Circadian expression of clock genes and angiotensin II type 1 receptors in suprachiasmatic nuclei of sinoaortic-denervated rats1
Introduction
The circadian clock system is responsible for the daily timing of behavior and physiological processes[1]. In mammals, the master circadian pacemaker controlling most of these rhythms is located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus, whose phase is mainly light-entrained directly through the retinohypothalamic tract[2]. Recent experiments have demonstrated an oscillatory system including a transcriptional-translational feedback loop of clock genes. These include 3 homologues of the Drosophila gene period (Per1, Per2, and Per3), 2 cryptochrome genes (Cry1 and Cry2), and the transcriptional activator genes: clock (the mammalian counterpart of the Drosophila cycle) and Bmal1 (a member of the basic helix-loop-helix/PER-ARNTSIM domain family of transcription factors)[3,4]. These clock genes are expressed in a pattern with circadian oscillations close to 24 h in the SCN. Among these clock genes, Per2 and Bmal1 are most important and were studied in the present work.
Intra-arterial blood pressure monitoring and non-invasive ambulatory blood pressure monitoring had shown that blood pressure exhibited variations consistent with circadian rhythm[5,6]. Most normotensive and essential hypertensive individuals show a diurnal pattern of blood pressure with markedly higher values during the active phase and lower values during the rest phase (dippers); however, a number of subjects display abnormal circadian blood pressure profiles characterized by an absence or even reversal in a nocturnal fall in blood pressure (non-dippers). Cross-sectional studies have indicated that target-organ damage is more pronounced in “non-dipper” than in “dipper” patients with comparable clinical blood pressure levels[7]. The transgenic hypertensive TGR(mREN2)27 rats harboring the murine Ren2 gene manifest an inverted circadian rhythm of blood pressure similar to “non-dippers”, while 24 h profiles of motor activity and heart rate remain undisturbed[8]. Moreover, the circadian rhythm of clock gene mRNA expression in the SCN of TGR(mRen2)27 rats was abolished[9].
Arterial baroreflex (ABR) is one of the most important mechanisms in the regulation of cardiovascular activities, especially in the stabilization of blood pressure. ABR function is impaired in hypertension and closely related to hypertensive organ damage[10]. In addition, the interruption of ABR by sinoaortic denervation could lead to irreversible organ damage such as cardiac lesion, vascular remodeling, and renal damage in rats[11]. Furthermore, it was found that sinoaortic denervation changed the rhythmicity of blood pressure from a 24 h period to a 12-h period[12]. However, it is not clear whether the function of baroreflex influences the circadian expression of clock genes in the central nervous system. The present work was therefore designed to observe the possible effects of sinoaortic denervation on the mRNA and protein expression of 2 main clock genes: Per2 and Bmal1 in the rat SCN.
Angiotensin II (Ang II), a key molecule for cardiovascular regulation, influences the activity of brain areas involved in the determination of circadian-dependent variations of blood pressure[13]. Therefore, the expression of Ang II type 1 (AT1) receptors in the same brain area of rats was also studied.
Materials and methods
Preparation of sinoaortic denervated (SAD) rats Male Sprague-Dawley (SD) rats were provided by Sino-British SIPPR/BK Lab Animal Ltd (Shanghai, China). At the age of 12 weeks, sinoaortic denervation was performed with a previously used method[14,15]. Briefly, the rats were anesthetized with a mixture of ketamine (50 mg/kg) and diazepam (5 mg/kg), intraperitoneally, and were then medicated with atropine sulfate (0.5 mg/kg, intraperitoneally) and procaine benzylpenicillin (60000 U, intramuscularly). After a midline cervical incision and bilateral isolation of the neck muscles, aortic baroreceptor denervation was carried out bilaterally by cutting the superior laryngeal nerves near the vagi, removing the superior cervical ganglia, and sectioning the aortic depressor nerves. The carotid sinus baroreceptors were denervated bilaterally by stripping the carotid bifurcation and its branches followed by the application of 10% phenol (in 95% ethanol) to the external, internal, and common carotid arteries and the occipital artery. In the control rats, the sham operation was performed under the same conditions, without cutting and removing the nerves and carotid adventitia. The animals were allowed to recover spontaneously after the denervation. All procedures were in accordance with institutional animal care guidelines, and approved by the local institutional committee.
Blood pressure measurement Blood pressure and heart period were continuously recorded using previously described techniques[16,17]. Briefly, the rats were anesthetized with a combination of ketamine (50 mg/kg) and diazepam (5 mg/kg). A floating polyethylene catheter was inserted into the lower abdominal aorta via the left femoral artery for blood pressure measurement. The catheters were exteriorized through the interscapular skin. After a 2 d recovery period, the animals were placed for blood pressure recording in individual cylindrical cages containing food and water. The aortic catheter was connected to a blood pressure transducer via a rotating swivel that allowed the animals to move freely in the cage. After about 4 h of habituation, the blood pressure signal was digitized by a microcomputer. Blood pressure and heart period values from every heartbeat were determined online. The mean values of these parameters during a period of 4 h were calculated. The standard deviation of the mean values was also calculated and served as the variabilities of blood pressure or heart period.
Experimental protocol Four weeks after the operation, blood pressure and heart period were measured in the conscious state in a group of sham-operated (n=10) and SAD rats (n=9). The remaining 72 (36 SAD rats and 36 sham-operated) rats were brought up in a separate, environmentally-controlled room (temperature 23–25 °C) with ventilated chambers under a 12/12 h light/dark cycle (8:00−20:00 light, 20:00−8:00 dark) for at least 10 d with food and water available ad libitum. The light was provided by white fluorescent bulbs (220 mW/cm2). Under entrained conditions, lights on was defined as Zeitgeber time (ZT) 0. Six SAD rats and 6 sham-operated rats were killed every 4 h from ZT0 to ZT20 by ether anesthesia. The mRNA and protein expression of the clock genes (Per2, Bmal1) and AT1 receptors were examined by RT-PCR and Western blotting, respectively. The brain was removed from the skull and snap-frozen in liquid nitrogen until use. The SCN region was punched out with a 2 mm diameter needle that was inserted to a 1 mm depth into the surface of the coronal plane, an area that included the SCN[17,18]. The animals were killed at each time point on 3 separate days to ensure reproducibility of the gene expression cycle.
RNA extraction and RT-PCR Total RNA was extracted from the SCN using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA concentrations were determined by spectrophotometry at 260 nm, and total RNA quality was assessed by electrophoresis on 1.3% denaturing formaldehyde agarose gels. Total RNA (<1 µg) was reverse transcribed using an RT system kit from Promega (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocols. Primers were designed according to cDNA sequences reported in the GenBank database and by computer analysis using the Primer premier 5.0. (PREMIER Biosoft International, Palo Alto, CA, USA) and synthesized at the SBS Genetech Co (Beijing, China). The sequences of primers are described in Table 1. PCR was performed with the 2×Taq PCR MasterMix (Tianwei Biotech, Beijing, China) using a Peltier Thermal Cycler 200 (MJ Research Inc, Watertown, MA, USA). The PCR protocol for Per2 was as follows: initial incubation at 94 °C for 2 min, 35 cycles of denaturation at 94 °C for 40 s, annealing at 54 °C for 1 min, and extension 72 °C for 1 min, and finally a prolonged extension step at 72 °C for 10 min. The PCR protocol for Bmal1 and AT1 differed slightly from that of Per2; annealing was performed at 58 °C or 56 °C, respec-tively. The relative amount of each mRNA was normalized to the housekeeping gene, β-actin, mRNA that the expression levels remained constant throughout the day. The PCR products were resolved by electrophoresis on a 2.0% agarose gel, stained with ethidium bromide (EB), and their relative quantities were determined using the Gel-pro Analyzer 4.5 software (Media Cybernetics, Silver Spring, MD, USA).
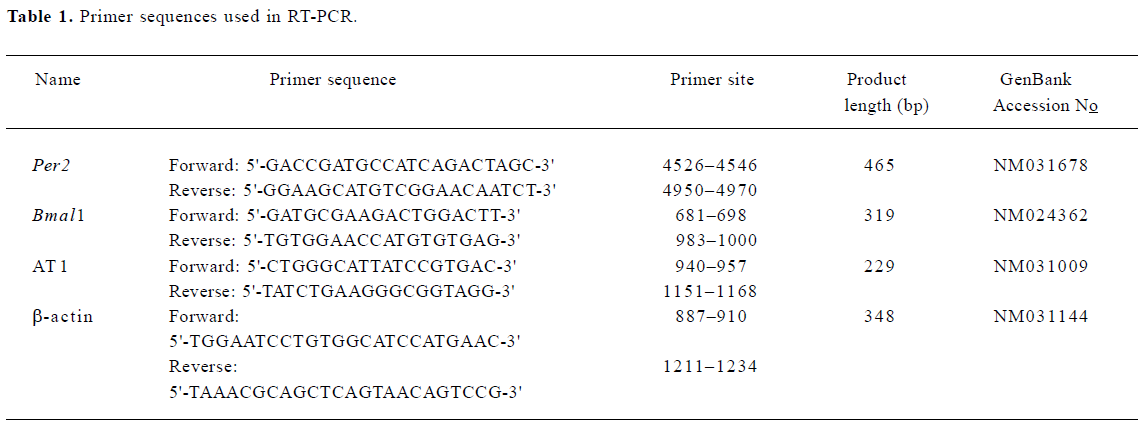
Full table
Western blotting Microdissected SCN tissue was homogenized in lysis buffer [50 mmol/L Tris-HCl (pH 7.5)], 150 mmol/L NaCl, 5 mmol/L EDTA, 1% NP-40, 1 mmol/L PMSF, plus a protease inhibitor cocktail (pH 7.4) for 30 min on ice. After removal of tissue debris by centrifugation (12 000×g, 5 min), the protein concentration of each lysate sample was determined using the BCA-100 protein assay kit (Boshide Biotech, Wuhan, China) according to the manufacturer’s instructions. The samples were boiled in ×2 SDS sample buffer and loaded with 50 µg proteins per lane onto 8% SDS-polyacrylamide gels. Following separation at 125 V for 120 min, the proteins were transferred onto nitrocellulose membranes, which were then blocked with 5% nonfat dried milk in TBS-Tween (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.25% Tween-20) for 4 h at room temperature. After brief washes, the membranes were incubated with polyclonal anti-mouse Per2 (1:100) and Bmal1 (1:200) antibodies (Abcam Inc, Cambridge, MA, USA) at 4 °C overnight. Immunoreactive bands were visualized using anti-rabbit IgG-HRP antibody and an ECL chemiluminescence system (Santa Cruz Biotech. Inc, CA, USA). The exact quantities of Per2 and Bmal1 were normalized against actin as a constitutively expressed internal control using optical density determined by the Gel-pro Analyzer 4.5 software (Media Cybernetics, USA).
Statistical analysis The statistical analyses were conducted directly with SPSS11.5 software. All data are express-ed as mean±SD. Multi-group mean values were compared by using ANOVA followed by Student Newman-Keuls test, and two-group mean values were compared by using an unpaired Student’s t-test. A significant difference among mean values was assigned at P<0.05.
Results
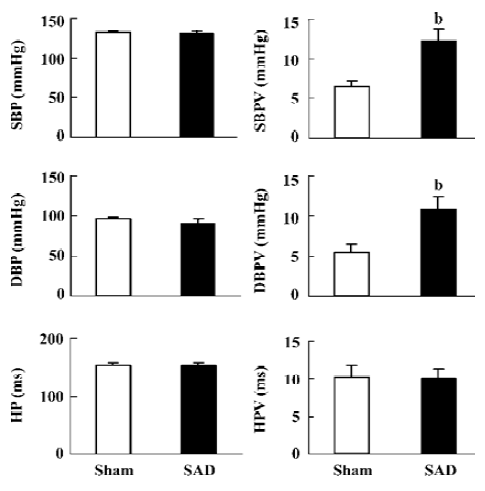
Blood pressure and heart period in SAD rats It was found that blood pressure levels in the SAD rats were similar to those of the sham-operated rats. However, blood pressure variabilities significantly increased in the SAD rats compared with the sham-operated rats (Figure 1). Heart period and heart period variability were not modified by sinoaortic denervation operation.
Circadian expression of Per2 mRNA in the SCN of SAD rats The Per2 gene exhibited a robust circadian expression pattern in the SCN of the sham-operated SD rats, as previously reported in Wistar rats[18]. By visual inspection of gel electropherogram, high intensity DNA bands were observed at ZT12, ZT16, and ZT20, and weak, but still recognized bands, at ZT0, ZT4, and ZT8 (Figure 2A). Clear circadian transcription profiles of Per2 mRNA were detected by the relative quantitative analyses, with higher transcription levels around the light-to-dark transition (ZT12) and lower levels during the early daytime (ZT0-4). The peak expression was observed at ZT12 and was 2.19-fold stronger than the trough value at ZT0 (Figure 2B).
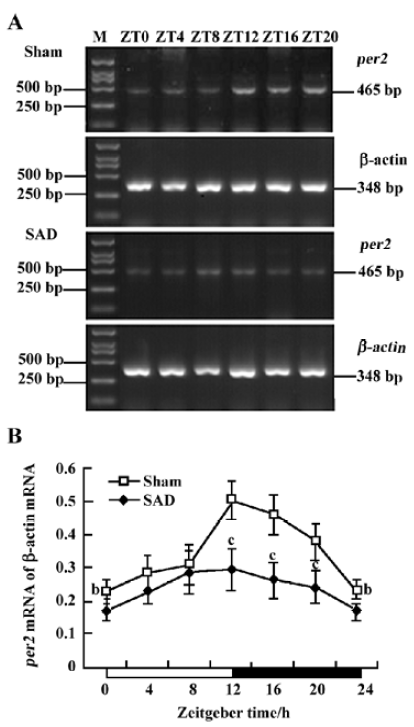
In the SAD rats, the Per2 gene showed a similar, but attenuated, circadian oscillation. The intensity of the DNA bands was weaker throughout the day than in the sham-operated rats (Figure 2A). The Per2 mRNA levels in the SCN were significantly lower in the SAD than in the sham-operated rats at most time points, except for ZT4 and ZT8 (Figure 2B).
Circadian expression of Bmal1 mRNA in the SCN of SAD rats A clear circadian expression of the Bmal1 gene was observed in the SCN of the sham-operated rats, which is in accordance with the finding in Wistar rats[19]. By visual inspection of gel electropherogram, high intensity DNA bands were detected at ZT16, whereas moderate intensity bands were detected at ZT0, ZT4, and ZT20. The intensity of the bands was faint at ZT8 and ZT12 (Figure 3A). The relative quantitative analyses displaying Bmal1 mRNA expression reached its peak early at night at ZT16 and descended to a trough late in the day at ZT8. The expression pattern was anti-phase to that observed for Per2 mRNA (Figure 3B).
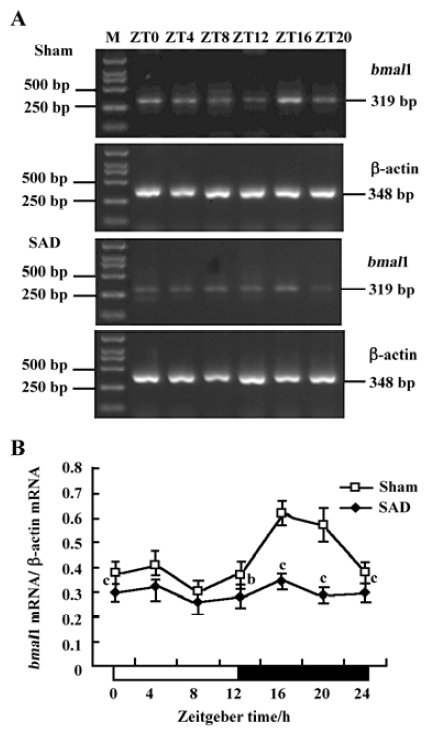
In the SAD group, the Bmal1 gene also presented syn-chronous, but subdued, circadian oscillation. There was scarcely any difference among the intensity of DNA bands at most time points by visual inspection of electropherogram (Figure 3A). Bmal1 mRNA expression in SAD rats significantly decreased throughout the day compared with the sham-operated rats, except at ZT8 (Figure 3B).
Circadian expression of the Per2 and Bmal1 proteins in the SCN of SAD rats The proteins extracted from the microdissected SCN were probed to detect Per2 and Bmal1. The anti-Per2 antibody consistently identified a major band of -130 kDa in the sham-operated and SAD rats, in accordance with the predicted mass of 136 kDa (Figure 4A). The intensity of the Per2-immunoreactivity (ir) bands exhibited a robust circadian oscillation in the sham-operated groups, with high expression during the early dark phase (ZT12−16), and low levels at the early light phase (ZT0−4), as previously reported in mice[20]. In the SAD rats, the intensity of the Per2-ir bands was significantly reduced at every time point compared with the controls, but a circadian pattern persisted. The quantitative analysis of normalizing the Per2-ir bands against actin-ir bands demonstrated that levels peaked at ZT16 and declined to a trough at ZT4 in both groups. There was a trend for decreased Per2 levels in the SCN of the SAD rats at most time points; the significant difference was only observed at ZT12 (Figure 4B).
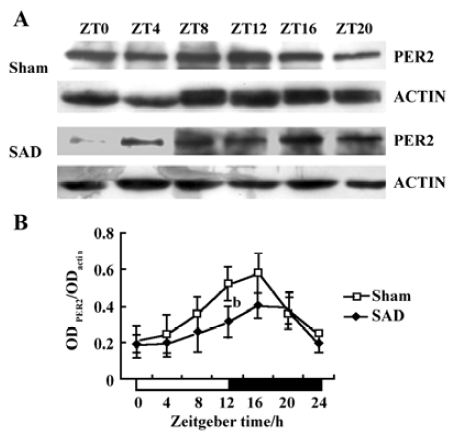
The anti-Bmal1 antibody identified a band around 68 kDa in the same SCN samples of the sham-operated and SAD rats (Figure 5A). The intensity of the Bmal1-ir band varied with a circadian pattern synchronized in both groups, with peak expression during late night at ZT16−20 and a nadir early in the subjective day at ZT0−4, accorded with the findings in Wistar rats[21]. The quantitative analysis of normalizing the Bmal1-ir bands against actin-ir bands showed that Bmal1 expression reached its peak at ZT20 and descended to a trough at ZT4. The Bmal1 levels significantly decreased at ZT8 and ZT20 in the SCN of the SAD rats compared with the sham-operated groups (Figure 5B).
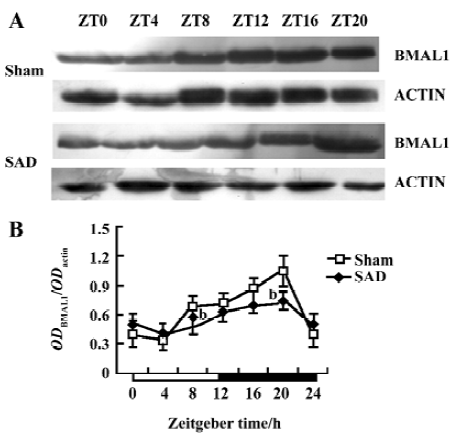
These results reinforced the findings that the circadian rhythm of some clock gene cycles in the SCN were attenuated after interruption of the arterial baroreflex.
Circadian expression of AT1 receptor mRNA in the SCN of SAD rats The circadian expression pattern of AT1 receptor mRNA expression in the SCN of the sham-operated rats was obvious. High-intensity bands were detected during the dark phase (ZT12−20), while weak intensity bands were detected in the light phase (ZT0−8, Figure 6A). AT1 receptor expression peaked at ZT16 and descended to a trough at ZT8 in the sham-operated rats (Figure 6B).
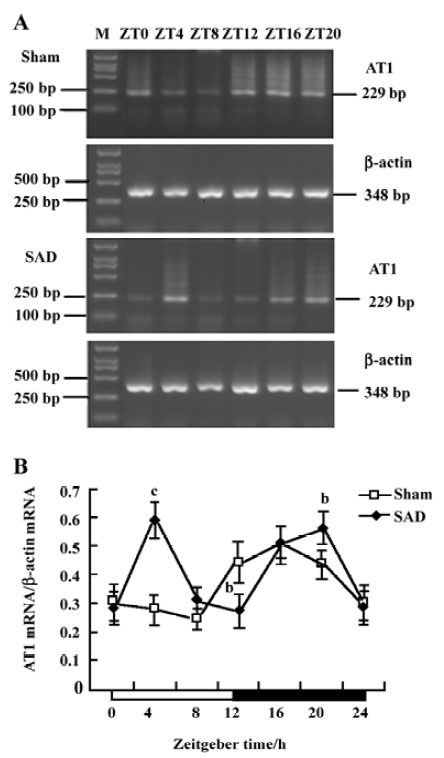
In contrast, the circadian expression profile of AT1 receptor mRNA changed to a bimodal pattern in the SCN of the SAD rats. High-intensity bands were detected abnormally at ZT4 (Figure 6A). AT1 receptor mRNA levels in the SAD group significantly increased in the light phase, greater than in the sham-operated group (Figure 6B).
Discussion
The present work shows, for the first time, that arterial baroreflex dysfunction may dramatically attenuate the circadian variation of clock gene expression in the SCN. The main findings of this study are as follows: (i) although Per2 and Bmal1 mRNA oscillated synchronously in the SCN of the SAD and sham-operated rats, the expression of mRNA levels and amplitude of oscillation were remarkably depressed in the SAD rats; (ii) the contents of the Per2 and Bmal1 proteins and the extent of the circadian variations were distinctly weakened in the SCN of the SAD rats compared with the controls; and (iii) AT1 receptor mRNA expressions in the SCN were abnormally upregulated in the light phase and altered the circadian rhythm of AT1 receptors in the SAD rats.
Circadian clock is normally entrained by periodic environmental cues, with the daily light-dark cycle being the most potent entraining signal in mammals. The clock showed phase delay and phase advance shifts in response to light exposure in early and late night, respectively. Per1 and Per2 have been demonstrated to be light-inducible in different rodent species[22,23]. In the present study, Per2 and Bmal1 genes oscillated synchronously in the SCN of the SAD and sham-operated rats. The peak level of Per2 mRNA was observed at ZT12 and at the trough level at ZT0, while Bmal1 mRNA transcription reached a peak at ZT16 and a nadir at ZT8. However, the Per2 and Bmal1mRNA levels and the amplitude of oscillation were dramatically reduced in the SCN of the SAD rats. The phenomena were confirmed by a Western blot analysis of Per2 and Bmal1 proteins in the same samples. Circadian profiles of the Per2 protein exhibited evident oscillation with a peak at ZT16; the Bmal1 protein showed a nocturnal peak at ZT20, indicating that the protein cycles were delayed by about 4–6 h, relative to the mRNA cycles under the light-dark conditions in the SCN. Similarly, the contents of Per2 and Bmal1 proteins at certain time points and the extent of circadian variations were distinctly weakened in the SCN of the SAD rats compared with the controls. The post-transcriptional regulation mechanisms may implicate the difference between the levels of mRNA and proteins of the clock genes. Our findings suggested that interruption of the arterial baroreflex could attenuate the circadian expression and rhythmicity of some central clock genes.
The ABR is most important in the regulation of heart rate and the stabilization of blood pressure. When ABR function is destroyed by sinoaortic denervation operation, blood pressure becomes unstable and blood pressure variability is great. However, the blood pressure level remains unchanged[14,15]. ABR function is impaired in hypertension, heart failure, and diabetes and has been found to be a crucial determinant of sudden death after acute myocardial infarction[24]. In hypertensive rats, the target organ damage correlates negatively with ABR function[10,25]. Interruption of ABR by sinoaortic denervation induced aortic and cardiac hypertrophy independent of the mean level of blood pressure[26–28]. In the present study, it was found that dysfunction of the ABR attenuated the circadian expression of clock genes in the SCN. It has been reported that mean blood pressure was significantly elevated during the light period in the SAD rats, thus the 24 h rhythmicity in mean blood pressure was suppressed and a bimodal pattern was observed under light–dark cycles, whereas the heart rate and locomotor activity were not dramatically affected in the same rats[12]. Although the precise mechanisms responsible for the selective elimination of the circadian rhythm of mean blood pressure in the SAD rats have not yet been satisfactorily clarified, the alteration of central clock genes induced by the disruption of ABR may be related with the phenomenon. These results further support the finding that ABR is not only important in maintaining the stability of blood pressure in the short term, but also in regulating the diurnal variation in blood pressure during a 24 h cycle.
It is well established that the central nervous system plays an important role in controlling ABR function. The major baroreceptor reflex pathway exists in the brain stem, such as the nucleus tractus solitarii and the caudal and rostral ventrolateral medulla[29]. Although these brain regions do not project directly to the SCN, the multisynaptic connections between them are much more extensive. Using the retrograde transneuronal tracer, the pseudorabies virus (PRV), which localizes the afferent inputs to the SCN in SD rats, has demonstrated that cells in the nucleus tractus solitarii, C3 catecholamine region, rostral ventrolateral medulla, peria-queductal gray matter, and lamina I and dorsomedial area of the spinal trigeminal nucleus are higher order sites in pathways which affect the SCN[30]. The presence of PRV-infected neurons in the brain stem indicates a pathway associated with regulatory mechanisms of arterial pressure which can influence SCN function and even clock gene expression. However, there is insufficient evidence showing a direct action of ABR on gene expression in SCN.
The renin-angiotensin system (RAS) is one of the major humoral mechanisms regulating cardiovascular function. Ang II plays a major role in the modulation of blood pressure, stimulation of vasopressin and aldosterone release, sodium appetite, and sympathetic facilitation. Most of these effects show a day–night variation and are mediated via AT1 receptors. Furthermore, the SCN contains not only Ang II-immunoreactive cells and fibers, but also a high density of Ang II receptors[31]. It was recently described that Ang II induces oscillatory expression of clock genes in vascular smooth muscle cells through the AT1 receptor subtype[32] and alters the ABR through peripheral (vascular and renal) and central effects[33]. Impaired ABR function is usually accompanied by overactivation of tissue RAS. Previous studies have confirmed that plasma Ang II levels did not increase in the chronic phase of sinoaortic denervation; in contrast, cardiac, aortic, and renal Ang II contents increased and AT1 receptor mRNA expression in the left ventricle and aorta was upregulated after sinoaortic denervation[34,35]. In the present work, the SCN AT1 receptor mRNA expression was abnormally upregulated in the light phase, resulting in the circadian rhythm of AT1 receptors from a 24 h cycle to a 12 h cycle in the SAD rats. This is in accordance with the changes of blood pressure rhythm induced by the interruption of ABR.
It is reasonable to hypothesize that ABR dysfunction may activate tissue RAS, including the SCN. Ang II acts as a candidate entraining signal, altering circadian expression of central clock genes through AT1 receptors. However, the cause–effect relationship between the changes in clock genes and RAS is not clear at this stage of study.
In conclusion, the circadian variation of the 2 central clock genes was attenuated in the SAD rats. ABR dysfunction also induced a disturbance in the expression of AT1 receptors in the SCN. The precise mechanisms underlying ABR dysfunction inducing an attenuated expression of the 2 clock genes remain to be elucidated in the future.
References
- Whitmore D, Cermakian N, Crosio C, Foulkes NS, Pando MP, Travnickova Z, et al. A clock organ. Biol Chem 2000;381:796-8.
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature 2002;418:935-41.
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng BH, et al. Interacting molecular loops in the mammalian circadian clock. Science 2000;288:1013-9.
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 2001;63:647-76.
- Basset A, Laude D, Laurent S, Elghozi JL. Contrasting circadian rhythms of blood pressure among inbred rat strains: recognition of dipper and non-dipper patterns. J Hypertens 2004;22:727-37.
- Finta E, Laude D, Alfoldi S, Farsang C, Elghozi JL. Effects of rilmenidine on 24-h rhythmicity of blood pressure and spontaneous baroreflex sensitivity in essential hypertensive subjects. J Hypertens 2006;24:1619-25.
- Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, et al. Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure. J Hypertens 2004;22:273-80.
- Lemmer B, Mattes A, Böhm M, Ganten D. Circadian blood pressure variation in transgenic hypertensive rats. Hypertension 1993;22:97-101.
- Lemmer B, Witte K, Enzminger H, Schiffer S, Hauptfleisch S. Transgenic TGR(mREN2)27 rats as a model for disturbed circadian organization at the level of the brain, the heart, and the kidneys. Chronobiol Int 2003;20:711-38.
- Xie HH, Shen FM, Miao CY, Su DF. Blood pressure, baroreflex sensitivity, and end organ damage in hybrid offspring of spontaneously hypertensive rats and Sprague-Dawley rats. Acta Pharmacol Sin 2005;26:1049-56.
- Shan ZZ, Dai SM, Su DF. Arterial baroreflex deficit induced organ damage in sinoaortic denervated rats. J Cardiovasc Pharmacol 2001;38:427-37.
- Makino M, Hayashi H, Takezawa H, Hirai M, Saito H, Ebihara S. Circadian rhythms of cardiovascular functions are modulated by the baroreflex and the autonomic nervous system in the rat. Circulation 1997;96:1667-74.
- Braga ANG, Lemos MS, Silva JR, Fontes WRP, Santos RAS. Effects of angiotensins on day-night fluctuations and stress-induced changes in blood pressure. Am J Physiol Regul Integr Comp Physiol 2002;282:R1663-71.
- Gu XW, Xie HH, Wang J, Shen FM, Su DF. Arterial baroreflex is not involved in salt preference in rats. Clin Exp Pharmacol Physiol 2006;33:607-11.
- Wang J, Shen FM, Wamg MW, Su DF. Effects of nine antihypertensive drugs on blood pressure variability in sinoaortic-denervated rats. Acta Pharmacol Sin 2006;27:1013-7.
- Zhan LS, Guan YF, Su DF, Miao CY. Blood pressure variability and baroreflex sensitivity are not different in spontaneously hypertensive rats and stroke-prone spontaneously hypertensive rats. Acta Pharmacol Sin 2005;26:959-62.
- Han P, Chu ZX, Shen FM, Xie HH, Su DF. Synergism of hydrochlorothiazide and nitrendipine on the reduction of blood pressure and blood pressure variability in spontaneously hypertensive rats. Acta Pharmacol Sin 2006;27:1575-9.
- Yan L, Takekida S, Shigeyoshi Y, Okamura H. Per1 and per2 gene expression in the rat suprachiasmatic nucleus circadian profile and the compartment-specific response to light. Neuroscience 1999;94:141-50.
- Oishi K, Sakamoto K, Okada T, Nagase T, Ishida N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem Biophys Res Commun 1998;253:199-203.
- Zheng BH, Albrecht U, Kaasik K, Sage M, Lu WQ, Vaishnav S, et al. Nonredundant roles of the mper1 and mper2 genes in the mammalian circadian clock. Cell 2001;105:683-94.
- Tamaru T, Isojima Y, Yamada T, Okada M, Nagai K, Takamatsu K. Light and glutamate-induced degradation of the circadian oscillating protein BMAL1 during the mammalian clock resetting. J Neurosci 2000;20:7525-30.
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2 to light. Cell 1997;91:1055-64.
- Dardente H, Poirel VJ, Klosen P, Pevet P, Masson-Pevet M. Per and neuropeptide expression in the rat suprachiasmatic nuclei: compartmentalization and differential cellular induction by light. Brain Res 2002;958:261-71.
- La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, et al. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 2001;103:2072-7.
- Wang DS, Xie HH, Shen FM, Cai GJ, Su DF. Blood pressure variability, cardiac baroreflex sensitivity and organ damage in experimentally hypertensive rats. Clin Exp Pharmacol Physiol 2005;32:545-52.
- Shen FM, Zhang SH, Xie HH, Jing Q, Su DF. Early structural changes of aortic wall in sinoaortic denervated rats. Clin Exp Pharmacol Physiol 2006;33:358-63.
- Miao CY, Su DF. The importance of blood pressure variability in rat aortic and left ventricular hypertrophy produced by sinoaortic denervation. J Hypertens 2002;20:1865-72.
- Miao CY, Xie HH, Zhan LS, Su DF. Blood pressure variability is more important than blood pressure level in determination of end-organ damage in rats. J Hypertens 2006;24:1125-35.
- Dampney RAL. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 1994;74:323-64.
- Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience 2002;110:73-92.
- Thomasa MA, Fleissnerb G, Stohrb M, Hauptfleischa S, Lemmer B. Localization of components of the renin-angiotensin system in the suprachiasmatic nucleus of normotensive Sprague-Dawley rats. Brain Res 2004;1008:212-35.
- Nonaka H, Emoto N, Ikeda K, Fukuya H, Rohman MS, Raharjo SB, et al. Angiotensin II induces circadian gene expression of clock genes in cultured vascular smooth muscle cells. Circulation 2001;104:1746-8.
- Sanderford MG, Bishop VS. Central mechanisms of acute ANG II modulation of arterial baroreflex control of renal sympathetic nerve activity. Am J Physiol Heart Circ Physiol 2002;282:H1592-602.
- Shan ZZ, Dai SM, Fang F, Su DF. Changes of central norepine-phrine, β-endorphin, leu-enkephalin, peripheral arginine-vasopressin, and angiotensin II levels in acute and chronic phases of sino-aortic denervation in rats. J Cardiovasc Pharmacol 2004;43:234-41.
- Miao CY, Zhang LM, Yuan WJ, Su DF. Angiotensin II and AT1 receptor in hypertrophied ventricles and aortas of sinoaortic-denervated rats. Acta Pharmacol Sin 2003;24:812-8.
