Calcitonin gene-related peptide inhibits interleukin-1β-induced interleukin-8 secretion in human type II alveolar epithelial cells1
Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are chronic inflammatory lung diseases associated with progressive airflow limitation. They are characterized by the presence of leukocytes in the lungs[1]. Type II alveolar epithelial (AEII) cells function in synthesis, secretion, and recycling of all components of the surfactant. Results from a number of published studies have shown that pulmonary epithelial cells, especially type II cells, help recruit inflammatory cells to the lungs by generating the chemokines interleukin (IL)-8 and monocyte chemoattractant protein-1 (MCP-1) in response to inflammatory factors such as tumor necrosis factor (TNF)-α and IL-1β. They have also found that AEII cells can play an important role in lung inflammatory diseases[2,3]. As well as expressing surfactants and chemokines, AEII cells also express adhesive molecules and other immune-related factors[4]. AEII cells may act as immunoregulatory cells to modulate the physiological and pathological functions of the lungs.
The suppression of immune responses and improvement of alveolar fluid clearance has received much attention. Numerous endogenous agents have been verified to protect cell damage from pro-inflammation. In our previous data, we demonstrated that AEII cells secrete neuropeptide calcitonin gene-related peptide (CGRP) and that IL-1β induces CGRP secretion[5,6]. Our initial results showed that AEII cell-derived CGRP could suppress inflammatory chemokine IL-8 secretion induced by IL-1 in an autocrine/paracrine mode. CGRP is a potent vasodilator neuropeptide localized in peripheral and central nerves. It also has broad anti-inflamatory effects, especially in the immune and related systems[6,7]. In the lungs, CGRP-like immunoreactivity is localized in the nerve fibers of the airway mucosa and around vascular smooth muscle[8,9]. CGRP modulates airway functions in health and disease. These functions include vasoregulation, broncho-regulation, anti-inflammatory actions and tissue repair[10]. It is interesting to note that CGRP is also expressed in AEII cells. Non-specific immune cells and the enhanced release of CGRP under inflammatory stress may play a negative feedback role in the local immune reaction[5].
In the pathogenesis of many inflammatory lung diseases such as acute respiratory distress syndrome, COPD and asthma, neutrophils have been implicated to play an important role. The CXC chemokine IL-8 is a potent neutrophil-recruiting and activating factor. Although lipopolysaccharide, IL-1β and TNF-α are IL-8 inducers, IL-8 exerts its effects on neutrophils by binding with high affinity to 2 chemokine receptors, CXCR1 and CXCR2, on the cell surface. In bronchoalveolar lavage of children with chronic respiratory diseases, IL-8 levels are significantly associated with IL-1β, and the detection of IL-8 in clinical samples from patients with these diseases has led clinicians to believe that the antagonism of IL-8 may be a practicable therapeutic strategy for disease management[11].
The present study was designed to confirm the inhibitory effect of AEII-derived CGRP on IL-1β-induced IL-8 secretion in the human AEII cell line (A549) and to investigate the mechanisms of the inhibitory effects.
Materials and methods
Materials A rabbit anti-human CGRP antibody (Ab) and recombinant human αCGRP, hCGRP8–37 and an anti-CGRP antibody were purchased from Peninsula Laboratory (Belmont, CA, USA). Recombined human IL-1β was purchased from PeproTech House (W6 Bll, London, United Kingdom). Trizol was purchased from Promega (Madison, WI, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased from Hyclone Co (Logan, UT, USA). All other chemicals and drugs were purchased from Sigma Chemical Co (St Louis, MO, USA) and Chinese Chemical Co (Beijing, China).
Cell culture A549 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were grown in DMEM supplemented with 10% FBS and penicillin/streptomycin (0.1 µL) in a humidified 37 °C incubator. Before being treated with chemicals, the cells were washed with DMEM twice and maintained in DMEM without FBS at 37 °C for 3 to 4 h. To avoid peptide degradation, 1 mg·L-1 aprotinin was added to every experimental group when the cells were incubated with hCGRP or hCGRP8−37.
ELISA detection of IL-8 Ninety-six well-microtiter plates (Nunc, Gmb H & Co. KG, Wiesbaden, Germany) were coated with 50 µL containing 1 mg·L-1 of the goat anti-mouse IL-8 antibody (R&D Systems, BorsigstraBe, Wiesbaden, Germany) in PBS at 4 °C for 48 h. After removing the excess capture Ab, the wells were filled with 50 µL of Casine/PBS (Pierce, Biotechnology, Inc, Rockford, IL, USA) and incubated at room temperature for 1 h to saturate excess binding sites. After being washed 3 times with wash buffer (0.125% Triton X-100/PBS), serial dilutions of the experimental samples diluted in 10% Casine/PBS were added to the plates, which were incubated at room temperature for 2 h. After 3 washes, 100 µL of the biotinylated detector anti-hIL-8 Ab was added to the wells and incubated for 1 h at room temperature. After an additional 3 washes, the plates were incubated with peroxidase-conjugated streptavidin for 0.5 h. After 3 final washes, the solutions were developed with hydrogen peroxide and tetramethylbenzidine (Sigma, St Louis, Mo, USA) and then stopped by the addition of 1.5 mol/L H2SO4. Titrations of recombinant human MCP-1 (R&D Systems, BorsigstraBe, Wiesbaden, Germany) were included in each experiment for standardization.
Stable transfection of CGRP gene into A549 cells A pcDNA3.1 plasmid containing human CGRP cDNA was constructed as previously described[12] and transferred to the A549 cells with the use of Lipofectin (Invitrogen, Carlsbad, CA, USA). After transfection, the cells were cultured with G418 (800 mg·L-1) for 4 d, and a stably transfected cell line was selected by subculturing the cells from a monoclone.
RNA extraction and RT-PCR The DMEM was removed and the cells were washed twice with PBS. The RNAs were then extracted by use of 1 mL Trizol and 0.2 mL chloroform and precipitated with 0.5 mL isopropanol. The suspension was kept at -70 °C for 30 min and centrifuged at 12 000×g at 4 °C for 15 min. The pellet was washed with 0.5 mL 70% ethanol and dried. The RNA was stored at -70 °C.
As previously reported[5], the RNA was reverse transcribed with oligo (dT) 15–17. The oligonucleotide primers for IL-8 and β-actin were synthesized on a DNA synthesizer (Model 49a, Applied Biosystems Inc., Foster, CA, USA). The nucleotide sequences of β-actin were sense, 5'-CATCTCTT-GCTCGAAGTCCA-3' and antisense, 5'-ATCATGTTTGAGA-CCTTCAACA-3'; the PCR product was 300 bp; human IL-8 primers were sense, 5'-ATTTCTGCAGCTCTGTGTGAAGG-TGC-3' and antisense, 5'-TGACCTTTGACCTAGTTTTG-3'; the product was 749 bp[13]. The PCR amplification process consisted of denaturation at 94 °C for 30 s, primer annealing at 58 °C (β-actin) or 60 °C (IL-8) for 30 s, and extension at 72 °C for 1.5 min. PCR was run for 22 cycles for β-actin and 24 cycles for IL-8.
Inhibition of CGRP expression by small interfering RNA (siRNA) As we described previously[14], RNA-interfering plasmid for the β-CGRP gene was transferred to the A549 cells with the use of Lipofectin (Invitrogen, Carlsbad, CA, USA). For stable inhibition of CGRP, 2 µg of pSuper-CGRP was cotransfected with 1 µg of pBABE-puro plasmid. The transfected cells were selected with 1 µg/mL puromycin for 7 d. Monoclones were chosen and expanded. Reverse-transcription PCR and radioimmunoassay showed decreased CGRP mRNA levels and protein secretion by 50%–70% in transfected cells.
Measurement of intracellular ROS generation The production of ROS, especially H2O2, by A549 cells was detected with luminol plus horseradish peroxide-derived chemiluminescence in a lit box and a luminescence analyzer (BPCL Ultra-weak, Beijing, China) at 37 °C as previously described[15]. Photon counts were integrated over 1 s and shown on a computer monitor. A549 cells were incubated with IL-1β or/and other chemicals for indicated times and the cells were then washed twice with 1 mL 37 °C PBS. Horseradish peroxide 10 µg/mL and 0.5 mmol/L luminol were then added. The luminol plus horseradish peroxidase-derived chemiluminescence was initiated by adding 3 mmol/L NADH as a substrate. All experiments were repeated at least 3 times; this reflected the formation of ROS.
Statistics The results are expressed as mean±SD. Data analysis involved the use of GraphPad Prism software (GraphPad software, Inc., San Diego, CA, USA). One-way ANOVA, Student-Newman-Keuls test (comparisons between multiple groups), or unpaired Student’s t-test (between 2 groups) was used as appropriate. P<0.05 was considered significant.
Results
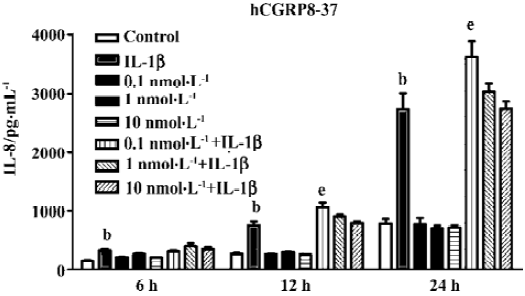
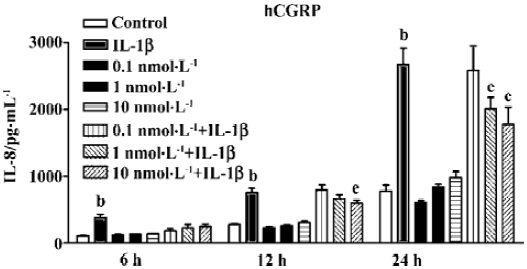
Suppression of IL-8 secretion by endogenous CGRP To investigate the effect of IL-1β-induced endogenous CGRP on IL-1β-induced IL-8 secretion, we treated A549 cells with hCGRP8−37 (0.1 nmol·L-1), a CGRP-1 receptor antagonist, simultaneously with IL-1β (1 ng/mL) for different times. As shown in Figure 1, hCGRP8−37 at a very low concentration of 0.1–1 nmol·L-1 significantly enhanced the IL-1β-induced IL-8 secretion, and even increased it to 130% at 24 h.
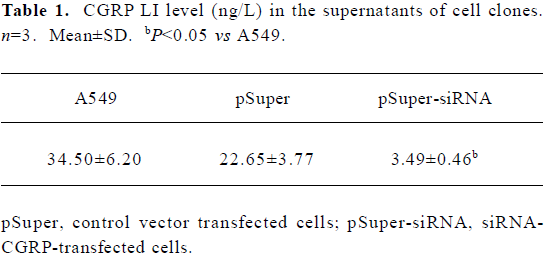
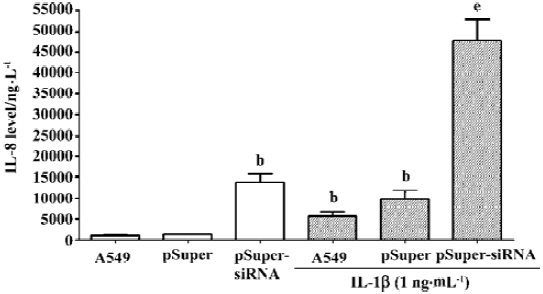
To further confirm that the endogenous CGRP has an inhibitory effect on IL-1β-induced IL-8 secretion, we used molecular and genetic manipulation of the CGRP expression. As previously reported, the knockdown of β-CGRP by siRNA[15] specifically attenuated both the CGRP mRNA and protein levels in A549 cells (Table 1). As shown in Figure 2, the inhibition of CGRP expression by siRNA significantly increased IL-8 secretion on IL-1β stimulation. These data strongly suggest that endogenous CGRP inhibits chemokine IL-8 expression induced by proinflammatory IL-1β.
Full table
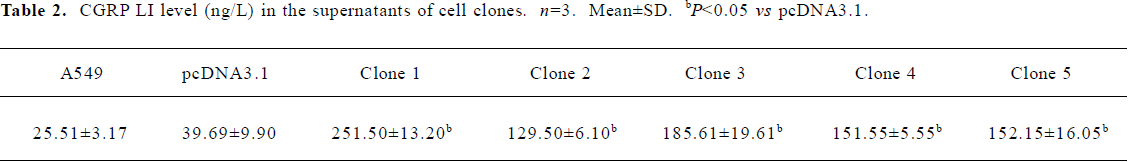
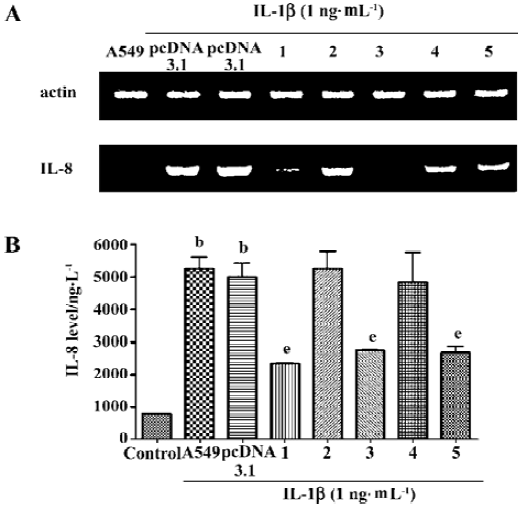
Suppression of IL-8 secretion by exogenous CGRP Exogenous CGRP (0.1–10 nmol·L-1) reduced IL-1β-induced IL-8 secretion in a concentration-dependent manner, and the inhibition reached more than 30% at 24 h (Figure 3). Moreover, A549 cells stably transfected with the CGRP gene showed an elevated CGRP protein level (Table 2). Compared with the control and empty vector clone, after IL-1β adminis-tration, both the mRNA and protein levels of IL-8 were greatly inhibited in the CGRP high-expression clones (Figure 4A,4B). The CGRP inhibitory effect mimicked exogenous CGRP administration in cells.
Full table
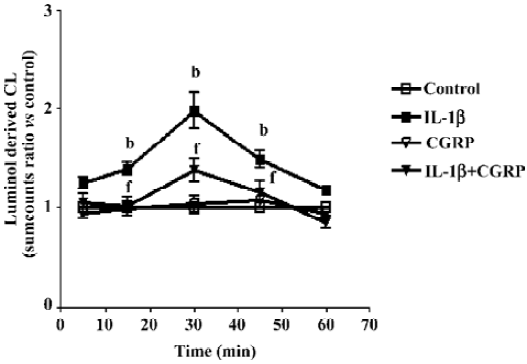
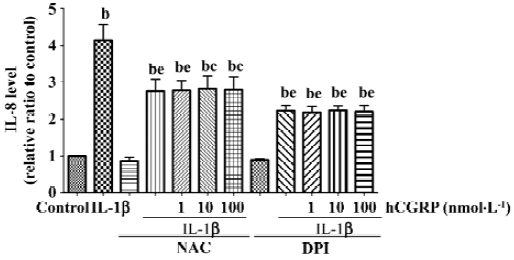
Effect of CGRP on IL-1β-induced ROS formation Chemiluminescence assay in A549 cells showed that CGRP (100 nmol·L-1) significantly inhibited IL-1β-induced ROS production in A549 cells (Figure 5). N-acetyl-L-cysteine (NAC), a thiol-based antioxidant, and ROS scavenger or diphenylene-iodonium (DPI), an inhibitor of mitochondrial NADPH-ubiquinone oxido-reductase, also reduced in part IL-1β-evoked IL-8 production and abolished the CGRP effect, which indicates that CGRP exerts its inhibitory function via a ROS pathway (Figure 6).
Discussion
In the present study, we demonstrate that endogenously expressed CGRP in the AEII cell line A549 suppresses IL-1β-induced IL-8 secretion in an autocrine/paracrine mode by inhibiting the ROS pathway.
As an approximately 70 to 72-residue mature protein, IL-8 can be produced by leukocytic cells (monocytes, T cells, neutrophils, and natural killer cells) and nonleukocytic somatic cells (endothelial cells, fibroblasts, and epithelial cells)[16]. IL-8 binds to 2 distinct receptors, CXCR1 and CXCR2, with a similar high affinity, and the receptors are mainly expressed on the cell surface of neutrophils[17,18]. After ligand binding, the receptors are internalized and subsequently recycled and reappear on the cell surface rapidly within 60 min[19]. More-over, ligand binding activates pertussis toxin-sensitive and receptor-coupled G proteins, particularly Gαi proteins[20]. G proteins then activate phosphatidylinositol 3-kinase-γ (PI3K-γ), which in turn generates phosphatidylinositol 3,4,5-trisphosphate (PIP3)[21]. PIP3 activates protein kinase B (Akt) as well as GTPases, resulting in directed cell migration.
In lung infection, including bacterial and viral infection, lung injury, including reperfusion injury, transplantation and other physical conditions, and lung acute and chronic inflammatory diseases, such as acute respiratory distress syndrome, asthma and COPD, IL-8 works as a key mediator in neutrophil-mediated acute inflammation because of its potent actions on neutrophils. In our previous and present data, proinflammatory factor IL-1β also stimulated CGRP secretion from AEII cells and endogenous/exogenous CGRP was found to inhibit IL-1β-induced IL-8 elevation in an autocrine/paracrine manner. These data indicate that AEII cells, together with neurogenic CGRP, may restrict lung inflammation at least in part, eliminating injury chemockine secretion.
CGRP is considered an immunomodulatory neuropeptide. We have demonstrated that CGRP can attenuate multiple low-dose streptozotocin-induced insulitis and reduce the occurrence of diabetes in mice[12]. As well as this, in vitro experiments have found that lymphocyte-derived CGRP can inhibit Con A-induced proliferation and IL-2 production in rat thymocytes in an autocrine/paracrine mode[22]. In the present study, a very low concentration of hCGRP8–37 (0.1 nmol·L-1), an antagonist of the CGRP1 receptor, at approximately 10 times more than that of endogenous CGRP, significantly magnified the IL-1β-induced IL-8 secretion (Figure 1) between 6 and 24 h. The knockdown of β CGRP gene expression by siRNA in A549 cells did not change the basal level of IL-8, but greatly potentiated IL-1β-induced IL-8 secretion. These data indicate that endogenous CGRP may play an inhibitory effect on the inflammatory process. Stably transfected, high-level CGRP clones seem to attenuate the cell responsibility of IL-8 secretion to IL-1β (Figure 4). In lung or airway inflammatory diseases, CGRP released from terminal afferents, neuroendocrine cells, and AEII cells might lead to a high local concentration of CGRP. We predict that CGRP will not only inhibit the immunoreactivity of the lung epithelium to inflammatory factors by reducing IL-8 secretion, but also enhance the phagocytosis of the peripheral macrophages to attenuate the inflammation[23].
IL-1β has been shown to stimulate the production of ROS, a class of highly reactive, diffusible, and ubiquitous molecules, in various cell types. ROS are involved in aging and many diseases such as cancer, diabetes mellitus, atherosclerosis, neurological degeneration, angiogenesis, and tumor invasion[24,25]. IL-1β primes and triggers O2¯· formation by activation of the NADPH oxidase and thereby provides an oxidative burst[26]. Numerous evidence have reported that ROS scavenger or NADPH oxidase inhibitor significantly reduces the IL-1β-induced expression of target genes[26,27]. IL-8 is one of the target genes of IL-1β[28]. Our previous report stated that CGRP gene therapy suppressed ROS in the pancreas and protected mice against autoimmune diabetes[29], thus, CGRP may protect β cells against ROS damage. In the present study, our findings that endogenous and exogenous CGRP inhibit IL-1β-induced ROS production in A549 cells imply that CGRP may have a broad inhibitory effect on ROS formation.
It is interesting to note that IL-1β simultaneously induces the secretion of IL-8 and CGRP, whereas CGRP itself acts on A549 cells and inhibits IL-8 production. On the one hand, in a seemingly autocrine feedback loop, IL-1β, as a stimulatory factor attracts inflammatory cells to the local area via chemo-kine IL-8. On the other hand, it also triggers the protective mechanism by CGRP release to confine the inflammatory response and avoid excessive injury. This kind of autocrine feedback loop may be a protective mechanism of tissue.
In conclusion, we have shown that in A549 human AEII cells, intrinsic and extrinsic peptide CGRP inhibits IL-1β-induced IL-8 secretion in an autocrine/paracrine mode via ROS suppression. Our data provide a new insight into the immunomodulatory effect of AEII-derived CGRP and suggests a novel therapeutic target in lung inflammatory disease.
References
- Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res 2001;2:33-46.
- Standiford TJ, Kunkel SL, Phan SH, Rollins BJ, Strieter RM. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem 1991;266:9912-8.
- Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP III, Toews GB, et al. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest 1990;86:1945-53.
- Cunningham AC, Kirby JA. Regulation and function of adhesion molecule expression by human alveolar epithelial cells. Immunology 1995;86:279-86.
- Li W, Hou L, Hua Z, Wang X. Interleukin-1beta induces beta-calcitonin gene-related peptide secretion in human type II alveolar epithelial cells. FASEB J 2004;18:1603-5.
- Bell D, McDermott BJ. Calcitonin gene-related peptide in the cardiovascular system: characterization of receptor populations and their (patho) physiological significance. Pharmacol Rev 1996;48:253-88.
- Dakhama A, Larsen GL, Gelfand EW. Calcitonin gene-related peptide: role in airway homeostasis. Curr Opin Pharmacol 2004;4:215-20.
- Lundberg JM, Franco-Cereceda A, Hua X, Hokfelt T, Fischer JA. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol 1985;108:315-9.
- Cadieux A, Springall DR, Mulderry PK, Rodrigo J, Ghatei MA, Terenghi G, et al. Occurrence, distribution and ontogeny of CGRP immunoreactivity in the rat lower respiratory tract: effect of capsaicin treatment and surgical denervations. Neuroscience 1986;19:605-27.
- Springer J, Geppetti P, Fischer A, Groneberg DA. Calcitonin gene-related peptide as inflammatory mediator. Pulm Pharmacol Ther 2003;16:121-30.
- Clementi G, Amico-Roxas M, Caruso A, Catena Cutuli VM, Prato A, Maugeri S, et al. Effects of CGRP in different models of mouse ear inflammation. Life Sci 1994;54:PL119-24.
- Sun W, Wang L, Zhang Z, Chen M, Wang X. Intramuscular transfer of naked calcitonin gene-related peptide gene prevents autoimmune diabetes induced by multiple low-dose streptozotocin in C57BL mice. Eur J Immunol 2003;33:233-42.
- Yamaguchi N, Yamashita Y, Ikeda D, Koga T. Actinobacillus actinomycetemcomitans serotype b-specific polysaccharide antigen stimulates production of chemotactic factors and inflammatory cytokines by human monocytes. Infect Immun 1996;64:2563-70.
- Wang W, Jia L, Wang T, Sun W, Wu S, Wang X. Endogenous calcitonin gene-related peptide protects human alveolar epithelial cells through protein kinase Cepsilon and heat shock protein. J Biol Chem 2005;280:20325-30.
- Allen RC. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol 1986;133:449-93.
- Mukaida N. Pathophysiological roles of interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell Mol Physiol 2003;284:L566-77.
- Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science 1991;253:1278-80.
- Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science 1991;253:1280-3.
- Samanta AK, Oppenheim JJ, Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem 1990;265:183-9.
- Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev 2000;177:175-84.
- Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000;287:1037-40.
- Xing L, Guo J, Wang X. Induction and expression of beta-calcitonin gene-related peptide in rat T lymphocytes and its signifi-cance. J Immunol 2000;165:4359-66.
- Ichinose M, Sawada M. Enhancement of phagocytosis by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Peptides 1996;17:1405-14.
- Harris GK, Shi X. Signaling by carcinogenic metals and metal-induced reactive oxygen species. Mutat Res 2003;533:183-200.
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 2003;27:277-84.
- Brigelius-Flohe R, Banning A, Kny M, Bol GF. Redox events in interleukin-1 signaling. Arch Biochem Biophys 2004;423:66-73.
- Piette J, Piret B, Bonizzi G, Schoonbroodt S, Merville MP, Legrand-Poels S, et al. Multiple redox regulation in NF-kappaB transcription factor activation. Biol Chem 1997;378:1237-45.
- Wu HM, Wen HC, Lin WW. Proteasome inhibitors stimulate interleukin-8 expression via Ras and apoptosis signal-regulating kinase-dependent extracellular signal-related kinase and c-Jun N-terminal kinase activation. Am J Respir Cell Mol Biol 2002;27:234-43.
- She F, Sun W, Mao JM, Wang X. Calcitonin gene-related peptide gene therapy suppresses reactive oxygen species in the pancreas and prevents mice from autoimmune diabetes. Acta Physiol Sin 2003;55:625-32.
