Internal ribosome entry site of Rhopalosiphum padi virus is functional in mammalian cells and has cryptic promoter activity in baculovirus-infected Sf21 cells1
Introduction
Translation initiation in eukaryotes is governed either by a cap structure-dependent mechanism or a cap-independent mechanism through an internal RNA element termed the internal ribosomal entry site (IRES). IRES elements were first discovered in the RNA of the virus family Picornaviridae, and have a long, highly-structured 5´untranslated region (5´UTR) that lacks a cap structure at the 5´ end[1,2]. In these viruses, translation is driven by the complex RNA secondary structures in the IRES within their 5´UTR, which confer cap-independent translation. Previous studies have shown that IRES elements are not restricted to picornaviruses, but are also found in retroviruses and DNA viruses; for example, HIV and herpes simplex viruses were reported to contain an IRES element in their genomes[3]. Furthermore, IRES elements have also been found in the 5´UTR of several cellular mRNA. Importantly, IRES-dependent translation has been reported for cellular mRNA when their cap-dependent translation is impaired (eg under conditions of apoptosis, heat shock stress, and viral infection, and at the G2/M phase of the cell cycle)[4–6]. In addition, some studies have suggested that the IRES may increase translation efficiency at postsynaptic sites after synaptic activation[7].
In addition to disclosing the unusual translational mechanism, IRES have been successfully introduced between 2 cistrons to construct bicistronic vectors. For example, it was demonstrated that the simultaneous co-expression of 2 proteins, one being a selection marker or a reporter gene and the other the gene of interest, by IRES-based bicistronic expression vectors is important for biotechnological applications[8,9]. Thus identifying and developing useful IRES elements for expression vectors have become an important issue in biotechnology. Recently, many very unusual IRESs have been found in the invertebrate viral family, Dicistroviridae. The family Dicistroviridae (genus Cripavirus) was formerly known as cricket paralysis virus-like viruses[10]. Dicistroviruses have been detected in a range of insects, from aphids and fire ants to Drosophila[11]. The viruses in this family share physicochemical properties with members of the Picornaviridae[12] and possess a large, single-stranded, positive-sense RNA genome (approximately 9–10 kb) with 2 open reading frames (ORF) that encode 2 polyproteins separated by an intergenic region (IGR). It has been shown that the 5´UTR as well as the IGR of these dicistroviruses contain IRES elements[13–16]. One of the recently described IRES in the dicistroviruses, the 579 nucleotide (nt)-long 5´UTR of Rhopalosiphum padi virus (RhPV), possesses cross-kingdom IRES activity that functions efficiently in mammalian-, plant-, and insect-derived in vitro translation systems[16,17], although RhPV infection is restricted to aphid species[14]. This cross-kingdom RhPV IRES therefore has the potential for utilization in eukaryotic cell expression vectors.
In this study, we showed that the RhPV IRES can mediate bicistronic gene expression in mammalian cells either by plasmid transfection or recombinant baculovirus transduction. Unexpectedly, we found that the RhPV IRES possesses cryptic promoter activity in baculovirus-infected Sf21 cells, but not in mammalian cells. The cryptic promoter activity may be explained by the presence of six TAAG motifs in the RhPV IRES, which could be recognized as baculovirus late transcription initiation sequences[18].
Materials and methods
Cells The Spodoptera frugiperda IPBL-Sf21 (Sf21) cell line was cultured in TNM-FH insect medium containing 8% heat-inactivated fetal bovine serum. CHO-K1 (Chinese hamster ovary cells), COS1 (an African green monkey kidney fibroblast-like cell line), and HeLa (a human cervix carcinoma cell line) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St Louis, MO, USA) containing 10% fetal bovine serum. HepG2 (human hepatocytes) and MDCK (a Canis familiaris synonymous kidney cell line) were maintained in minimum essential medium (MEM; Sigma, USA) supplemented with 10% fetal bovine serum. U2OS (a human osteogenic sarcoma cell line) cells were grown in McCoy’s medium supplemented with 10% fetal bovine serum.
Construction of plasmids DNA was prepared, and manipulations were performed using standard methods as described by Sambrook et al[19] or by the manufacturers of the reagents used. To construct a vector with dual fluorescent protein genes to monitor the RhPV IRES activity in mammalian cells, we first digested the pBacDRhirE plasmid[20] with NheI and SalI and subcloned the 2.6 kb DsRed-IRES–enhanced green fluorescent protein gene (EGFP) DNA fragment into a NheI- and Sal I-digested pEGFP-C1 plasmid (ClonTech, Mountain View, CA, USA). The resulting plasmid was named pCMV-DRhirE (Figure 1C). In addition, this 2.6 kb DsRed-IRES–EGFP DNA fragment was also subcloned into a SpeI- and XhoI-digested pIB vector (Invitrogen, Carlsbad, CA, USA) to construct a baculovirus-independent, insect cell transient expression vector, pIE2-DRhirE (Figure 1H). A positive control plasmid containing the DsRed-EGFP fusion gene was generated through the digestion of the plasmid pDsRed1-N1 (ClonTech, USA) with NheI and EcoRI to release the DsRed gene fragment and then cloned into the NheI and EcoRI sites of the pEGFP-C1 plasmid (ClonTech, USA). For this ligation product, named pD-E/O (Figure 1B), the DsRed gene was not fused with the EGFP gene; we first digested pD-E/O with EcoRI and then treated it with mung bean nuclease (New England Biolab, NEB) before the ligation reaction. A construct in which DsRed and EGFP were fused in frame was named pD-E (Figure 1A). In addition, the polyhedrin promoter in the pBac-DRhirE plasmid was replaced with a human cytomegalovirus (CMV) immediate early promoter, and the resulting plasmid was named pCMV-DRhirE. To construct pCMV-DRhirE, the CMV promoter was first amplified from the pEGFP-C1 plasmid by PCR with the forward primer, 5´-GCC
To facilitate the quantification of RhPV IRES translational activity in mammalian cells, we fused the secretory alkaline phosphatase (SEAP) with the EGFP gene in the pCMV-DRhirE (Figure 1C) and generated the plasmid, pCMV-DRhirSE (Figure 1D). Briefly, the SEAP gene fragment was first amplified from the pGS-HCV plasmid[21] by PCR with the forward primer, 5´-ATATA
Transfection studies and SEAP activity measurements Transfections of mammalian cells, including CHO-K1, COS1, HeLa, HepG2, MDCK, and U2OS cells, were performed using Lipofectine reagent (Invitrogen, USA). The cells (at 9×104–9×105/well) were plated onto 24-well plates. Before transfection, the cells were repeatedly washed with serum-free media to remove all traces of sera. One microgram of pCMV-DRhirSE plasmid (Figure 1D) was diluted in 200 μL serum-free DMEM or MEM medium, and 1 μL of the Lipofectine reagent was added. The DNA–Lipofectin mix was incubated for 15 min for DNA–Lipofectin complex formation. Then the DNA–Lipofectin complex solution was transferred to the cells at a total volume of 0.5 mL by adding serum-free medium. After 12 h, the medium was removed and 1 mL fresh medium with 10% fetal bovine serum was added. Two days post-transfection, supernatants were harvested and analyzed for SEAP activity. The SEAP activity in the culture media was measured using BD Great EscApe SEAP detection kits (ClonTech, USA). The chemiluminescent intensities reflecting relative SEAP activities were detected with a chemical luminescence counter (Mithras LB 940; Berthold Technologies).
The pIE2-DRhirE plasmid (Figure 1H) was transfected into Sf21 insect cells (2× 105 cells/well) by Cellfectin reagent at a 1:1 ratio (ml reagent:mg DNA) on 24-well plates. After transfection for 2 d, the transfected cells were observed under fluorescent microscopy (Nikon).
Recombinant virus production and titer determination Using Cellfectin (1 μL), the Sf21 cells (2×105 cells/well in a 24-well plate) were cotransfected with the linearized viral DNA Bac-N-Blue (0.25 μg; Invitrogen, USA) and 0.8 μg of one of the transfer vectors, either pCMV-DRhirE or pBacΔ-DRhirE. The resulting viruses were respectively named vAcCMV-DRhirE (Figure 1E) and vAcΔ-DRhirE (Figure 1G). For the Bac-N-Blue viral DNA containing the LacZ gene controlled by the ETL promoter, the recombinant virus was identified by X-gal staining according to the manufacturer’s protocol. The recombinant viruses were selected and purified by a series of 3 end-point dilutions. Sf21 monolayers were used for virus propagation, and all viral stocks were prepared and titers determined according to the end-point dilution as described before[20]. The recombinant viruses, vAc-DCrirE and vAc-DRhirE (Figure 1F), were used in the Northern blot analysis. vAc-DCrirE contains the DsRed and GFP ORF separated by the cricket paralysis virus (CrPV) IGR IRES. In the recombinant vAc-DRhirE, the RhPV IRES is present between these ORF. These 2 recombinant baculoviruses were prepared and processed as described before[22].
Northern blot analysis An EGFP gene fragment (366 bp) was amplified by PCR from the pBac-DRhirE plasmid using the primer set, EGFP forward (5'-ACGACTTCTTCAAGTCCGCC-3') and EGFP reverse (5'-TGCTCAGGTAGTGGTTGTCG-3'). The fragment was then cloned into a pGEM-T easy vector (Promega), which contains T7/SP6-opposed promoters. DIG–RNA probes were prepared by in vitro transcription with a commercial kit (DIG–RNA labeling kit; Roche) according to the instructions provided by the manufacturer. Total RNA transcripts were extracted from vAcCMV-DRhirE-, vAc-DCrirE- and vAc-DRhirE-infected Sf21 cells at 4 d postinfection (dpi) and also from uninfected Sf21 cells. Extracts were electrophoresed in a 1% agarose gel containing formaldehyde, blotted onto a nylon membrane (Hybond-N; Amersham), and probed with the EGFP probe according to the standard procedure of Sambrook et al[19]. Standard chemiluminescent detection was performed according to the manufacturer’s instructions (Roche), and the blot was exposed to X-ray film (Kodak XAR-5).
Western blot analysis Proteins were separated by SDS–PAGE on a mini Protein III system (Bio-Rad, Hercules, CA, USA). After SDS–PAGE fractionation, proteins were electrotransferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The resulting membrane was blocked with Tris-buffered saline (TTBS; 100 mmol/L Tris [pH 7.4], 100 mmol/L NaCl, and 0.1% Tween 20) containing 5% (v/v) non-fat, dry milk at room temperature for 1 h with gentle shaking. Subsequently, the membrane was incubated with 1:2000-diluted, anti-EGFP or anti-DsRed antibodies (ClonTech, USA) in TBS with 0.5% (v/v) non-fat, dry milk at 4 °C overnight. Unbound antibodies were removed by 3 washes each of 5 min in TTBS buffer at room temperature with shaking. Then the membrane was incubated with 1:2500-diluted horseradish peroxidase (HRP)-conjugated secondary antibodies (Chemicon) for 1 h at room temperature. The HRP on the membrane was detected by an enhanced chemiluminescence kit (Pierce, Rockford, IL, USA) following the protocol provided by the manufacturer.
Transduction of mammalian cells The cells were seeded in 24-well plates at 5×103 cells/well. The culture medium was removed and replaced with virus inoculate at a multiplicity of infection of 150 plaque-forming units/cell, and centrifuged at 2000 r/min for 1 h. Then the supernatant was removed, and fresh medium containing 10 mmol/L sodium butyrate was added and cultured at 37 °C.
Results
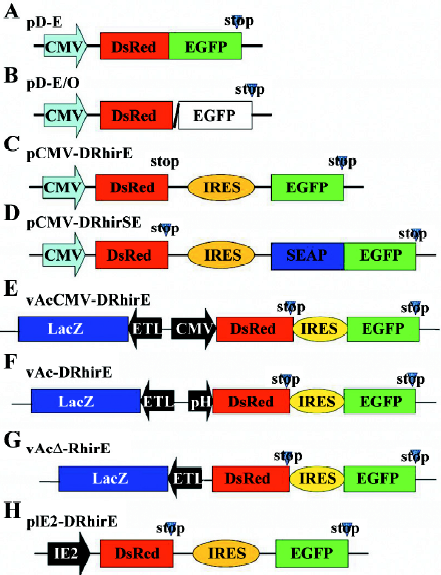
RhPV IRES is functional in mammalian cells The 579 nt-long 5´UTR of RhPV is recognized as a cross-kingdom IRES that functions efficiently in in vitro mammalian, insect, and plant translation systems[16,17,23]. To substantiate the observation of mammalian rabbit reticulocyte lysate in in vitro translation, we constructed the bicistronic reporter plasmid, pCMV-DRhirE (Figure 1C), which contains the CMV promoter to drive the transcription of DsRed-RhPV IRES–EGFP bicistronic mRNA in mammalian cells. The cap-dependent translation of the bicistronic mRNA was monitored by measuring the red fluorescence and the activity of the IRES was assessed from the green fluorescence. Meanwhile, we also constructed a vector, pD-E (Figure 1A), which contains the DsRed-EGFP fusion gene controlled by the CMV promoter as a positive control. When CHO cells were transiently transfected with pD-E, we found that both the red and green fluorescence were revealed in the same cells under a fluorescent microscope (Figure 2A). Similarly, pCMV-DRhirE-transfected CHO cells also revealed both red and green fluorescence (Figure 2B). However, CHO cells transfected with the plasmid pD-E/O (Figure 1B), in which the DsRed gene was not in-frame fused with the EGFP gene (see Material and methods), only revealed the red fluorescence, but without green fluorescence (data not shown). This result suggested that the RhPV IRES can mediate the cap-independent translation in CHO cells, and confirmed the results of previous in vitro translation experiments[16,17].
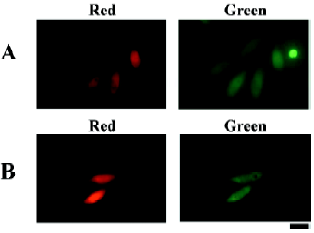
To quantitatively assess the translational activity of the RhPV IRES in different mammalian cells, the pCMV-DRhirSE plasmid (Figure 1D) was constructed. The pCMV-DRhirSE plasmid contains a SEAP reporter gene fused in-frame with an egfp fluorescent gene. Thus the activity of the RhPV IRES can easily and sensitively be monitored from the medium by the SEAP activity[24]. Six different cell lines were transiently transfected with the pCMV-DRhirSE plasmid, and all transfected cells could be identified by the cap-dependent translation of the DsRed gene under a fluorescent microscope (data not shown). After being normalized with the transfection rate by counting the number of red fluorescent-emitted cells, the SEAP–EGFP activities in the culture medium differed among these cell lines (Figure 3). The RhPV IRES exhibited significantly higher translation efficiency in the CHO and HeLa lines than that in HepG2, COS-1, and MDCK cell lines. This result suggests the RhPV IRES may be not functionally equal in these tested mammalian cells, although it has been proven to be a cross-kingdom IRES[17]. However, it can not be excluded that variations in the CMV promoter activity in different cell lines are responsible for these observations (Figure 3).
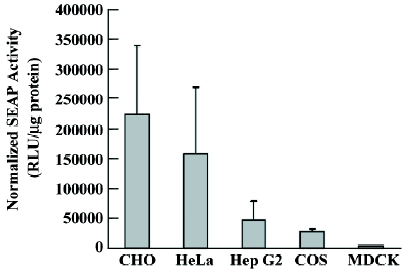
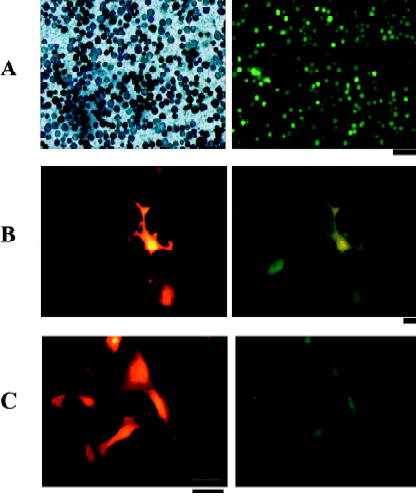
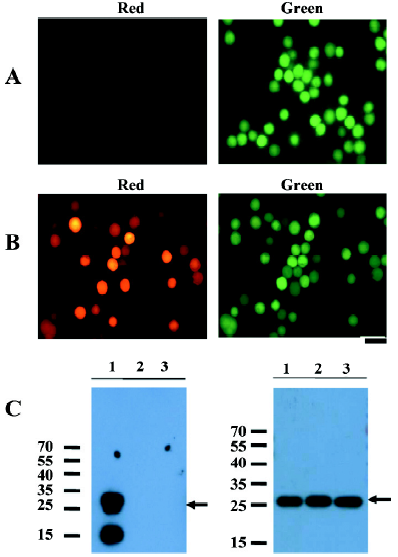
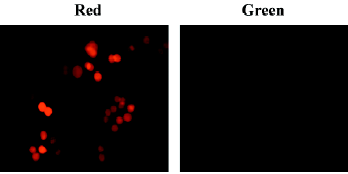
RhPV IRES has cryptic promoter activity in baculovirus-infected Sf21 cells Recombinant baculoviruses with mammalian cell-active promoter elements have successfully been used for transient and stable gene delivery in a broad spectrum of primary and established mammalian cell lines and are proving to be a valuable gene therapy vector[25,26]. For gene therapy, the development of polycistronic vectors that allow cells to be genetically modified through the introduction of several therapeutic genes in 1 step has become increasingly important. Therefore, we incorporated the CMV-DsRed-RhPV IRES–EGFP expression cassette into the baculovirus genome to construct the mammalian cell gene-delivery bicistronic recombinant baculovirus vector, vAcCMV-DRhirE (Figure 1E). Previous studies have shown that the CMV promoter is not functional in insect cells[27], so the recombinant virus was isolated by X-gal staining for the vAcCMV-DRhirE genome containing the LacZ gene driven by the ETL promoter (Figure 4A, left). As expected, the vAcCMV-DRhirE-transduced COS-1 cells revealed both red and green fluorescence under a fluorescent microscope (Figure 4B). Our previous studies showed that the bone-derived cell lines are more accessible to baculoviruses than COS-1 cells[28], so we also transduced the bone-derived cell line U2OS with vAcCMV-DRhirE. Figure 4C shows that both red and green fluorescence were also expressed in the transduced cells. These results indicate that the RhPV IRES is functional in mammalian cells either in a plasmid vector or in a recombinant viral genome. Interestingly, vAcCMV-DRhirE-infected Sf21 cells revealed green fluorescence (Figure 4A, right) in the absence of red fluorescence (data not shown). These unexpected results imply that the RhPV IRES may possess cryptic promoter activity or that DsRed-RhPV IRES–EGFP bicistronic mRNA undergoes RNA cleavage in vAcCMV-DRhirE-infected Sf21 cells. To clarify these questions, a Northern blot analysis and a promoterless assay were performed. Figure 5A indicates that a transcript of around 0.8 kb, corresponding to the EGFP gene transcript, was detected in vAcCMV-DRhirE-infected Sf21 cells. However, the predicted size of the bicistronic RNA transcript (approximately 2 kb) containing the DsRed gene (680 bp), the RhPV IRES (579 bp), and the EGFP gene (798 bp) in vAcCMV-DRhirE-infected Sf21 cells was not found. This result implied that the CMV promoter did not mediate the transcription of the bicistronic transcript in the baculovirus-infected Sf21 cells, and that the expression of green fluorescent proteins may be driven by a promoter within the RhPV IRES. Interestingly, we found that 6 TAAG motifs are present in the DNA sequence, which corresponded to the RhPV IRES (Figure 5B). TAAG sequences are relatively rare in the AcMNPV genome and are found primarily in late or very late promoter regions[29]. Thus the 6 TAAG motifs in the DNA sequence of RhPV 5´UTR IRES may be responsible for the promoter activity in baculovirus-infected Sf21 cells. These results implied that vAc-DRhirE (Figure 1F)-infected insect cells will generate 2 transcripts: one containing the bicistronic transcript and the other containing only the EGFP transcript. To test this hypothesis, a Northern blot analysis was performed with a DIG-labeled, GFP-specific probe and we found that both a 2 and 0.8 kb transcript were detected in the vAc-DRhirE infected Sf21 cells (Figure 5C, lane 2). In contrast, in the cell lysates of the vAc-DCrirE-infected Sf21 cells containing the CrPV IRES, a species of RNA with a size of approximately 2 kb was detected (Figure 5C, lane 1). These Northern blot analyses suggested that a cryptic promoter may occur in the RhPV IRES and could mediate the EGFP gene transcription in the baculovirus-infected Sf21 cells. To further confirm this suggestion, a promoterless bicistronic vector, pBacΔ-DRhirE, formed by simply removing the CMV promoter from pCMV-DRhirE, was constructed, and the resulting recombinant baculovirus was named vAcΔ-DRhirE (Figure 1G). This promoterless recombinant virus should not generate bicistronic mRNA after infection of Sf21 cells due to the lack of a CMV or a polyhedrin promoter. Thus the activity of the RhPV IRES element in this promoterless recombinant baculovirus should not be detected, and any expression of the second cistron egfp gene from vAcΔ-DRhirE-infected Sf21 cells will be due to the existence of a promoter in the RhPV IRES sequence. Figure 6A shows that vAcΔ-DRhirE-infected Sf21 cells, like vAcCMV-DRhirE-infected Sf21 cells, revealed only green fluorescence and no red fluorescence. In contrast, vAc-DRhirE-infected Sf21 cells, in which the bicistronic construct was made from the polyhedrin promoter, expressed both green and red fluorescence (Figure 6B). A Western blot analysis also confirmed the expression of fluorescent proteins in these baculovirus-infected Sf21 cells. As shown in Figure 6C, the DsRed protein was only expressed in the vAc-DRhirE-infected Sf21 cells, but the EGFP protein can be detected in vAc-DRhirE-, vAcCMV-DRhirE-, and vAcΔ-DRhirE-infected Sf21 cells. The combined Northern and Western blot analyses demonstrated that the RhPV IRES can mediate gene expression in baculovirus-infected Sf21 cells through a cryptic promoter. However, neither green nor red fluorescence appeared in CHO and U2OS cells transiently transfected with the promoterless pBacΔ-DRhirE bicistronic vector or transduced with vAc-DRhirE (data not shown). These results implied that this cryptic promoter, like the TAAG-containing baculovirus late promoter, depends on baculovirus early gene expression. Thus as we transfected Sf21 cells with pCMV-DRhirE (Figure 1C), neither the red or green fluorescence was observed (data not show). To further confirm this observation and rule out any experimental bias (eg failed plasmid transfection can also get this result), we generated another construct, pIE2-DRhirE (Figure 1H), to analyze the RhPV IRES activity in insect cells without baculovirus infection. In the pIE2-DRhirE-transfected Sf21 cells, the transcription of the bicistronic transcripts, containing the DsRed and EGFP OFP sequence flanking the RhPV IRES, is mediated by the baculovirus-independent immediate early promoter, ie2 promoter. We found that only the cap-dependent translation of DsRed was observed; however, the green fluorescence was barely detected under fluorescent microscopy (Figure 7). Thus the expression of the green fluorescent protein EGFP from the vAc-DRhirE-infected Sf21 cells (Figure 6B,6C) may be mostly mediated by the cryptic promoter activity of the RhPV IRES rather than the cap-independent, IRES-mediated translation activity. Taken together, these results show that the DNA sequence of the RhPV IRES exhibits a cryptic promoter in baculovirus-infected Sf21 cells, but not in mammalian cells.

Discussion
IRES from different origins largely differ in factors of their structural organization, sequence, length, and requirements for translation initiation or auxiliary mRNA-binding proteins[30]. Thus it is generally accepted that there are kingdom-specific limitations on viral IRES activity. For example, the picornavirus-derived IRES do not work in insect cells[16,31], and none of the animal virus-derived IRES elements seem to be active in yeast cells[5]. However, a polypurine (A)-rich sequence found in the IRES of the crucifer-infecting tobamovirus can promote cross-kingdom conservation of internal ribosome entry and mediate translation initiation in yeast, plants, and HeLa cells[30].
In this work, we demonstrated that the IRES element derived from the aphid infective virus RhPV has cap-independent translational activity in mammalian cells according to a bicistronic DNA transient transfection assay (Figures 2,3). These results, combined with previous studies, show that the RhPV IRES is functional in baculovirus-infected insect cells[20,32,33]. We concluded that the RhPV IRES exhibits cross-kingdom in vivo IRES activity for cap-independent translation, although RhPV is an insect virus with a narrow host range, infecting aphids of the Rhopalosiphum and Schizaphis families[14,16,34]. To explore the application of the RhPV IRES in a bicistronic vector, we constructed a bicistronic baculovirus expression cassette containing the CMV promoter to drive the expression of bicistronic mRNA containing the RhPV IRES in mammalian cells. Although these recombinant baculovirus (vAcCMV-DRhirE)-transduced COS-1 and U2OS cells revealed expected results, that is, the RhPV IRES can function as an IRES element in mammalian cells (Figure 4B,4C), the vAcCMV-DRhirE-infected Sf21 insect cells, however, revealed that only the EGFP gene downstream of the RhPV IRES was expressed (Figure 4A, right). Furthermore, the Northern blot analysis (Figure 5A,5C) showed that a 0.8 kb transcript was generated in the vAcCMV-DRhirE- and vAc-DRhirE-infected Sf21 cells, respectively. These results implied that the RhPV IRES may function as a cryptic promoter and induce the transcription of the EGFP gene. However, Pijman et al did not observe this phenomenon in their Northern blot analysis of transcripts from the RhPV IRES–EGFP-containing recombinant baculovirus-infected Sf21 cells[32]. When we compared the methods to perform the Northern blot analysis, 2 major differences were found. First, Pijman et al isolated the polyadenylated (poly[A]) mRNA instead of total RNA in their study. Second, they isolated the poly(A) mRNA at 48 h pi, but we isolated total RNA at 96 h pi. Thus the transcript from the RhPV IRES may be deficient of the polyadenylation, or RNA degradation in our isolation protocol may result in this conflict. If the RNA transcribed by this cryptic promoter lacked a poly(A) tail would be very interesting, as the transcripts of histone genes are the only known cellular mRNA without the poly(A) tail. In addition, it was reported that the polyhedrin gene mRNA of the Spodoptera exigua nuclear polyhedrosis virus did not appear to be poly(A)[35]. However, we did not have any evidence to support this and more work is needed to clarify this issue. To further confirm the cryptic promoter activity of the RhPV IRES in baculovirus-infected Sf21 cells, a promoterless assay (Figure 6) and a baculovirus-free, plasmid-based transient transfection assay (Figure 7) were performed and showed that the DNA sequence of the RhPV IRES possesses cryptic promoter activity that can be activated and can mediate gene expression in baculovirus-infected insect cells.
Many studies have indicated that the IRES derived from cellular mRNA; for example, p27kip1[36] and pim-1[37], contains cryptic promoter activity. Furthermore, previous studies have shown that promoter activity is present in the DNA sequence corresponding to the hepatitis C virus IRES[38]. Interestingly, we found that 6 TAAG motifs are present in the DNA sequence which corresponded to the RhPV IRES (Figure 5B). TAAG sequences are relatively rare in the AcMNPV genome and are found primarily in late or very late promoter regions[29]. Linker-scan mutational analyses revealed that the TAAG motif is absolutely essential for transcriptional initiation to occur and also appears to be the primary determinant of transcriptional initiation from baculovirus late promoters[18]. For example, the vp39 late promoter actually has 3 transcriptional initiation sites, each starting within a TAAG site. So it has been proposed that if TAAG sequences in an appropriate context are present within a gene, transcription can be initiated within that gene. Thus the 6 TAAG motifs in the DNA sequence of the RhPV IRES may be responsible for the promoter activity in baculovirus-infected Sf21 cells. Furthermore, we surveyed the IRES sequences in the Dicistroviridae family (which consists of 12 invertebrate members so far[11]) and found that these IRES sequences contain many of the TAAG motifs (Table 1). Although the IGR IRES of CrPV contains 1 TAAG motif, it did not exhibit cryptic promoter activity (Figure 5C). This CrPV IRES also had no activity in baculovirus-infected Sf21 cells[22]. It would be interesting to know whether these IRES sequences can also act as late promoters in the baculovirus-infected insect cells, like the RhPV IRES. Recently, Ongus et al found that an IRES element in the Varroa destructor virus 1 (genus Iflavirus) contained baculovirus early gene motifs: a CAGT motif is preceded by 2 TATA boxes[39]. They also suggest that this CAGT motif may be active in insect cells and gives rise to transcripts initiating within the IRES element. Interestingly, we also found 3 TAAG motifs in the IRES element of Iflavirus. These combined findings imply that in order to evaluate the IRES activity in insect cells, especially in baculovirus-infected insect cells, the cryptic promoter activity should be considered and may be inactivated by mutating the CAGT or TAAG motifs.
Full table
In addition to their application for the production of recombinant proteins, baculoviruses are also regarded as promising mammalian gene delivery vectors. In previous studies, we identified a mammalian-insect shuttle promoter, the ETL promoter[28]. Recently, an ie1 promoter of white spot syndrome virus (WSSV, a pathogenic Whispovirus in shrimps) had also been shown to act as an efficiently mammalian-insect shuttle promoter[40]. Thus this novel RhPV IRES that was functional in mammalian cells and worked as a baculovirus late promoter will facilitate the development of bicistronic baculovirus gene therapy vectors. In addition to carrying the gene of interest, these novel bicistronic baculovirus vectors can be selected through antibiotic selection or traced by fluorescent markers in both insect (through cryptic promoter transcriptional activity) and mammalian cells (through IRES translational activity) controlled by this novel RhPV IRES sequence.
References
- Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988;334:320-5.
- Jang SK, Pestova TV, Hellen CU, Witherell GW, Wimmer E. Cap-independent translation of picornavirus RNAs: structure and function of the internal ribosomal entry site. Enzyme 1990;44:292-309.
- Hellen CU, Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev 2001;15:1593-612.
- Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat Cell Biol 1999;1:190-2.
- Martinez-Salas E, Ramos R, Lafuente E, Lopez de Quinto S. Functional interactions in internal translation initiation directed by viral and cellular IRES elements J Gen Virol 2001;82:973-84.
- Sachs AB. Cell cycle-dependent translation initiation: IRES elements prevail. Cell 2000;101:243-5.
- Pinkstaff JK, Chappell SA, Mauro VP, Edelman GM, Krushel LA. Internal initiation of translation of five dendritically localized neuronal mRNAs. Proc Natl Acad Sci USA 2001;98:2770-5.
- Martinez-Salas E. Internal ribosome entry site biology and its use in expression vectors. Curr Opin Biotechnol 1999;10:458-64.
- van Oers MM. Vaccines for viral and parasitic diseases produced with baculovirus vectors. Adv Virus Res 2006;68:193-253.
- Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol 2002;147:1655-63.
- Jan E. Divergent IRES elements in invertebrates. Virus Res. 2006;119:16-28.
- Domier LL, McCoppin NK. In vivo activity of Rhopalosiphum padi virus internal ribosome entry sites. J Gen Virol 2003;84:415-9.
- Nakashima N, Sasaki J, Toriyama S. Determining the nucleotide sequence and capsid-coding region of himetobi P virus: a member of a novel group of RNA viruses that infect insects. Arch Virol 1999;144:2051-8.
- Domier LL, McCoppin NK, D’Arcy CJ. Sequence requirements for translation initiation of Rhopalosiphum padi virus ORF2. Virology 2000;268:264-71.
- Wilson JE, Powell MJ, Hoover SE, Sarnow P. Naturally occurring dicistronic cricket paralysis virus RNA is regulated by two internal ribosome entry sites. Mol Cell Biol 2000;20:4990-9.
- Woolaway KE, Lazaridis K, Belsham GJ, Carter MJ, Roberts LO. The 5’untranslated region of virus contains an internal ribosome entry site which functions efficiently in mammalian, plant, and insect translation systems. J Virol 2001; 75: 10 244–9.
- Terenin IM, Dmitriev SE, Andreev DE, Royall E, Belsham GJ, Roberts LO, Shatsky IN. A cross-kingdom internal ribosome entry site reveals a simplified mode of internal ribosome entry. Mol Cell Biol 2005;25:7879-88.
- Thiem SM, Miller LK. Differential gene expression mediated by late, very late and hybrid baculovirus promoters. Gene 1990;91:87-94.
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press: 2001.
- Chen YJ, Chen WS, Wu TY. Development of a bi-cistronic baculovirus expression vector by the Rhopalosiphum padi virus 5' internal ribosome entry site. Biochem Biophys Res Commun 2005;335:616-23.
- Lee JC, Wu TY, Huang CF, Shih SR, Hsu TA. High-efficiency protein expression mediated by enterovirus 71 internal ribosome entry site. Biotechnol Bioeng 2005;90:656-72.
- Wu TY, Wu CY, Chen YJ, Chen CY, Wang CH. The 5´untranslated region of Perina nuda virus (PnV) possesses a strong internal translation activity in baculovirus-infected insect cells. FEBS Lett 2007;581:3120-6.
- Groppelli E, Belsham GJ, Roberts LO. Identification of minimal sequences of the Rhopalosiphum padi virus 5’untranslated region required for internal initiation of protein synthesis in mammalian, plant and insect translation systems. J Gen Virol 2007;88:1583-8.
- Teng CY, Wu TY. Secretory fluorescent protein, a secretion green fluorescent fusion protein with alkaline phosphatase activity as a sensitive and traceable reporter in baculovirus expression system. Biotechnol Lett 2007;29:1019-24.
- Hu YC. Baculovirus vectors for gene therapy. Advances Virus Res 2006;68:287-320.
- Condreay JP, Kost TA. Baculovirus expression vectors for insect and mammalian cells. Curr Drug Targets 2007;8:1126-31.
- Wu TY, Liono L, Chen SL, Chen CY, Chao YC. Expression of highly controllable genes in insect cells using a modified tetracycline-regulated gene expression system. J Biotechnol 2000;80:75-83.
- Liu YK, Chu CC, Wu TY. The baculovirus ETL promoter acts as a shuttle promoter between insect cells and mammalian cells. Acta Phamacol Sin 2006;27:321-7.
- O’Reilly DR, Miller LK, and Luckow VA. Baculovirus Expression Vector: a laboratory manual. NY: WH Freeman and Co; 1992.
- Dorokhov YL, Skulachev MV, Ivanov PA, Zvereva SD, Tjulkina LG, Merits A, et al. Polypurine (A)-rich sequences promote cross-kingdom conservation of internal ribosome entry. Proc Natl Acad Sci USA 2002;99:5301-6.
- Finkelstein Y, Faktor O, Elroy-Stein O, Levi BZ. The use of bi-cistronic transfer vectors for the baculovirus expression system. J Biotechnol 1999;75:33-44.
- Pijman GP, Roode EC, Fan X, Roberts LO, Belsham GJ, Valk JM, et al. Stabilized baculovirus vector expressing a heterologous gene and GP64 from a single bicistronic tanscript. J Biotechol 2006;123:13-21.
- Royall E, Woolaway KE, Schacherl J, Kubick S, Belsham GJ, Roberts LO. The Rhopalosiphum padi virus 5' internal ribosome entry site is functional in Spodoptera frugiperda 21 cells and in their cell-free lysates: implications for the baculovirus expression system. J Gen Virol 2004;85:1565-9.
- Pal N, Boyapalle S, Beckett R, Miller WA, Bonning BC. A baculovirus-expressed dicistrovirus that is infectious to aphids. J Virol 2007;81:9339-45.
- van Strien EA, Zuidema D, Goldbach RW, Valk JM. Nucleotide sequence and transcriptional analysis of the polyhedrin gene of Spodoptera exigua nuclear polyhedrosis virus. J Gen Virol 1992;73:2813-21.
- Liu Z, Dong Z, Han B, Yang Y, Liu Y, Zhang JT. Regulation of expression by promoters versus internal ribosome entry site in the 5'-untranslated sequence of the human cyclin-dependent kinase inhibitor p27kip1. Nucleic Acids Res 2005;33:3763-71.
- Wang Z, Weaver M, Magnuson NS. Cryptic promoter activity in the DNA sequence corresponding to the pim-1 5'-UTR. Nucleic Acids Res 2005;33:2248-58.
- Dumas E, Staedel C, Colombat M, Reigadas S, Chabas S, Astier-Gin T, et al. A promoter activity is present in the DNA sequence corresponding to the hepatitis C virus 5’UTR. Nucleic Acids Res 2003;31:1275-81.
- Ongus JR, Roode EC, Pleij CWA, Valk JM, van Oers MM. The 5´ non-translated region of Varroa destructor virus (genus iflavirus): structure prediction and IRES activity in Lymantria dispar cells. J Gen Virol 2006;87:415-9.
- Gao H, Wang Y, Li N, Peng WP, Sun Y, Tong GZ, Qiu HJ. Efficient gene delivery into mammalian cells mediated by a recombinant baculovirus containing a whispovirus ie1 promoter, a novel shuttle promoter between insect cells and mammalian cells. J Biotechnol 2007;131:138-43.
