Co-application of ricin A chain and a recombinant adenovirus expressing ricin B chain as a novel approach for cancer therapy
Introduction
Many toxins of bacteria and plant origins have 2 moieties, with one moiety to bind the cell and help the toxin to enter cell, and the other to be enzymatically active to exert the action[1,2]. These toxins have potential in cancer therapy. One promising approach is to use them in immunotoxins, where the active moiety of toxin is fused with cancer-specific monoclonal antibodies or other cancer cell-specific ligands[3,4]. However, this approach may be limited by the lack of highly specific monoclonal antibodies or other specific ligands for certain types of cancer, and the requirement of large amounts of fusion proteins due to the lack of the bystander effect.
As a 2-moiety toxin from the seeds of Ricinus communis, ricin is a ribosome inactivating protein highly toxic to mammalian cells. It contains 2 peptide chains, A and B, which join together with 2 disulfide linkages[5]. The ricin A chain (RTA) inhibits protein synthesis by removing an adenine residue from the exposed loop of 28S ribosomal RNA (eg A4323 in rat 28S RNA), thus resulting in cell death[6,7]. However, it is generally non toxic outside the cell due to the low efficiency to enter cells by itself[8]. Progress has been made to further reduce its side effect by point mutations[9]. Meanwhile, the ricin B chain (RTB) is an agglutinin that mediates binding of the toxin to the cell surface, transporting the molecule to an intracellular compartment. It is non toxic to cells by itself[10,11].
In the current study, a novel approach to use these 2-moiety toxins in cancer therapy is proposed and tested in vitro using ricin as an example. According to the design, RTB is expressed by an adenovirus vector targeting cancer tissues while RTA is applied in the form of a purified protein. When used in vivo, RTA is concentrated in the cancer tissue where RTB is located due to the strong affinity between RTA and RTB[3]. It enters cancer cells with the help of RTB, and exerts its cell-killing function. By-stander effect is also expected, since the killed cells release more RTB to form a complex with the external RTA. A prototype of the design, with no cancer-specificity, was preliminarily tested for its cytotoxicity in cultured cells and proved to be a potential alternative for the efficient application of this type of toxin.
Materials and methods
PCR amplification of RTA and RTB genes The leaves of Ricinus communis were collected from Ningbo, Zhejiang Province, China. Genomic DNA was extracted using the Plant Genomic DNA Isolation Kit (Sangon, Shanghai, China). Primers were designed according to the sequence on Genbank (N
Prokaryotic expression of RTA The plasmid pT-RTA was doubly digested with EcoR I and Hind III, and the RTA gene was recovered from agarose and ligated with pET32a (Novagen, San Diego, CA, USA) similarly digested with EcoR I and Hind III. The resulted plasmid was named pET32-HisRTA. The RTA production E coli strain was obtained by transforming BL21 with pET32-HisRTA. The RTA protein was produced by inducing with isopropyl-beta-D-thioga-lactoside (IPTG) at 1 mmol/L for 10 h at 20 oC. The expression of RTA was confirmed by SDS-PAGE and Western blotting with anti-6×His IgG following standard protocols.
Purification of the RTA protein Bacteria cells were pelleted by centrifugation at 10 000 r/min for 10 min at 4 oC, and lysed with sonication. A 6×His-tagged RTA protein was purified with Ni2+-nitrilotriacetic acid (Ni2+-NTA) affinity resin (Shanghai Shenergy Biocolor) under non-denaturing conditions and eluted with imidazole solution. The fractions with RTA were pooled and dialyzed in phosphate buffer saline (PBS) to remove imidazole. RTA was concentrated by dialysis against polyethylene glycol 8000, quantitated, and stored at -70 oC for later use.
Construction of RTB-expressing recombinant adenovirus The recombinant adenovirus was constructed using the system developed by He et al[13]. The RTB gene was digested from pT-RTB with Sal I and Xho I and ligated with the transfer vector pAdTrackCMV[13] digested with the same enzymes. The resulted plasmid, pAdCMV-RTB, was then linearized with PmeI and transformed into the E coli strain BJ5183 (RecA+) harboring the plasmid pAdEasy-1, a plasmid with an adenovirus genome[13]. Homologous recombination between linearized pAdCMV-RTB and pAdEasy-1 resulted in the production of pAdEasyCMV-RTB, with the RTB gene and a kanamycin resistant gene inserted within the adenovirus genome. The plasmid pAdEasyCMV-RTB was amplified in E coli DH5a and extracted with the Genomic DNA Isolation Kit (Shanghai Shenergy Biocolor) from a 200 mL culture. 5 µg of its DNA was linearized with Pac I and used to transfect human embryonic kidney cell line HEK293 cells with Lipofectamine Reagent (Invitrogen, San Diego, CA, USA). The recombinant adenovirus AdGFP-RTB was prepared by the repeated freezing and thawing of transfected cells 10 d post transfection. Since AdGFP-RTB had an enhanced green fluorescence protein (GFP) gene, virus infection could be visualized in infected HEK293 cells 24 h post infection (pi).
RTB expression and binding with RTA Lysates of the AdGFP-RTB-infected HEK293 cells were collected at 0, 12, 24, 36, and 48 h pi and dotted on a nitrocellulose membrane. The membrane was treated with 6×His-tagged RTA in PBS (10 µg/mL) for 1 h, followed by similar treatment with a rabbit polyclonal antibody against 6×His (Bioinforbody, Zhuhai, Guangdong, China, 1:1000) and alkaline phosphatase-conjugated mice anti-rabbit IgG (Sigma-Aldrich, St Louis, MO, USA, 1:30000). The blot was then visualized by adding substrates of nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP). The lysates of the cells infected with AdGFP, an adenovirus without the RTB gene[14], were used as a negative control. To confirm the release of RTB from the infected cells, the samples of culture medium from AdGFP-RTB were concentrated with vacuum freeze-drying, and assayed with an immuno dot blot as described earlier.
Presence of the RTA protein in AdGFP-RTB-infected cells To confirm the entry of the RTA protein in RTB-expressing cells, the RTA protein (40 µg/mL) was added to the AdGFP-RTB-infected HEK293 cells at 24 h pi, and the cells were collected 0, 24, and 48 h later. The total cell protein was analyzed by SDS-PAGE and Western blotting using the anti-6×His antibody as described earlier. Non-infected HEK293 cells were treated similarly with the RTA protein, collected 24 h later, and used as a control.
Cell-killing effect of the combined application of RTA and AdGFP-RTB HEK293 and human cervical carcinoma cell line HeLa were used to seed 24-well plates at a density of 1×105 cells/well. The cells were infected with the recombinant adenovirus AdGFP-RTB or AdGFP at an MOI (multiplicity of infection) of 100 pfu/cell, or mock infected. A certain amount of the RTA protein was added to the culture supernatant at 24 h pi. Cell mortality was examined with trypan blue staining 24 h later. The total cells and dead cells were recorded to calculate the cell mortality. All experiments were carried out in triplicate and the average mortality and standard deviation were calculated. MTT assay was also used to measure the cell viability in treated HeLa and human liver cancer cell lines Smmc7721 and HL7702 cells using a similar experimental design as described before, following standard protocol[14].
By-stander effect The HeLa cells were infected with AdGFP-RTB or AdGFP at an MOI of 100. RTA 5 µg/mL was added to the culture at 24 h pi. The supernatant of the culture was collected 4 d later, diluted, and moved to a 24-well plate seeded with fresh HeLa cells. The cell viability was determined by MTT assay 24 h later. The experiment was carried out in triplicate and the average mortality and standard deviation were calculated.
Results
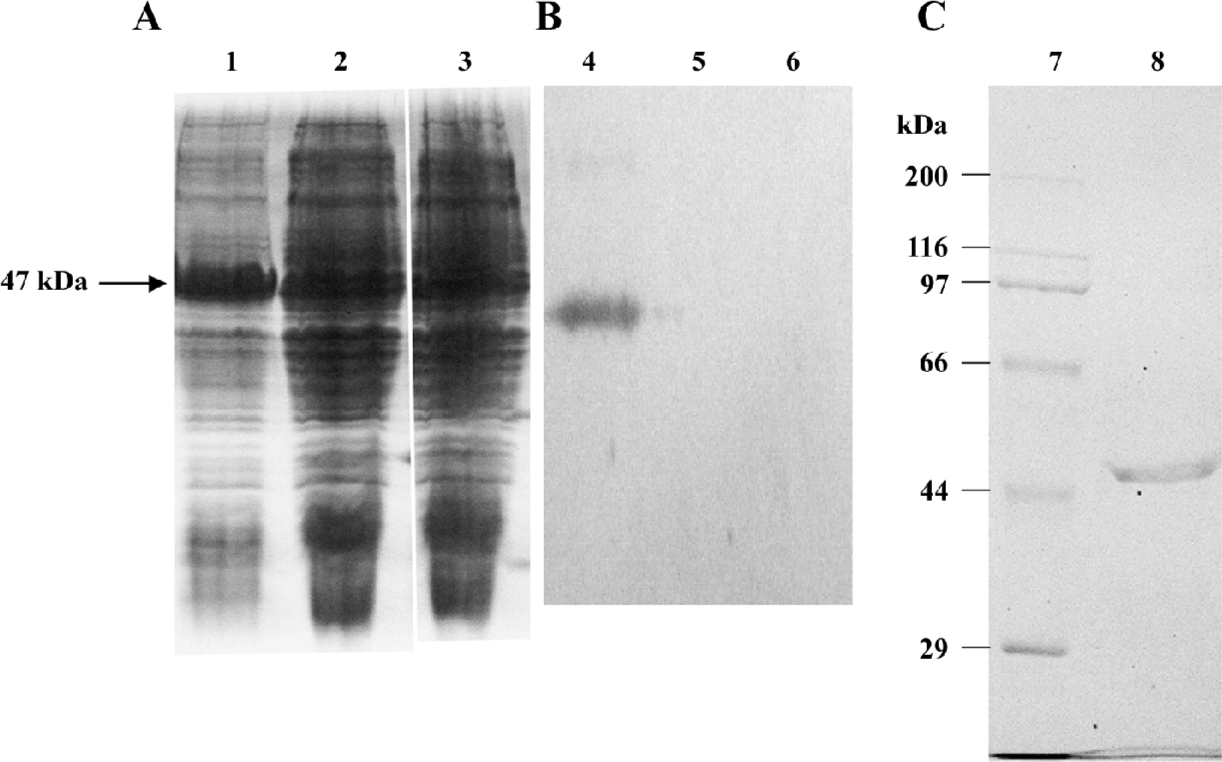
Prokaryotic expression and purification of RTA The expression of the RTA protein in the E coli BL21 strain was observed in SDS-PAGE, and Western blot analysis was performed using the specific primary antibody against 6×His (Figure 1). A strong band with a molecular weight of 47 kDa was presented in an IPTG-induced sample (Figure 1A), which was reactive to the anti-6×His antibody (Figure 1B). The purified RTA protein was obtained from Ni2+-NTA affinity chromatography eluted with imidazole at the concentration of 20 and 40 mmol/L (Figure 1C).
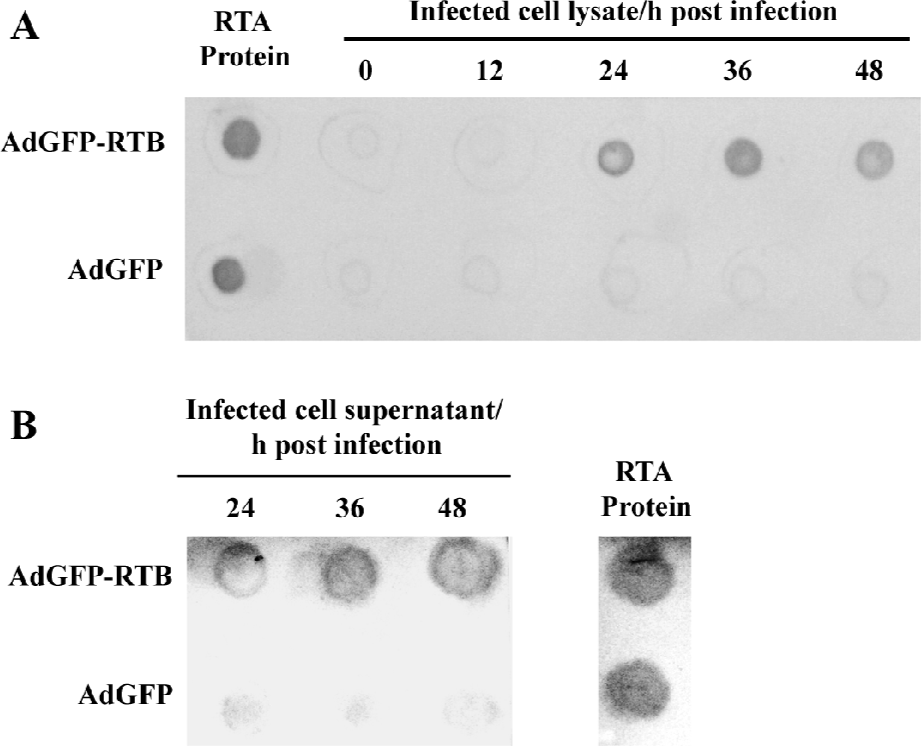
Construction of the recombinant adenovirus and expression of RTB The expression of RTB in AdGFP-RTB-infected HEK293 cells was confirmed with the dot blot assay using the RTA protein as an intermediate ligand. The RTB expression in AdGFP-RTB-infected cells was observed at 24 h pi, and peaked at 36 h pi, while no RTB expression was observed in AdGFP-infected cells (Figure 2). Since His-tagged RTA was used as an intermediate ligand for the detection of RTB, the result also proved that RTB expressed in cells has high binding activity to prokaryotically-expressed RTA. The RTB protein was also detected in the culture medium, concentrated 20 times, of AdGFP-RTB-infected cells, although the concentration was very low. This result indicated that the RTB protein was secreted into the medium, even though there was not a significant signal peptide in this protein.
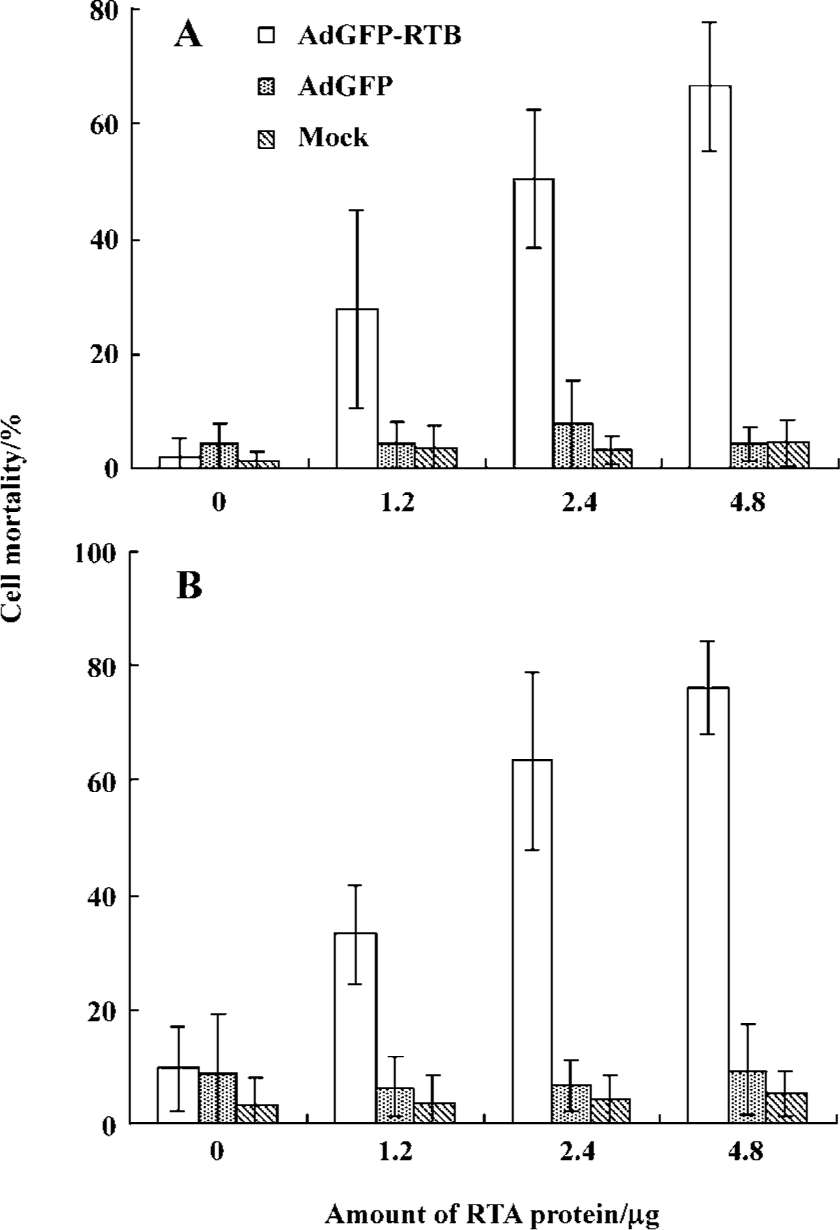
Cell-killing effect of RTA and AdGFP-RTB The cell-killing effect of RTA and AdGFP-RTB was first evaluated with trypan blue staining in HEK293 and HeLa cells. AdGFP-RTB infection did not result in significant cell death without RTA (RTA 0 µg), neither did the treatment of the RTA protein alone or in combination with AdGFP, even at the highest dose (Figure 3). However, when AdGFP-RTB and the RTA protein were used in combination, significant cell mortality was seen. The mortality increased with the RTA dose, which reached around 60% with 4.8 µg RTA protein, when the MOI of AdGFP-RTB was 100 pfu/cell. Similar results were observed using the MTT assay, where cell viability dropped by 50% or more when co-treated with AdGFP-RTB and the RTA protein in HeLa, HL7702, and SMMC-7721 cells (Figure 4).
Entry of the RTA protein into AdGFP-RTB-infected cells To confirm that the cell death observed above was a result of the entry of the RTA protein into the cell, HEK293 cells were infected with AdGFP-RTB, followed by the treatment of the RTA protein at 24 h pi. The RTA protein was detected in the cell lysate 24 and 48 h later with Western blotting (Figure 5). No RTA was detected in the non-infected cells similarly treated with the RTA protein for 24 h. These results indicated that infection of AdGFP-RTB prompted the entry of RTA into the cell.
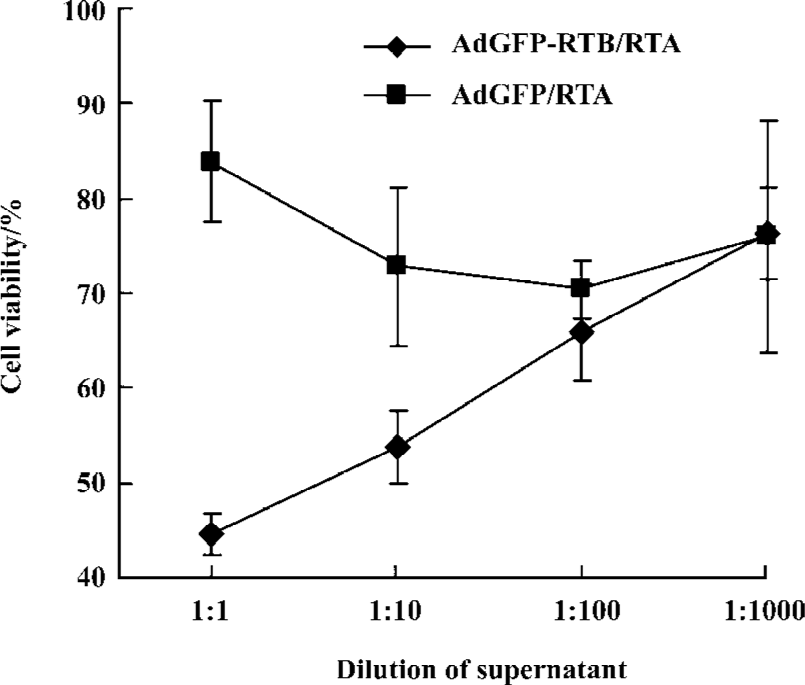
Bystander effect of the co-application of AdGFP-RTB and the RTA protein When the RTA protein and AdGFP-RTB were used in combination, it was expected that RTB expressed by the adenovirus and the RTA protein would combine to form the RTA-RTB complex, which would be highly toxic to cells. To prove the case, the supernatant was collected from HeLa cells infected with AdGFP-RTB in the presence of the RTA protein 48 h pi, and used to inoculate fresh HeLa cells. No green fluorescence was observed in treated cells 24 h later (data not shown) as expected indicating that there was no adenovirus in the supernatant. Meanwhile, cell viability decreased significantly when a high concentration of supernatant was used (Figure 6). No significant change of cell viability was seen in the control where cells were treated with supernatant from AdGFP and RTA-treated HeLa cells.
Discussion
Ricin is commonly used as a part of immunotoxins for clinical tumor research and application, although the detailed mechanism of its function is still under investigation[15,16]. Previous studies have shown that recombinant RTA produced in bacteria was highly active to form a complex with RTB and cause cytotoxicity[8]. Meanwhile, recombinant RTB expressed in eukaryotic cells has also been shown to be functional in transporting RTA into the cell[10,11]. In the current study, a novel approach for ricin-based tumor therapy was preliminarily tested in vitro to apply RTA as a purified protein, and RTB as a locally expressed protein in target cells.
Prokaryotically-expressed RTA was prepared, and an adenovirus expressing RTB was constructed. RTB expression and its binding with RTA were confirmed with an indirect dot blot assay. Further analysis showed, as expected, that although neither RTA protein nor AdGFP-RTB was cytotoxic when applied individually, they became highly toxic when applied simultaneously. Significant cell death or loss of cell viability was observed in all of the cell lines tested: HEK293, HeLa, HL7702, and SMMC7721; the cytotoxic effect correlated with the amount of RTA applied. The RTA protein was observed in AdGFP-RTB infected cells, but not in non-infected cells, which was consistent with the cytotoxicity data and proved that the cell death was due to the entry of RTA protein into the cells.
The mechanism on how RTA was transported into cells in this system has not been studied. One possibility is that some of the RTB produced by cell was leaked to the medium, formed the RTA-RTB complex with RTA outside the cell, and helped to move the complex into the cell. The observation of the RTB protein in the culture medium of AdGFP-RTB infected cells supported the hypothesis. It is presumed that the secretive expression of RTB may help to improve the cell-killing effect further.
The cytotoxic effect caused by the supernatant from AdGFP-RTB/RTA-treated HeLa cells suggested a potential bystander effect. It is believed to be due to the presence of the RTA-RTB complex in the supernatant rather than the production of more adenoviruses. AdGFP-RTB is a replication-deficient adenovirus with deletion in the E1A gene. It could only replicate in HEK293 cells, but not in HeLa cells. This was consistent with the absence of green fluorescence in HeLa cells treated by the supernatant.
In the current, we proprosed a novel approach to use ricin in cancer therapy and preliminarily tested it in vitro. Experiments in animal models will be necessary to further confirm the applicability of this approach in vivo. The method needs to be further developed in terms of specificity and effectiveness, possibly by using non-fusion native RTA proteins, cancer cell-specific and replication-competent recombinant adenoviruses[16], and by the secretive expression of RTB.
References
- Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer 2006;6:559-65.
- Johannes L, Decaudin D. Protein toxins: intracellular trafficking for targeted therapy. Gene Ther 2005;12:1360-8.
- Ng HC, Khoo HE. Cancer-homing toxins. Curr Pharm Des 2002;8:1973-85.
- Schnell R, Borchmann P, Staak JO, Schindler J, Ghetie V, Vitetta ES, et al. Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin’s lymphoma. Ann Oncol 2003;14:729-736.
- Olsnes S, Kozlov JV. Ricin. Toxicon 2001;39:1723-8.
- Robertus JD, Monzingo AF. The structure of ribosome inactivating proteins. Med Chem 2004;4:477-86.
- Jon R. The structure and action of ricin, a cytotoxic N-glycosidase. Cell Biol 1991;2:23-30.
- O’Hare M, Roberts LM, Thorpe PE, Watson GJ, Prior B, Lord JM. Expression of ricin A chain in Escherichia coli. FEBS Letter 1987;216:73-8.
- Smallshaw JE, Ghetie V, Rizo J, Fulmer JR, Trahan LL, Ghetie MA, et al. Genetic engineering of an immunotoxin to eliminate pulmonary vascular leak in mice. Nat Biotechnol 2003;21:387-91.
- Vitetta ES, Yen N. Expression and functional properties of genetically engineered ricin B chain lacking galactose-binding activity. Biochim Biophys Acta 1990;1049:151-7.
- Roberts LM, Lord JM. Ribosome-inactivating proteins: entry into mammalian cells and intracellular routing. Mini Rev Med Chem 2004;4:505-12.
- Ferrini JB, Martin M, Taupiac MP, Beaumelle B. Expression of functional ricin B chain using the baculovirus sytem. Eur J Biochem 1995;233:772-7.
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998;95:2509-14.
- Chen L, Yin J, Chen Y, Zhong J. Induction of Epstein-Barr virus lytic replication by recombinant adenoviruses expressing the zebra gene with EBV specific promoters. Acta Biochim Biophys Sin 2005;37:215-20.
- Stirpe F, Battelli MG. Ribosome-inactivating proteins: progress and problems. Cell Mol Life Sci 2006;63:1850-66.
- Rao PV, Jayaraj R, Bhaskar AS, Kumar O, Bhattacharya R, Saxena P, et al. Mechanism of ricin-induced apoptosis in human cervical cancer cells. Biochem Pharmacol 2005;69:855-65.
- Majhen D, Ambriovic-Ristov A. Adenoviral vectors – how to use them in cancer gene therapy? Virus Res 2006;119:121-33.
