Endogenously generated sulfur dioxide and its vasorelaxant effect in rats1
Introduction
Sulfur dioxide (SO2) is a common air pollutant that resulted from increased industrial activity over the past several decades. Inhaled SO2 can easily be hydrated to produce sulfurous acid in the respiratory tract, which subsequently dissociates to form its derivatives, sulfite and bisulfite (3:1 mole ratios in neutral fluids). The toxic effects of SO2 have been extensively studied[1–5]. However, SO2 was recently found to exert various biological effects on both animals and plants. For example, SO2 inhalation was found to cause changes in oxidative stress and antioxidative status in various organs of mice[6], and the serum sulfite level increased under physiopathological conditions[7]. Endogenous SO2 was reported to be generated in the liver during the normal processing of sulfur-containing amino acids such as L-cysteine[8]. L-cysteine is first oxidized to L-cysteine sulfinate, and the latter can develop through transamination by aspartate aminotransferase into β-sulfinylpyruvate, which decomposes spontaneously to pyruvate and SO2. SO2 is further hydrated to sulfite in vivo, oxidized by sulfite oxidase to sulfate and excreted in urine[9].
Up until now, the distribution of the endogenous SO2/aspartate aminotransferase pathway and its physiological and pathophysiological roles of endogenous SO2 in the regulation of cardiovascular system are unclear. In this study, therefore, we explored the characterization of endogenous SO2 production and the expression of aspartate aminotransferase mRNA in rat vascular tissues, and observed the influence of SO2 on vascular function.
Materials and methods
Reagents Sodium sulfite and sodium bisulfite (Na2SO3/NaHSO3, the SO2 derivatives), L-aspartate-β-hydroxamate (HDX, the inhibitor of aspartate aminotransferase), glibenclamide, nicardipine, Bay K8644, and monobromo-bimane (mBrB) were purchased from Sigma (St Louis, MO, USA). Trizol, M-MuLV reverse transcriptase, dNTP, Taq DNA polymerase, and oligo (dT)15 were bought from Promega (Madison, WI, USA). Other chemicals and reagents were of analytical grade.
Animal treatment Animal care and experimental protocols complied with the Animal Management Rule of the Ministry of Health, People’s Republic of China (2001) and the Animal Care Committee of Peking University First Hospital. Sixty male Wistar rats (body weight 250±5 g) were obtained from the Experimental Animal Center, Peking University Health Science Center. The rats were housed under special pathogen-free conditions, and kept at a temperature of 22 °C with 40% humidity and a 12-h light/12-h dark cycle.
Preparation of vascular tissue samples in rats Ten male Wistar rats were anaesthetized with 10% chloral hydrate (4 mL/kg, intraperitoneally). Vascular tissues, including aorta, pulmonary, mesenteric, renal, and tail arteries were rapidly isolated. Samples were snap frozen and stored in liquid nitrogen. The vascular tissues were homogenized in 0.1 mol/L phosphate-buffered saline (PBS; pH 7.4, 10 mL/g tissue) using a glass homogenizer, and then the homogenates were centrifuged at 12 000×g for 30 min at 4 °C. The supernatants obtained were stored at –70 °C for determination of SO2 content, aspartate aminotransferase activity, and protein assay. The protein level was measured according to the Bradford assay. For in situ hybridization, the rats were injected with 4% polyoxymethylene into their left ventricles, and the tissues were then fixed in polyoxymethylene.
Determination of SO2 content Sulfite is the hydrate form of SO2 in mammalian plasma and sera[10]. Therefore, we detected the sulfite concentration in plasma and vascular tissues to represent SO2 content indirectly. Sulfite determination was analyzed using high performance liquid chromatography with fluorescence detection (HPLC-FD)[8]. Briefly, 100 µL sample was mixed with 70 µL of 0.212 mol/L sodium borohydride in 0.05 mol/L Tris-HCl (pH 8.5) and incubated at room temperature for 30 min. The sample was then mixed with 10 µL of 70 mmol/L mBrB in acetonitrile, incubated for 10 min at 42 °C, and then mixed with 40 µL of 1.5 mol/L perchloric acid. Protein precipitate in the mixture was removed by centrifugation at 12 400×g for 10 min at 23 °C. The supernatant was immediately neutralized by adding 10 µL of 2 mol/L Tris-HCl (pH 3.0), and centrifuged at 12 400×g for 10 min. The neutralized supernatant was used for high performance liquid chromatography. The column (4.6×150 mm C18 reverse-phase column, Agilent series 1100; Agilent Technologies, Waldbronn, Karlsruhe, Germany) was first equilibrated with a buffer (methanol: acetic acid: water=5.00:0.25:94.75 by volume, pH 3.4). The sample loaded onto the column was resolved by a gradient of methanol for 0–5 min, 3%; 5–13 min, 3%–35%; 13–30 min, 35%–62%; 30–31 min, 62%–100%; 31–39 min, 100%; 39–40 min, 100%–3%; and 40–45 min, 3% at a flow rate of 1.0 mL/min. Sulfitebimane was measured by excitation at 392 nm and emission at 479 nm. Quantification was carried out by the standardization of sodium sulfite, and the sulfite content in the tissues was expressed as µmol/mg protein.
Measurement of aspartate aminotransferase activity Aspartate aminotransferase activity in plasma and tissue homogenates were determined by a Hitachi 7600 automatic biochemistry analyzer (Tokyo, Japan). Tissue aspartate aminotransferase activity was expressed as IU/g protein.
Determination of aspartate aminotransferase 1 and aspartate aminotransferase 2 mRNA in tissues by quantitative real-time RT–PCR Total RNA in tissues was extracted by Trizol reagent and reverse transcribed by oligo d(T)18 primer and M-MuLV reverse transcriptase. Primers and probes used are listed in Table 1. Quantitative real-time PCR was performed on an ABI PRISM 7300 instrument (ABI USA Sales Corp, Los Angeles, CA, USA). The PCR mixture contained 5 µL of 10×PCR buffer, 5 µL cDNA template or standard DNA, 4 µL of 2.5 mmol/L each dNTP, 5 U of Taq DNA polymerase, 1 µL 6-carboxy-X-rhodamine (ROX; category No12223-012; Invitrogen, Carlsbad, CA, USA), 15 pmol of each forward and reverse primers, and 10 pmol TaqMan probe in a total volume of 50 µL. Samples and standard DNA were determined in duplicate. The PCR condition was predenaturing at 95 °C for 5 min, then 95 °C for 15 s, and 60 °C for 1 min for 40 cycles. The amount of β-actin cDNA in the sample was used to calibrate the sample amount used for the determination[11].

Full table
In situ hybridization of aspartate aminotransferase 1 and aspartate aminotransferase 2 mRNA levels in tissues Digoxin-labeled oligonucleotides designed according to aspartate aminotransferase 1 and aspartate aminotransferase 2 mRNA were used as probes for the in situ hybridization (aspartate aminotransferase 1: 5’-CCT GTG GAA CCT GGG CAA AGA ATG ATG GA-3’ and 5’-ACA GGC GTG GAG GAC AAA GAT GGA GAA CT-3’; and aspartate aminotransferase 2: 5’-ACC GCC ATT TCC TTC CAC TGC TCT G-3’ and 5’-CTT GGC ATA GGA TTG GCA GAG GCA GAC AT-3’). The protocol was the same as that described previously[7]. Paraffin sections were treated with xylene and a series of descending ethanol and rinsed in PBS for 5 min. After being digested with proteinase K (0.05 g/L) for 12 min at 37 °C, the sections were prehybridized at 42 °C for 4 h with 20 µL hybridization mixture (70% formamide10 µL, 20×standard sodium citrate buffer 4 µL, 50% dextrin sulfate 2 µL, 50× Denhardt’s solution 2 µL, 10 g/L of single strand DNA 1 µL, and 1 mol/L dithiothreitol 1 µL). Then 5 µL labeled cDNA probes were applied into the hybridization mixture, and hybridized at 42 °C for 120 min. The sections were then immersed in 1:100 horse serum for 20 min to block the non-specific binding sites, incubated with rabbit antidigoxigenin antibody at 37 °C for 60 min, and biotin-conjugated goat antirabbit immunoglobulin G antibody at 37 °C for 20 min. After being rinsed 3 times, the sections were incubated with horseradish peroxidase-conjugated avidin at 37 °C for 30 min and developed using diaminobenzidine. The developed sections were counterstained with hematoxylin. Dark brown dots in the section represented positive signals of aspartate aminotransferase 1 or aspartate aminotransferase 2 mRNA in the tissue. At least 10 aortic arteries were assessed in each animal. Omission of cDNA probe on previously confirmed positive tissue sections was used as the negative control process in this study. And the sections of hepatic tissue were used as the positive control process in this study[12].
Measurement of rat aortic contractility The method used was as previously described with modification[13,14]. Briefly, male Wistar rats (n=30) were anesthetized, and the thoracic aorta was excised immediately and immersed in Krebs’ bicarbonate buffer. The adherent adipose and connective tissues of the aorta were removed, and the aorta was cut into rings of length 2–3 mm and mounted in 20 mL organ baths containing prewarmed Krebs’ bicarbonate buffer gassed with 95% O2/5% CO2 at 37 °C. Changes in tension were recorded using force transducers connected to a PowerLab (BL-Newcentrary, TaiMeng, Chengdu, China). The rings were first stretched passively to a tension of 9.8×10–3 Newt and allowed to equilibrate for 1 h before starting of the experiment. The endothelia of the rings were kept functionally undamaged and confirmed by the relaxation reactivity to acetylcholine (1 µmol/L). The aortic rings were pretreated with a submaximal dose of norepinephrine (NE, 1 µmol/L) to initiate contraction and then incubated with the SO2 derivatives (Na2SO3/NaHSO3 3:1 mole ratio, final content 25 µmol/L–12 mmol/L). Following incubation with the aspartate aminotransferase inhibitor HDX (0.1 µmol/L) to inhibit endogenous SO2, the vasorelaxant effect was observed.
To explore the effect of SO2 on the vasoconstrictive response to NE, the concentration-dependent (10 nmol/L–1 mmol/L) vasoconstrictive effects of NE on the aortic ring were studied in the absence or in the presence of SO2 derivatives (6 mmol/L) or aspartate aminotransferase inhibitor HDX (0.1 µmol/L) in the isolated bathed ring with a constant flow of Krebs’ buffer.
Additionally, to elucidate whether a KATP or an L-type calcium channel was the target of SO2 vascular action, the ring was pre-incubated with glibenclamide (1 µmol/L), a known KATP channel blocker, or nicardipine (1 µmol/L), a L-type calcium channel blocker, for 10 min before precontraction with NE and SO2 derivatives.
To further study the role of the L-type calcium channel in SO2-induced vasorelaxation, the aortic rings were incubated with nicardipine (1 µmol/L) or SO2 derivatives (25 µmol/L-12 mmol/L) for 10 min, then treated with Bay K8644 (final content 1, 5, and 10 µmol/L), an L-type calcium channel agonist, and the contraction of rings was observed.
Finally, endothelium was preremoved from the isolated aortic rings by rubbing with a glass stick. The absence of a functional endothelium was verified by the failure of acetylcholine (1 µmol/L) to induce relaxation of the NE-precontracted vascular tissues. The effects of the SO2 derivatives or HDX on the aortic rings were compared between intact and nude vascular rings, respectively.
Statistics Results are expressed as mean±SD. Data are presented as either the percentage of tension reduction or the absolute value of induced tension. Statistical analysis was performed using SPSS 11.5 (SPSS, Chicago, IL, USA). Student’s t-test for unpaired samples was used to compare results between 2 groups. For multiple group comparisons, ANOVA followed by a post-hoc analysis (Newman–Keuls test) was used. Statistical significance was set at P<0.05.
Results
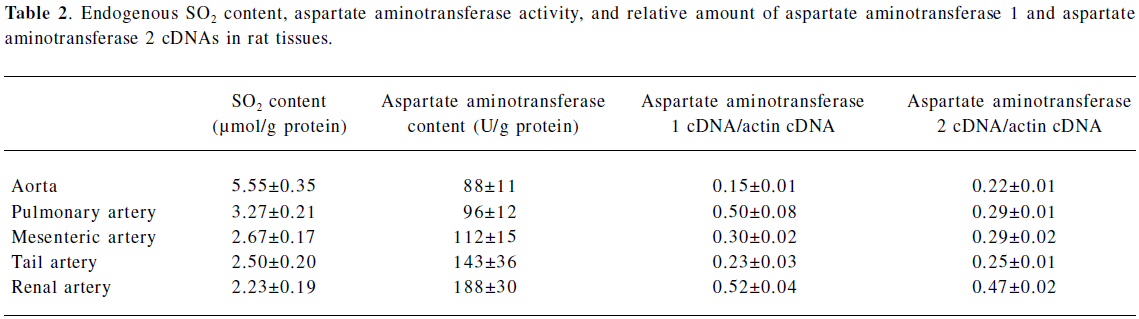
Characterization of the endogenous SO2 production in rat vascular tissues The plasma SO2 concentration was 15.54±1.68 µmol/L. In addition, a significant amount of SO2 was noted in the arteries. Among the arteries, the aorta had the highest concentration (5.55±0.35 µmol/g protein), followed by the pulmonary (3.27±0.21 µmol/g protein), mesenteric (2.67±0.17 µmol/g protein), tail (2.50±0.20 µmol/g protein), and renal arteries (2.23±0.19 µmol/g protein), respectively (Table 2).
Full table
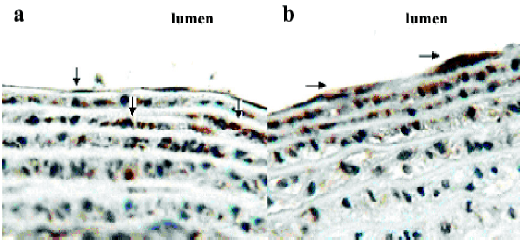
The tissue distribution of aspartate aminotransferase, a key SO2 generating enzyme, is shown in Table 2. Plasma aspartate aminotransferase activity was 87±18 U/L. Unlike the SO2 content, aspartate aminotransferase activity in the aorta (88±11 U/g protein) was the lowest, then the pulmonary arteries (96±12 U/g protein), mesenteric arteries (112±15 U/g protein), tail arteries (143±36 U/g protein), and renal arteries (188±30 U/g protein), respectively. Using quantitative real-time RT–PCR, aspartate aminotransferase 1 and aspartate aminotransferase 2 mRNA were detected in various tissues. The relative mRNA expression of aspartate aminotransferase 1 and aspartate aminotransferase 2 paralleled that of total aspartate aminotransferase. In situ hybridization was used to further locate aspartate aminotransferase 1 and aspartate aminotransferase 2 mRNA in the aortic wall. The results revealed aspartate aminotransferase 1 (Figure 1A) and aspartate aminotransferase 2 (Figure 1B) mRNA expression in endothelial cells and vascular smooth muscle cells beneath the endothelial layer.
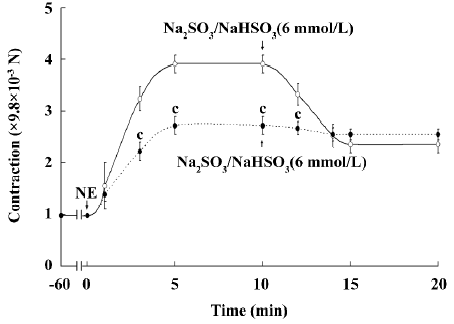
Vasorelaxant effect of SO2 on the isolated aortic ring Upon incubation with Na2SO3/NaHSO3 at a low concentration of 25–100 µmol/L, the NE-precontracted aortic ring showed a slight and temporary relaxation (Figure 2A). However, a high concentration of SO2 derivatives (1–12 mmol/L), much higher than that in plasma (approximately 0–30 µmol/L), induced a remarkable and persistent relaxant response in a concentration-dependent manner (Figure 2B). To further explore the vasorelaxant effect of SO2, HDX (0.1 µmol/L), a blocker for endogenous SO2 generation, was used. In this experiment, the SO2 concentration in the incubation solution was significantly lowered after HDX treatment [(1.85±0.11) vs (0.67±0.08) µmol/L, P<0.01]. Pretreatment with SO2 derivatives (6 mmol/L) for 10 min shifted the contraction curve in response to NE (10 nmol/L–1 mmol/L) to the right, whereas pretreatment with HDX for 10 min shifted the contraction curve in response to NE to the left (Figure 3).


Involvement of L-type calcium channel in the vascular effects of SO2 After pretreatment with the L-type calcium channel blocker, nicardipine (1 µmol/L), the vasorelaxant response of the NE-contracted rings to SO2 derivatives (6 mmol/L) was eliminated almost completely (Figure 4). Bay K8644, an L-type calcium channel agonist, contracted the aortic ring in a concentration-dependent manner. Meanwhile, pre-incubation with SO2 derivatives eliminated the vasoconstriction induced by Bay K8644. The SO2-induced vasorelaxant response was also mimicked by nicardipine (Figure 5).

Role of KATP channel in the vasorelaxation induced by SO2 In the present study, glibenclamide, a known KATP channel blocker, was used to treat aortic rings 10 min before the application of SO2 derivatives. The relaxant effect of SO2 was not affected by glibenclamide, which did not indicate the involvement of KATP channels (Figure 6).

Role of endothelium in the SO2-induced vasorelxation Removing endothelium from the aortic tissue did not alter the SO2 -induced vasorelaxation curve (P>0.05). HDX enhanced the vasoconstrictive response of either intact or nude aortic rings to NE to a similar extent (P>0.05; Figure 7).

Discussion
Recently, increasing experiments have demonstrated SO2 or sulfite with multiple biological actions. The culture of human peripheral lymphocytes with a chemically-defined, protein-free medium revealed that sulfite was an essential metabolite[15], and Na2SO3 is a modulator of cytokine production, but does not alter either chemotaxis or cell surface expression of the tested molecules[16]. The inhalation of exogenous SO2 was reported to reduce mean blood pressure in rats[17], but the endogenous SO2 production in the vascular system and its bioactivity on vessels have not been made clear yet. Our present study, therefore, was undertaken to provide direct experimental evidence of endogenously-generated SO2 in tissues, including vessels, and explore the vasorelaxant effect of SO2 in rats.
According to a previous report, total serum sulfite in normal volunteer was found to be 0–10 µmol/L as measured by the HPLC-FD method[18]. In our study, we found the production of endogenous SO2 in various vascular tissues. The aortic SO2 content was the highest among the arteries, then the pulmonary, mesenteric, tail, and kidney arteries.
SO2 and its hydrated form, sulfite/bisulfite, are generated during the normal processing of sulfur-containing amino acids, such as L-cysteine[8]. L-cysteine is first oxidized via cysteine dioxygenase to L-cysteine sulfinate. Then L-cysteine sulfinate undergoes transamination to form β-sulfinylpyruvate catalyzed by aspartate aminotransferase.β-Sulfinylpyruvate, the putative product, decomposes spontaneously to pyruvate and SO2[18]. Thus aspartate aminotransferase could be regarded as a key enzyme in controlling endogenous SO2 production.
Therefore, we further studied the aspartate aminotransferase activity and gene expression. We noticed that the aspartate aminotransferase activity was lowest in aorta, then pulmonary artery, mesenteric artery, tail, and renal arteries. Aspartate aminotransferase has two isoenzymes named for their intracellular location. Aspartate aminotransferase 1 is located in the cell cytoplasm, whereas aspartate aminotransferase 2 is located in the cell mitochondria. There were some functional differences between aspartate aminotransferase 1 and aspartate aminotransferase 2. For example, aspartate aminotransferase 1 and aspartate aminotransferase 2 are both were involved in the malate–aspartate shuttle. However, α-ketoglutarate and aspartate reacted together to form glutamate and oxaloacetate in a reaction catalyzed by aspartate aminotransferase 1, while glutamate and oxaloacetate reacted together to form α−ketoglutarate and aspartate in a reaction catalyzed by aspartate aminotransferase 2[19]. In this study, we explored the gene expressions of aspartate aminotransferase 1 and aspartate aminotransferase 2 in the various arteries. The data showed that the mRNA level of aspartate aminotransferase 1 and aspartate aminotransferase 2 was lowest in aorta, and that of renal artery was high. In the aorta, aspartate aminotransferase 1 mRNA and aspartate aminotransferase 2 mRNA were located in endothelia and vascular smooth muscle cells beneath the endothelial layer. No significant difference was found in the gene expression and localization in vascular tissues between the 2 isoenzymes.
The above data suggested that endogenous SO2 and aspartate aminotransferase comprise the SO2/aspartate aminotransferase pathway in the arteries. These observations implied the potential physiological functions of SO2 in the cardiovascular system.
To explore the possible vasoactive effect of SO2, we identified SO2-induced vasorelaxation in NE precontracted aortic rings, although it was temporal and slight under the physiological plasma concentration. Furthermore, we found that SO2 derivatives attenuated the NE-induced concentration-dependent vasoconstriction, whereas HDX, an aspartate aminotransferase inhibitor, enhanced the NE-induced vasoconstriction. The above in vitro data demonstrated that SO2 exerted a vascular regulatory function as a vasorelaxant factor.
Based on the above findings, we further examined the possible mechanisms responsible for SO2-induced vasorelaxation. Generally, calcium influx plays important physiological roles in mediating the contraction of vascular smooth muscle cells. During sarcolemmal membrane depolarization, the L-type calcium channel will open to permit calcium ion influx and trigger intracellular calcium-induced calcium release, leading to cell contraction[20]. Therefore, we observed the role of the L-type calcium channel in SO2-induced vasorelaxation. Pretreatment with an L-type calcium
Potassium ion channels in the cell membrane are well known to participate in the vascular relaxation response[21]. In our study, pre-inhibition of the KATP channel by glibenclamide did not alter SO2-induced vasorelaxation. These data do not support the involvement of the KATP channel in the SO2-induced vasorelaxation.
Carbonyl sulfide and SO2, which were detected by GC/MS analysis, were found in porcine artery rings and cardiac muscle tissue[22]. The authors suggested that the release of these 2 gases was related to a endothelium-derived hyperpolarizing factor. However, we observed that vascular endothelium and medial smooth muscles both generated endogenous SO2. Moreover, the vasorelaxant effect of SO2 was similar in intact and nude aortic rings, and HDX augmented the NE-induced vasoconstriction of either intact or nude aortic rings similarly. The results suggested that SO2-induced vasorelaxation is endothelium independent.
In summary, we demonstrated that SO2 could be endogenously generated in vascular tissues and could relax aortic rings, at least in part, through an L-type calcium channel, suggesting that it might act as a vasoactive molecule.
References
- Bush RK, Taylor SL, Holden K, Nordree JA, Busse WW. Prevalence of sensitivity to sulfiting agents in asthmatic patients. Am J Med 1986;81:816-20.
- Fine JM, Gordon T, Sheppard D. The role of pH and ionic species in sulfur dioxide- and sulfite-induced bronchoconstriction. Am Rev Respir Dis 1987;136:1122-6.
- Dales RE, Cakmak S, Doiron MS. Gaseous air pollutants and hospitalization for respiratory disease in the neonatal period. Environ Health Perspect 2006;114:1751-4.
- McLeod RL, Jia Y, McHugh NA, Fernandez X, Mingo GG, Wang X, et al. Sulfur-dioxide exposure increases TRPV1-mediated responses in nodose ganglia cells and augments cough in guinea pigs. Pulm Pharmacol Ther 2007;20:750-7.
- Nidhi JG. Air quality and respiratory health in Delhi. Environ Monit Assess 2007;135:313-25.
- Meng ZQ. Oxidative damage of sulfur dioxide on various organs of mice: sulfur dioxide is a system oxidative damage agent. Inhal Toxicol 2003;15:181-95.
- Mitsuhashi H, Ikeuchi H, Yamashita S, Kuroiwa T, Kaneko Y, Hiromura K, et al. Increased levels of serum sulfite in patients with acute pneumonia. Shock 2004;21:99-102.
- Ubuka T, Yuasa S, Ohta J, Masuoka N, Yao K, Kinuta M. Formation of sulfate from L-cysteine in rat liver mitochondria. Acta Med Okayama 1990;44:55-64.
- Stipanuk MH. Metabolism of sulfur containing amino acids. Annu Rev Nutr 1986;6:179-209.
- Gunnison AF, Benton AW. Sulfur dioxide: Sulfite. Interaction with mammalian serum and plasma. Arch Environ Health 1971;22:381-8.
- Kroupis C, Stathopoulou A, Zygalaki E, Ferekidou L, Talieri M, Lianidou ES. Development and applications of a real-time quantitative RT-PCR method (QRT-PCR) for BRCA1 mRNA. Clin Biochem 2005;38:50-7.
- Zhong GZ, Chen FR, Cheng YQ, Tang CS, Du JB. The role of hydrogen sulfide generation in the pathogenesis of hypertension in rats induced by inhibition of nitric oxide synthase. J Hypertens 2003;21:1879-85.
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 2001;20:6008-16.
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 1997;237:527-31.
- Pettit FH, Lyon D, Brown JR, Shive W. Evidence for sulfite as an essential metabolite for human peripheral lymphocytes. Biochem Biophys Res Commun 1991;179:611-4.
- Ratthe C, Pelletier M, Roberge CJ, Girard D. Activation of human neutrophils by the pollutant sodium sulfite: effect on cytokine production, chemotaxis, and cell surface expression of cell adhesion molecules. Clin Immunol 2002;105:169-75.
- Meng ZQ, Geng HF, Bai JL, Yan G. Blood pressure of rats lowered by sulfur dioxide and its derivatives. Inhal Toxicol 2003;15:951-9.
- Ji AJ, Savon SR, Jacobsen DW. Determination of total serum sulfite by HPLC with fluorescence detection. Clin Chem 1995;41:897-903.
- Scholz TD, Koppenhafer SL, Teneyck CJ, Schutte BC. Ontogeny of malate-aspartate shuttle capacity and gene expression in cardiac mitochondria. Am J Physiol 1998;274:C780-8.
- Kruse HJ, Bauriedel G, Heimerl J, Höfling B, Weber PC. Role of L-type calcium channels on stimulated calcium influx and on proliferative activity of human coronary smooth muscle cells. J Cardiovasc Pharmacol 1994;24:328-35.
- Farouque HM, Worthley SG, Meredith IT. Effect of ATP-sensitive potassium channel inhibition on coronary metabolic vasodilation in humans. Arterioscler Thromb Vasc Biol 2004;24:905-10.
- Balazy M, Abu-Yousef IA, Harpp DN, Park J. Identification of carbonyl sulfide and sulfur dioxide in porcine coronary artery by gas chromatography/mass spectrometry, possible relevance to EDHF. Biochem Biophys Res Commun 2003;311:728-34.
