Effect of curcumin on the adhesion of platelets to brain microvascular endothelial cells in vitro1
Introduction
Endothelial dysfunction is a key initiating event in the etiology of vascular diseases and has been linked to the pathogenesis of stroke. Ischemic stroke is thought to be the product of atherogenesis, thrombosis, and brain infarction[1]. Under normal physiological circumstances, resting endothelial cells generate an active antithrombotic surface[2]. The other important function of the normal microvasculature is to prevent the adhesion of platelets and subsequent formation of microthrombi, which can lead to impaired tissue perfusion. Endothelial cells help create this antithrombogenic surface by producing platelet inactivators (eg prostacyclin)[3]. Although the pathophysiological consequences of activated platelets in circulation are not yet fully understood, it is well established that increased platelet activation is associated with an enhanced risk of thrombotic complications in clinical disorders, such as stroke. Because activated, but not resting, platelets have been shown to adhere to the intact endothelium, it has been suggested that platelet thrombi may also occur in the absence of endothelial cell denudation, particularly in the microvasculature. However, while the platelet receptors involved in aggregate formation and matrix adhesion have been studied extensively, the pathways responsible for the interaction of platelets and the endothelium are not well characterized.
Curcumin, the major yellow pigment extracted from turmeric (the powdered rhizome of the herb Curcuma longa), has been used in indigenous medicine to treat a variety of inflammatory conditions and chronic diseases and is commonly used as a coloring and flavoring additive in foods. Epidemiological studies have raised the possibility that this molecule, used by the Asian/Indian population, plays a part in the significantly lower prevalence of Alzheimer’s disease in India compared to the USA (4.4 fold)[4]. Recent studies indicate that the dietary administration of curcumin may have beneficial effects in conditions, such as Alzheimer’s disease[5]. Some results also support curcumin’s potential for use in the treatment of neurodegenerative diseases[6]. Moreover, curcumin was found to protect the rat forebrain against ischemia/reperfusion (I/R) insult[7] and to prevent cerebral I/R injury by protecting blood–brain barrier integrity[8]. To investigate whether curcumin can protect the physiological function of platelets and endothelial cells, we tested their influence on the adhesion of platelets to cultured brain microvascular endothelial cells (BMECs).
Materials and methods
Reagents Curcumin and MCDB 131 were from Sigma–Aldrich (St Louis, MO, USA). TNF-α, thrombin, and the endothelial cell growth supplement (ECGS) were obtained from Sigma (USA). Fetal bovine serum, trypsin–versene mixture, penicillin–streptomycin, and L-glutamine were from Sangon (Shanghai). [3H]Adenine (25 Ci/mmol; 1 Ci=37 GBq) was purchased from Amersham (Minneapolis, MN, USA). P-selectin, glycoprotein IIb (GPIIb)/glycoprotein IIa (GPIIIa) monoclonal antibodies (mAb) were supplied by the Department of Immunity, Suzhou University Medical College (Suzhou, China). E-selectin was from Sigma–Aldrich (USA). Immunoglobulin G (IgG)–fluorescein isothiocyanate was from Immunotech (France).
Culture of BMECs Primary cultures of cerebral endothelial cells were prepared and characterized as previously described[9]. Wistar neonate rats (~5 d old) were killed by cervical dislocation, and the brains were rapidly removed and placed in prechilled phosphate-buffered saline (PBS). The cerebral cortexes were finely minced, to filter by 200 mesh (pore diameter 120 μm). The pellet containing the microvessels was washed twice in prechilled Hanks’ solution, and then incubated in 0.1% collagenase solution at 37 °C for 20 min in a shaking water bath. After incubation, 20% bovine serum MCDB 131 was added to the homogenate and centrifuged at 800 r/min for 3 min. The cell suspension was carefully seeded onto polylysine collagen-coated, 35 mm plastic dishes in MCDB 131 culture medium. The medium contained 20% (v/v) heat-inactivated fetal calf serum (FCS), 100 kU·L–1 benzylpenicillin, 100 mg·L–1 streptomycin, 2 mmol/L L-glutamine, 40 μg/mL ECGS, and 15 U/mL heparin, and maintained at 37 °C in humidified 95% air and 5% CO2. The medium was changed approximately every 23 d. Confluent BMECs were obtained after approximately 57 d. Endothelial cell identity was verified by morphology and by positive staining for factor VIII-related Ag.
Preparation of platelets The washed platelets were prepared from platelet-rich plasma (PRP) by the single centrifuging and dilution procedure described by Fontana et al[10]. Briefly, blood was taken from the inferior caval vein of healthy rats not taking any medication, and directly added into 3.8% sodium citrate (1:9 v/v blood), followed by centrifugation at 250 r/min for 10 min. The upper tier was the PRP, which was collected by centrifugation at 3000 r/min for 10 min. Then the platelets were washed and resuspended in Tyrode’s buffer (in mmol: 2.6 KCl, 4 MgCl2·6H2O, 135 NaCl, 12 NaHCO3, 0.42 NaH2PO4, 5 glucose, and 0.25% FCS, pH 7.4). The platelet concentrations were adjusted to 1×109/L.
Adhesion assay of platelets and BMECs The platelets were labeled with [3H]adenine, as described earlier[11–13]. To investigate platelet adhesion on endothelial cells, the platelets were then labeled by incubation with 10 μCi/mL [3H]adenine for 30 min at 37 °C. The unlabeled isotope was washed 3 times with Tyrode’s buffer. The labeled platelets were adjusted to 1×1010·L–1 and used for the adhesion test. The BMECs were grown until confluence on precoated 96-well plates. Then the labeled platelets were added to the BMECs. The adherent platelets were solubilized with 1% Triton X-100 (200 μL/well). Solubilized contents of each well were transferred to scintillation vials, and [3H]adenine was counted by liquid scintillation. Platelet adhesion, measured as [3H]adenine present in each well, was expressed as a percentage of the total [3H]adenine that had been added to each well. Because platelet adhesion was measured in a static system without stirring, the platelets did not aggregate during the adhesion experiments. The adherence rate was calculated as follows: Adherence rate (%)=bound platelets DPM/total platelets DPM×100.
1 Ci=3.7×1010 DPS=2.22×1012 DPM.
Curcumin pretreatment of BMECs Curcumin (30–240 μmol/L) was added to the BMECs and incubated at 37 °C for 30 min. Then 10 ng/mL TNF-α was added for 24 h. The control cells received only the complete medium without TNF-α. Before the labeled platelets were added, the BMECs were washed 3 times with Tyrode’s buffer, and the platelets were suspended in Tyrode’s buffer at a concentration of 1×109·L–1. The labeled platelets (100 μL per well) were then added and incubated for 30 min.
Curcumin–pretreated activated platelets Curcumin (30–240 μmol/L) was added to the platelets, which were labeled by [3H]adenine, and incubated at 37 °C for 30 min. Then 2 U/mL thrombin was added for 30 min. The control cells were received from the complete medium without thrombin. The pretreatment platelets were then added to the BMECs (96-well plate, 1×108 per well) and continued to culture at 37 °C for 30 min.
After washing out the unincorporated platelets, each well was treated with 3% NaNO3 (100 μL, dissolved in 0.1% NaOH) for 20 min. Lysates were collected, and radioactivity of each well was determined by Beckman liquid scintillation spectroscopy. All samples were performed in triplicate.
Semiquantitative RT–PCR The BMECs were cultured in a 6-well plate until confluence. Curcumin (30–240 μmol/L) was added to the BMECs and incubated at 37 °C for 30 min. Then 2 U/mL thrombin was added (but not to the control group), cocultivated with BMECs for 30 min, and then RNA was extracted. The cells were washed twice with cold PBS and total RNA was isolated by TRI reagent. Briefly, the cells were harvested and treated with 1 mL TRI reagent for 5 min at room temperature and then centrifuged at 12 000 r/min for 15 min at 4 °C. The aqueous portion containing RNA was transferred to another RNAase-free Eppendorf tube, and 0.5 mL isopronol was added and incubated for 10 min at room temperature, followed by the precipitation of total RNA with centrifugation at 12 000 r/min at 4 °C for 10 min. The pellet was then washed twice with 75% ethanol, dried at room temperature on ice for 5 min, and the total RNA pellet was dissolved in 20 μL H2O.
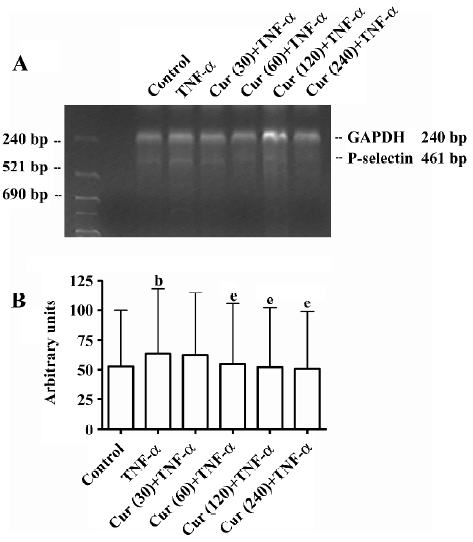
GAPDH was used as an internal control. Aliquots of the RT reaction were amplified with the rat P-selectin and GAPDH primers shown in Table 1.
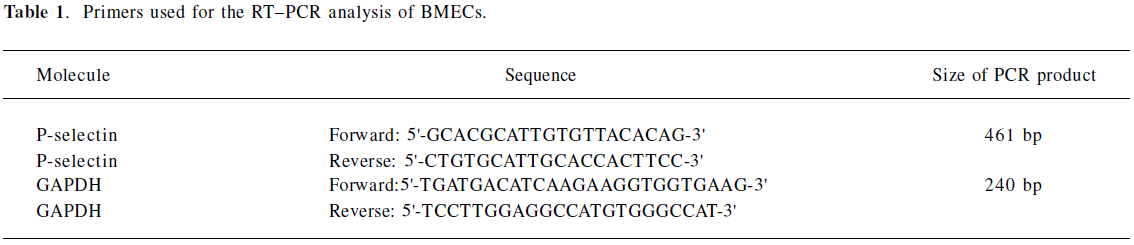
Full table
RT reaction system (40 μL) The RT reaction system consisted of 5×4 μL buffer solution, 4 μL dNTP (10 mmol/L), 4 μL (15 mmol/L) MgCl2, 2 μL random primer, 1 μL M-MLV Temin enzyme, cDNA, 10 μL production, and 15 μL DEPC. The reaction conditions were as follows: 94 °C for 5 min, 37 °C for 60 min, and 72 °C for 10 min.
PCR (P-selectin) reaction system (50 μL) The PCR (P-selectin) reaction system consisted of 10×5 μL buffer solution, 5 μL (10 mmol/L) dNTP, 5 μL (15 mmol/L) MgCl2,2 µL P-selectin (forward), 2 μL P-selectin (reverse), 2 μL GAPDH (sense), 2 μL GAPDH (antisense), 1 μL Taq enzyme, which were added to ddH2O. The reaction conditions were as follows: 94 °C 5min, then 35 cycles of 94 °C for 30 s, 56 °C for 30 s, 72 °C 30 s, and a final extension at 72 °C for 7 min.
Products were separated by electrophoresis gels stained with ethidium bromide, illuminated with UV light, and measured by quantitative scanning densitometry of autoradiographs. All measurements of the P-selectin mRNA expression are reported as a ratio of optical density of the P-selectin and GAPDH bands calculated for each experimental sample, in which both P-selectin and GAPDH were measured in parallel. Preliminary experiments in which we repeated the PCR analysis on the same sample (during the determination of the optimal number of PCR cycles) showed a measurement variability of laser microscopy.
Flow cytometry detection expressions of P-selectin and GPIIb/GPIIIa on the activation of platelets The blood platelet suspension was incubated with curcumin (30–240 μmol/L) for 30 min (each sample contained 1×106 platelets), followed by activation with 2 U/mL thrombin for 30 min. Confluent BMECs treated with curcumin and thrombin as described earlier were detached from the plate (6-well plate) by PBS/EDTA (5 mmol/L). The cells were washed with MCDB 131 plus 20% FCS. The platelets were stained with anti-P-selectin mAb, GPIIb/GPIIIa mAb followed by fluorescein-conjugated goat antimouse IgG. A cell immunofluorescence analysis was performed with an EPICS XL cytofluorimeter (Coulter, USA). Five thousand cells were analyzed for each experiment.
Flow cytometry detection of P-selectin expression on BMECs BMECs were cultured in a 6-well plate until confluence. Curcumin (30–240 μmol/L) was added to the BMECs and incubated at 37 °C for 30 min, then 10 ng/mL TNF-α was added for 24 h (but not for the control group). Confluent BMECs treated with curcumin were detached from the plate (6-well plate) by PBS/EDTA (5 mmol/L). The cells were washed with MCDB 131 plus 20% FCS. BMECs were stained with anti-P-selectin mAb followed by fluorescein-conjugated goat antimouse IgG. A cell immunofluorescence analysis was performed with an EPICS XL cytofluorimeter. Five thousand cells were analyzed for each experiment.
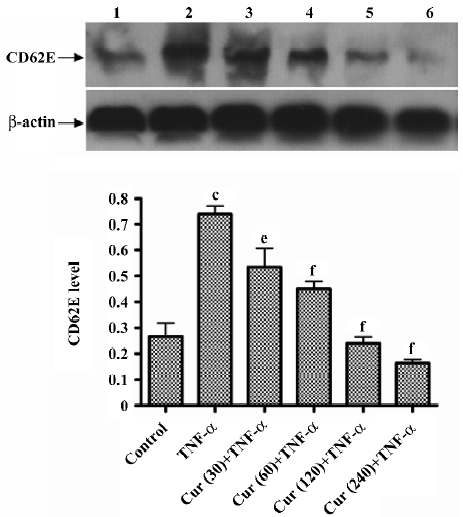
Western blot analysis of E-selectin expression BMECs were cultured in a 6-well plate until confluence. Curcumin (30–240 μmol/L) was added to the BMECs and incubated at 37 °C for 30 min, then 10 ng/mL TNF-α was added for 4 h (but not to the control group). Confluent BMECs treated with curcumin were detached from the plate (6-well plate) by PBS/EDTA (5 mmol·L–1). The cells were washed 3 times with cold PBS, lysed with lysis buffer (25 mmol/L Tris-HCl, pH 7.4, 0.15 mol/L NaCl, and 1% Triton X-100), and complete protease inhibitor cocktail (Roche), and then placed on a shaker at 4 °C for 1 h. After centrifugation at 12 000 r/min for 10 min, the protein concentration of the supernatant was measured by the Bio-Rad protein assay (Bio-Rad Laboratories, Inc, USA). Proteins (40 μg) were separated by 10% SDS–PAGE. The proteins were transferred to nitrocellulose membranes, and immunoblots were blocked with blocking buffer (5% non-fat milk, 10 mmol/L Tris, pH 7.5, 100 mmol/L NaCl, and 0.1% Tween-20) at room temperature for 1 h. CD62E was detected at dilution of 1:100 of the monoclonal mouse antihuman CD62E antibody and incubated overnight at 4 °C. Then the membrane was washed by TBS-T (1×Tris-buffered saline and 1% Tween-20) 3 times for 5 min. The second antibody (at a dilution of 1:5000) was incubated at room temperature for 2 h. Immunoreactivity was detected with enhanced chemiluminescent autoradiography (ECL kit, Amersham, Arlington Heights, IL, USA) according to the manufacturer’s instructions.
Statistics analysis Pooled data were analyzed using Microsoft Excel. All values were expressed as mean±SD and compared with the t-test.
Results
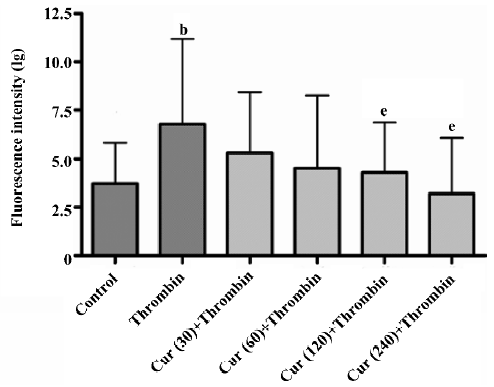
Curcumin inhibits the activation of platelet adhesion to BMECs To determine whether curcumin can affect the physiological function of platelets, we tested their influence on the adhesion of platelets to cultured endothelial cells. The platelet adhesion was induced by thrombin-stimulated platelets onto BMECs. Platelet treatment with thrombin resulted in an increased basal adhesion of platelets to BMECs. In the adhesion experiments, the pretreatment of platelets with 60 μmol/L curcumin for 30 min inhibited platelet adhesion to BMECs by 24.03%±2.19% (n=6, P<0.01). This was a dose-dependent effect that reached significant levels at a dose of 240 μmol/L (15.81%±1.37%, n=6, P<0.01; Table 2; Figure 1).
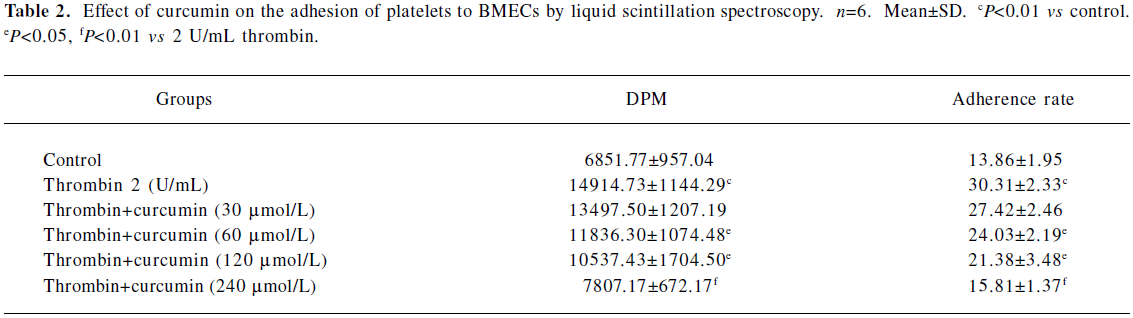
Full table
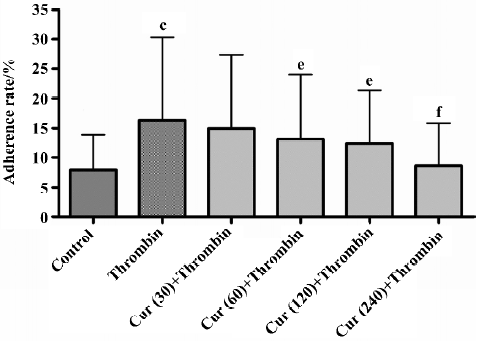
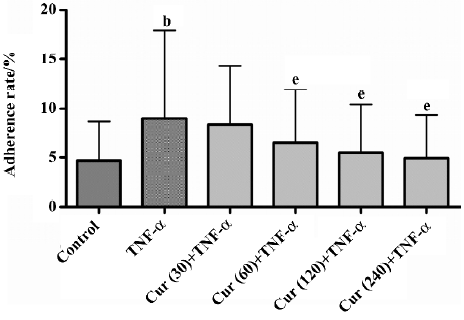
Curcumin inhibits platelet adhesion to TNF-a-activated BMECs TNF-α-stimulated endothelial cells promoted adherent and labeled platelets, the rate of adhesion reaching 17.92%. For the control group without TNF-α, the rate of adhesion was only 8.69%. The pretreatment of TNF-α-activated BMECs with 60 μmol/L curcumin for 30 min inhibited platelet adhesion to BMECs by 11.95%±1.06% (n=6, P<0.01). This was a dose-dependent effect that reached significant levels at a dose of 240 μmol/L (9.36±0.42%, n=6, P<0.01; Table 3; Figure 2).
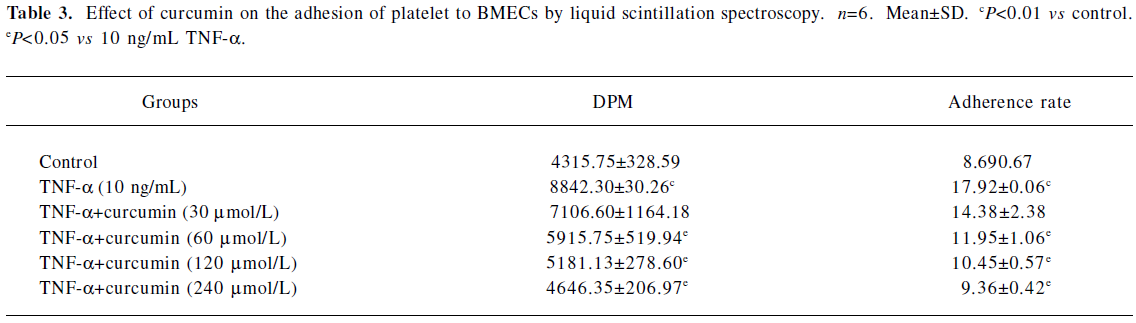
Full table
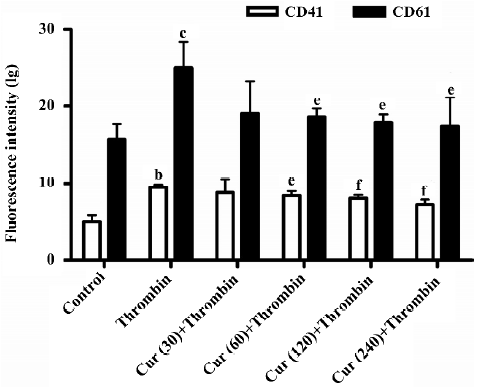
Curcumin inhibits P-selectin and GPIIb/GPIIIa(CD41/CD61) expressions in the activation of platelets As previously demonstrated, flow cytometry is a very sensitive and accurate method to measure the heterotypic adhesion of platelets. Its use allowed us to exclude the involvement of matrix proteins in the adhesive interaction. The fluorescence-activated cell sorter (FACS) analysis demonstrated that the fluorescence associated with unstimulated platelets was not different from the background fluorescence of washed platelets alone. The treatment of platelets with 2 U/mL thrombin for 30 min resulted in a marked increase of P-selectin and GPIIb/GPIIIa expressions in the mean fluorescence intensity of the total platelet population. The pre-incubation of platelets with curcumin (30–240 μmol/L) for 30 min reduced thrombin-induced P-selectin and GPIIb/GPIIIa expressions in a concentration-dependent manner (P<0.05; Figures 3,4).
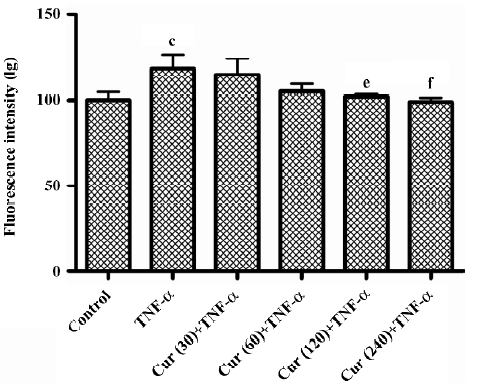
Curcumin inhibits P-selectin expression on TNF-α-activated BMECs The treatment of BMECs with 10 ng/mL TNF-α for 24 h resulted in a marked increase of P-selectin expression. In the control group without TNF-α treatment, BMECs had much lower P-selectin expression as compared to other types of cells. The pretreatment of BMECs with curcumin (30–240 μmol/L) for 30 min reduced TNF-α-induced P-selectin expression in a concentration-dependent manner (P<0.01; Figure 5).
Curcumin inhibits P-selectin mRNA expression on activated BMECs The P-selectin mRNA levels of BMECs were determined by RT–PCR. In the control group without TNF-α treatment, BMECs had a much lower P-selectin expression compared to other types of cells. Thus TNF-α significantly enhances the P-selectin mRNA expression in endothelial cells. In response to the concentration-dependent treatment of curcumin, the P-selectin mRNA expression, as normalized with GAPDH mRNA, dramatically decreased (Figure 6A). Compared to the controls (100%), P-selectin mRNA levels were inhibited with the treatment of curcumin (30–240 μmol/L) (Figure 6B).
Curcumin inhibits the expression of the E-selectin on activated BMECs E-selectin levels of BMECs were determined by Western blotting. In the control group without TNF-α treatment, BMECs had a much lower E-selectin expression compared to other types of cells. Thus TNF-α significantly enhances the E-selectin expression in endothelial cells. In response to the concentration-dependent treatment of curcumin, the E-selectin expression dramatically decreased. Compared to the controls (100%), E-selectin levels were inhibited with the treatment of curcumin (30–240 μmol/L), representing reductions, respectively (Figure 7).
Discussion
The interaction between platelets and endothelial cells plays a crucial role in the dysfunction and pathogenesis of cerebrovascular diseases, such as stroke. When a blood vessel is injured, the adhesion of circulating platelets to the site of damage occurs rapidly. The repair of endothelial cell dysfunction is very important to maintain the normal vascular tone and biological function. The understanding and illustration of endothelial dysfunction will indicate a new direction to the treatment of cerebrovascular diseases. Adherent molecules play an important role in the pathophysiological processes of inflammatory reaction and thrombosis. The selectin family is closely associated with cerebrovascular diseases. Studies have revealed that platelets, like leukocytes, can roll along and firmly adhere to the venular endothelium. Furthermore, a variety of different adherent glycoproteins, expressed on the surface of platelets and/or endothelial cells, have been implicated in platelet-endothelial cell adhesion within venules, including P-selectin and GPIIb/GPIIIa[14]. We know that P-selectin is a transmembrane glycoprotein expressed on the activated vascular endothelium and that GPIIb and GPIIIa are expressed on activated platelets. They are believed to play an important role in atherogenesis and plaque inflammation by attracting leukocytes from the circulation[15]. Although the pathophysiological consequences of activated platelets in circulation are not yet fully understood, it is well established that increased platelet activation is associated with an enhanced risk of thrombotic complications in stroke. Platelet adhesion, activation, and aggregation play a major role for the thromboembolic complications of advanced atherosclerosis[16,17].
In the present study, we investigated the effects of curcumin on the adhesion of platelets with BMECs. Curcumin, which is derived from the root of the plant Curcuma longa, for the treatment of different inflammatory diseases, has been used in traditional Chinese medicine for thousands of years. The beneficial effects of curcumin appear as a promising class of compounds for brain protection. Elucidation of their mechanism of action should provide new insights for new targets for neuroprotective drugs. Our data indicate that the pre-incubation of platelets with curcumin (30–240 μmol/L) for 30 min reduced thrombin-induced P-selectin and GPIIb/GPIIIa expressions in a concentration-dependent manner (Figures 3, 4). We know that the platelet integrin GPIIb/GPIIIa not only mediates platelet aggregation, but also contributes substantially to firm platelet adhesion to subendothelial matrix proteins after endothelial denudation. The loss of GPIIb/GPIIIa profoundly reduced platelet-endothelium and platelet-subendothelium adhesion in vivo[18], thus our study indicates reduced platelet-endothelium and platelet-subendothelium adhesion in vitro.
Curcumin also inhibits platelet adhesion to TNF-α-activated BMECs. P-selectin and E-selectin comprise a family of adherent glycoproteins that mediate leukocyte–endothelial cells and leukocyte–platelet adhesive interactions. All selectins are believed to play a significant role in the initial “rolling” interaction of leukocytes on activated endothelium. P-selectin is constitutively expressed in Weibel–Palade bodies of endothelial cells and granules of platelets, which can be rapidly mobilized to the endothelial cell surface after stimulation with thrombin or histamine.
E-selectin is an endothelial cell-specific surface molecule which is transiently expressed upon activation[19]. It is critically involved in mediating leukocyte rolling, a prerequisite for leukocyte adhesion at sites of inflammation[20–22]. The transient expression of E-selectin is induced by a number of pro-inflammatory mediators, such as interleukin-1 (IL-1) and TNF-α. The E-selectin expression on the cell surface occurs within 1 and 2 h of cytokine treatment, reaching its maximum at 4–6 h and then rapidly declining[23]. Our data demonstrate that the pretreatment of BMECs with curcumin (30–240 μmol/L) for 30 min reduced TNF-α-induced P-selectin and E-selectin expressions in a concentration-dependent manner (Figures 5, 7). P-selectin mRNA levels were inhibited with the treatment of curcumin. The results in Figures 5 and 7 show how curcumin inhibits the adhesion of platelets to BMECs.
In summary, the selective blockade of key signaling pathways of the adhesion on platelets and BMECs has a different impact on stroke outcome and bleeding complications. The inhibition of early steps of platelet adhesion to the ischemic endothelium may offer a novel and safe treatment strategy in acute stroke. Our view is that curcumin can exert beneficial effects on the protection of the brain not only through its antioxidant potential, but also through the modulation of different pathways. Because of the low toxicity of curcumin, its ability to inhibit the induction of adherent molecules suggests that it should be explored for its therapeutic value in stoke and neurodegenerative diseases.
References
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999;340:115-26.
- Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 1998;91:3527-61.
- van Zanten GH, de Groot PG, Sixma JJ. Platelet adhesion JJ. In: von Bruchhausen F, Walter U, editors. Platelets and their factors. Berlin, Heidelberg: Springer-Verlag; 1997. p 61–81.
- Ganguli M, Chandra V, Kamboh MI, Johnston JM, Dodge HH, Thelma BK, et al. Apolipoprotein E polymorphism and Alzheimer’s disease: the Indo–US Cross-National Dementia Study. Arch Neurol 2000;57:824-30.
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci 2001;21:8370-7.
- Shin HJ, Lee JY, Son E, Lee DH, Kim HJ, Kang SS, et al. Curcumin attenuates the kainic acid-induced hippocampal cell death in the mice. Neurosci Lett 2007;416:49-54.
- Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res 2002;46:273-9.
- Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood–brain barrier damage. Eur J Pharmacol 2007;561:54-62.
- Kis B, Kaiya H, Nishi R, Deli MA, Abraham CS, Yanagita T, et al. Cerebral endothelial cells are a major source of adrenomedullin. J Neuroendocrinol 2002;14:283-93.
- Fontana S, Olmedo DG, Linares JA, Guglielmotti MB, Crosa ME. Effect of platelet-rich plasma on the peri-implant bone response: an experimental study. Implant Dent 2004;13:73-8.
- Sneddon JM, Vane JR. Endothelium-derived relaxing factor reduces platelet adhesion to bovine endothelial cells. Proc Natl Acad Sci USA 1988;85:2800-4.
- Zhu Y, Gu ZL, Liang ZQ, Zhang HL, Qin ZH. Prostaglandin A1 inhibits increases in intracellular calcium concentration, TXA2 production and platelet activation. Acta Pharmacol Sin 2006;27:549-54.
- Joseph R, Welch KM, Grunfeld S, Oster SB, D’Andrea G. Baseline and activated platelet cytoplasmic ionized calcium in acute ischemic stroke. Stroke 1988;19:1234-8.
- Andre P, Denis CV, Ware J, Saffaripour S, Hynes RO, Ruggeri ZM, et al. Platelets adhere to and translocate on von Willebrand factor presented by endothelium in stimulated veins. Blood 2000;96:3322-8.
- Frijns CJM, Kappelle LJ. Inflammatory cell adhesion molecules in ischemic cerebrovascular disease. Stroke 2002;33:2115-22.
- Lusis AJ. Atherosclerosis. Nature 2000;407:233-41.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352:1685-95.
- Massberg S, Schürzinger K, Lorenz M, Konrad I, Schulz C, Plesnila N, et al. Platelet adhesion via glycoprotein IIb integrin is critical for atheroprogression and focal cerebral ischemia: an in vivo study in mice lacking glycoprotein IIb. Circulation 2005;112:1180-8.
- Bevilacqua MP. Endothelial–leukocyte adhesion molecules. Annu Rev Immunol 1993;11:767-804.
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specifity and diversity. Cell 1991;67:1033-6.
- Springer T. Traffic signals for lymphocytes recirculation and leukocyte emigration: the multistep paradigm. Cell 1994;76:301-14.
- Kansas GS. Selectins and their ligands: Current concepts and controversies. Blood 1996;88:3259-87.
- Bevilacqua MP, Stengelin S, Gimbrone MA Jr, Seed B. Endothelial leukocyte adhesion molecule: 1. An inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science 1989;243:1160-5.
