-57.
- Gerstein HC, Garon J, Joyce C, Rolfe A, Walter CM. Pre-prandial vspost-prandial capillary glucose measurements as targets for repaglinide dose titration in people with diet-treated or metformin-treated Type 2 diabetes: a randomized controlled clinical trial. Diabet Med 2004;21:1200-3.
- Van Gaal LF, Van Acker KL, De Leeuw IH. Repaglinide improves blood glucose control in sulphonylurea-naive type 2 diabetes. Diabetes Res Clin Pract 2001;53:141-8.
- Moses RG, Gomis R, Frandsen KB, Schlienger JL, Dedov I. Flexible meal-related dosing with repaglinide facilitates glycemic control in therapy-naive type 2 diabetes. Diabetes Care 2001;24:11-5.
- Li J, Tian H, Li Q, Wang N, Wu T, Liu Y, et al. Improvement of insulin sensitivity and beta-cell function by nateglinide and repaglinide in type 2 diabetic patients - a randomized controlled double-blind and double-dummy multicentre clinical trial. Diabetes Obes Metab 2007;9:558-65.
- Liu Z, Zhang YW, Feng QP, Li YF, Wu GD, Zuo J, et al. Association analysis of 30 type 2 diabetes candidate genes in Chinese Han population. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2006;28:124-8.
- Ji L, Han X, Wang H. Sulfonylurea receptor gene polymorphism is associated with non-insulin dependent diabetes mellitus in Chinese population. Zhonghua Yi Xue Za Zhi 1998;78:774-5. (in Chinese).
- Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 2003;52:568-72.
- Nielsen EM, Hansen L, Carstensen B, Echwald SM, Drivsholm T, Glumer C, et al. The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes 2003;52:573-7.
- Riedel MJ, Steckley DC, Light PE. Current status of the E23K Kir6.2 polymorphism: implications for type-2 diabetes. Hum Genet 2005;116:133-45.
- Schwanstecher C, Meyer U, Schwanstecher M. K. (IR)6.2 polymorphism predisposes to type 2 diabetes by inducing overactivity of pancreatic beta-cell ATP-sensitive K(+) channels. Diabetes 2002;51:875-9.
- Zychma MJ, Gumprecht J, Strojek K, Grzeszczak W, Moczulski D, Trautsolt W, et al. Sulfonylurea receptor gene 16-3 polymorphism-association with sulfonylurea or insulin treatment in type 2 diabetic subjects. Med Sci Monit 2002;8:512-5.
- Hart LM, Dekker JM, van Haeften TW, Ruige JB, Stehouwer CD, Erkelens DW, et al. Reduced second phase insulin secretion in carriers of a sulphonylurea receptor gene variant associating with Type II diabetes mellitus. Diabetologia 2000;43:515-9.
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest 2002;32 Suppl 3:14-23.
- Rizzo MR, Barbieri M, Grella R, Passariello N, Paolisso G. Repaglinide has more beneficial effect on cardiovascular risk factors than glimepiride: data from meal-test study. Diabetes Metab 2005;31:255-60.
- Elbein SC, Sun J, Scroggin E, Teng K, Hasstedt SJ. Role of common sequence variants in insulin secretion in familial type 2 diabetic kindreds: the sulfonylurea receptor, glucokinase, and hepatocyte nuclear factor 1alpha genes. Diabetes Care 2001;24:472-8.
