Therapeutic effect of osthole on hyperlipidemic fatty liver in rats1
Introduction
Non-alcoholic fatty liver (NAFL) is one of the most common causes of chronic liver disease. The disease is a spectrum that is initiated with steatisis. It can progress to nonalcoholic hepatosteatitis and later fibrosis, cirrhosis, and potentially end-stage liver failure and/or hepatocellular carcinoma[1–4]. Although several promising medications are on the horizon, there are currently no ideal pharmacological reagents that can prevent or reverse this disease[5].
Osthole (7-methoxy-8-isopentenoxycoumarin) is an active constituent isolated from the fruit of Cnidium monnieri (L) Cusson, one of the Chinese herbal medicines which possesses a variety of pharmacological properties[6,7] and has been administered to humans in clinics for many years. Modern pharmacological studies have proven that osthole has many functions such as anti-inflammation[8], anti-oxidation[9], anti-tumor[10], anti-apoptosis[11], estrogen-like effects, and so on. It is considered to have potential therapeutic applications, but there is no report about the treatment of hyperlipidemic fatty liver. We found that the level of serum triglyceride (TG) in ovariectomized rats decreased after treatment with osthole for 12 weeks (0.30±0.13 mmol/L in the model group vs 0.19±0.07 mmol/L in the 10 mg/kg osthole group) accidentally, so we began to study the therapy for hyperlipidemic fatty liver. Lipanthyl can lower serum total cholesterol (TC), and TG, and be used for treatment of hyperlipidemia and hyperlipidemic fatty liver in clinics. Therefore, it acted as a positive drug in the experiment.
Materials and methods
Drugs and reagents Osthole was kindly provided by Dr Jia ZHOU (Xi’an Green Fount Natural Product Co Ltd, Xi’an, China); the purity was >98% as determined by HPLC. Lipanthyl was procured from Laboratories Fournier SA (Chenove, France). The assay kits for serum TC, TG, and high density lipoprotein-cholesterol (HDL-C) were purchased from Suzhou Tianneng Biology-Technology Company (Suzhou, China). The assay kits for liver tissue TC, TG, malondialdehyde (MDA), superoxide dismutase (SOD), plasma lipoprotein lipase (LPL), and hepatic lipase (HL) were the products of Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Animals Sprague-Dawley rats (male, 200±20 g) and Kunming mice (male, 18±2 g) were obtained from the Animal Breeding Center of Soochow University (Suzhou, China). The animals were housed under standard conditions (20± 1 °C room temperature, 60%±10% humidity, and light from 6:00 to 18:00) in regular cages and allowed free access to food and water. All animal studies were conducted according to the regulations for the use and care of experimental animals.
Establishment of rat hyperlipidemic fatty liver model[12–14] A hyperlipidemic fatty liver rat model was generated by feeding fatty milk (containing 6% cholesterol, 15% lard, 0.2% propylthiouracil, 2% bile salt, 20% propylene glycol, and 20% Tween-80) at 1 mL/100 g (body weight per d) for 6 weeks. Three rats were then killed and the livers were taken for assessment of fatty hepatic deve-lopment. After the model developed, the rats were randomly divided into 5 groups (n=10): the fatty hepatic model group, the 5, 10, and 20 mg/kg osthole groups, and the 20 mg/kg lipanthyl group. Control and model animals were treated with an equivalent volume of 0.5% sodium carboxymethyl cellulose solution. These medications were taken orally for 6 weeks. Finally, all of the rats were sacrificed, blood was obtained, and hepatic tissues were collected for measure-ment.
Establishment of the mouse hyperlipidemic model[15] The hyperlipidemic mouse model was generated by feeding fatty milk (containing 10% cholesterol, 20% lard, 1% thimecil, 2% bile salt, 20% propylene glycol, and 20% Tween-80) at 0.2 mL/10 g (body weight per d). The experimental mice were randomly divided into 5 groups, (n=10): the control group, hyperlipidemic model group, 10 and 20 mg/kg osthole groups, and the 40 mg/kg lipanthyl group. These mice were administered in the morning and treated with fatty milk in the afternoon for 3 weeks. Control and model animals were treated with an equivalent volume of 0.5% sodium carboxymethyl cellulose solution. After 3 weeks, all mice received an injection of heparin (312.5 U/kg, iv) 15 min before being sacrificed. Post-heparin plasma was obtained for the measurement of LPL and HL activities.
Measurement of serum TC, TG, HDL-C, and the coefficient of hepatic weight in rats Rat blood was obtained after 12 h of overnight fasting. Serum TC, TG, and HDL-C were determined by colorimetric methods according to the procedure provided. Low density lipoprotein-cholesterol (LDL-C) was obtained by the Friedewald calculation, namely, LDL-C=TC-(TG/2.2+HDL-C). The coefficient of hepatic weight was calculated according to liver weight (g) divided by body weight (100 g).
Measurement of plasma LPL, HL, and total lipase (TL) activities in mice Activities of LPL and HL in mouse post-heparin plasmas were determined by enzymatic methods according to the procedure provided, and the TL was obtained by LPL plus HL.
Measurement of TC and TG in rat liver tissue[16] The hepatic lipid was extracted from the liver tissue using a chloroform/methanol mixed solution (1/1, v/v). The prepared sample was then centrifuged at 1 200×g for 10 min; the obtained supernatant was used for the measurement of TC and TG according to colorimetric methods.
Measurement of SOD, MDA, and protein in rat liver tissue The liver tissue was taken at the time of slaughter and rapidly broken to pieces in ice-cold saline, then the tissue homogenate (10%, w/v) was prepared. The contents of SOD and MDA were determined by colorimetric methods according to the procedure provided, respectively. Protein in liver was measured by colorimetric methods.
Histological observation[17] For the histological study, rat liver specimens were fixed in 10% formaldehyde and embedded in paraffin for hematoxylin (HE) staining. The degree of fatty degeneration is generally determined by evaluating the proportion of hepatocytes containing fat droplets and graded and expressed as “–, +, ++, +++”; no fat present as “–”, less than 1/3 of the hepatic lobule as “+”, 1/3 to 2/3 as “++”, and more than 2/3 as “+++”. The histological evaluation of the liver sections was performed blindly.
Statistical analysis Data are expressed as mean±SD. One-way ANOVA was performed to determine difference between the experimental groups and χ2-test was used to evaluate the significances of histopathological evaluation. For all tests, P<0.05 was considered statistically significant.
Results
General condition of the rats At the end of the experiment, the rats’ body weight and hepatic weight were measured and the coefficient of hepatic weight was calculated. Hepatic weight and its coefficient in the model group were significantly higher than those in the control group, but the rat’s body weight had no difference among the groups. After treatment with 5−20 mg/kg osthole for 6 weeks, compared with the model group, the coefficient of hepatic weight in the osthole groups significantly decreased (P<0.05 or P<0.01), hepatic weight also decreased, especially in the 10 and 20 mg/kg osthole groups (P<0.05 or P<0.01). In the lipanthyl group, hepatic weight and its coefficient inversely increased (P<0.01; Table 1).
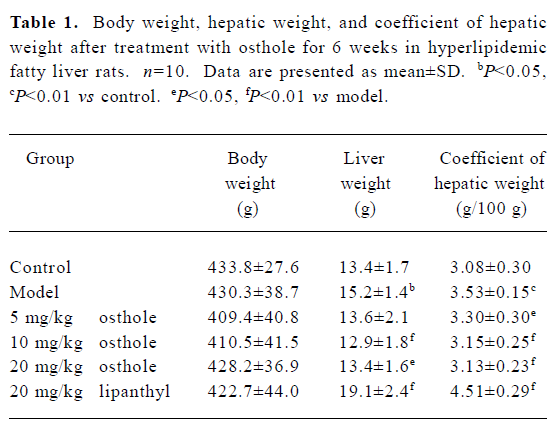
Full table
Effects on serum levels of TC, TG, HDL-C, and LDL-C in fatty liver rats The serum levels of TC, TG, and LDL-C in the model group were significantly higher than those in the control group (P<0.05 or P<0.01); the level of HDL-C between the different groups had no difference. After 6 weeks of administration, serum TC, TG, and LDL-C levels were significantly lower in the osthole groups than in the model group (P<0.05 or P<0.01). The serum HDL-C level increased to some degree, especially in the 10 mg/kg osthole group (P< 0.01). In the lipanthyl group, the serum levels of TC, TG, and LDL-C significantly decreased, but the serum level of HDL-C was not increased (Table 2).
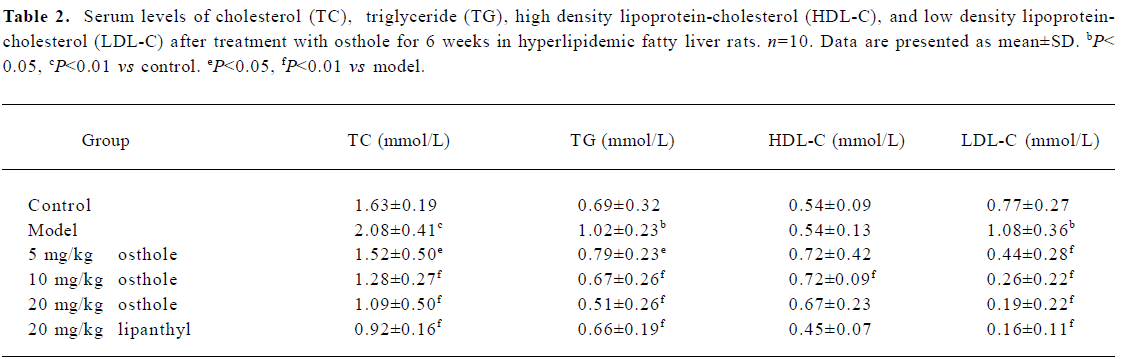
Full table
Effects on plasma activities of LPL, HL, and TL in hyperlipidemic mice The post-heparin plasma activities of LPL, HL, and TL in the mouse model group were markedly higher than those in the control group (P<0.01). After administration of osthole for 3 weeks, post-heparin plasma LPL, HL, and TL activities in the mice further increased (P<0.05 or P<0.01); the percentages increased by 11.8%−30.1% for LPL, 41.3%−77.5% for HL, and 20.4%−44.0% for TL, respectively. In the lipanthyl group, mice plasma activities of LPL, HL and TL also increased significantly (P<0.01; Table 3).
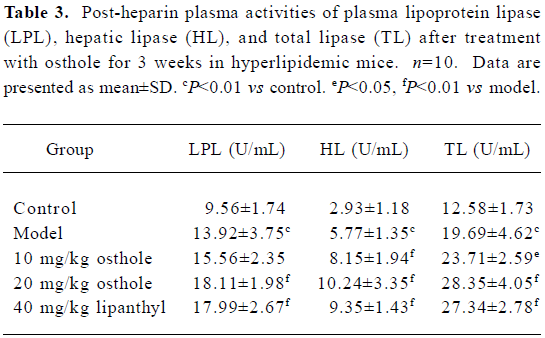
Full table
Effects on hepatic tissue TC, TG, SOD, and MDA contents in fatty liver rats The contents of TC, TG, and MDA in the hepatic tissue in the model group were significantly higher than those in the control group. After treatment with 5−20 mg/kg osthole for 6 weeks, the contents of TC, TG, and MDA in the hepatic tissue were significantly lowered as compared with the model group (P<0.05 or P<0.01) and decreased by 12.5%−32.2%, 25.0%−34.3%, and 20.6%−26.5%, respectively. In the lipanthyl group, the levels of TC, TG, and MDA also decreased. However, the activity of SOD between the model group and the drug-treated group was not significantly different (Table 4).

Full table
Rat liver histological changes The degree of liver fatty degeneration was severer in the model group than in the control group. After administration of osthole for 6 weeks, the degree of fatty degeneration in the liver was significantly improved (P<0.01), especially in the 10 and 20 mg/kg osthole groups; liver fatty degenerations in partial rats were reversed. In the lipanthyl group, the severity of liver fatty degeneration also significantly decreased. A histological evaluation of the liver specimen demonstrated osthole dramatically decreased lipid accumulation (Table 5; Figure 1). Thus, our results demonstrated a therapeutic effect of osthole on fatty milk-induced fatty liver.
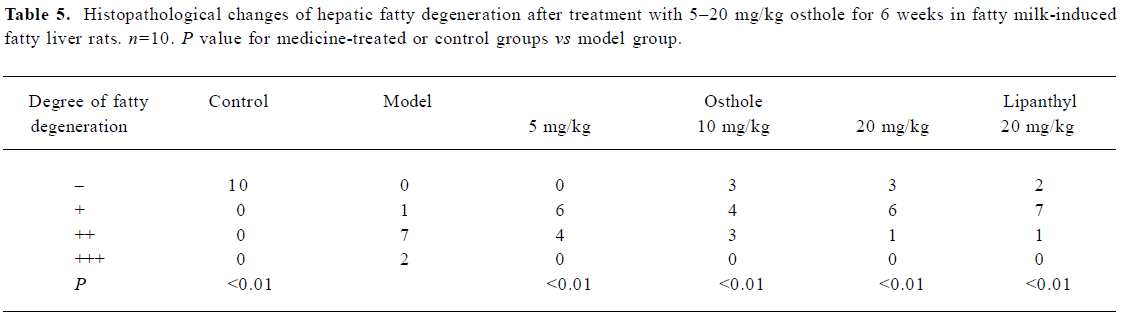
Full table

Discussion
At present, there are many different therapeutic approaches for NAFL, but the strategies of therapy include two different directions; one is towards the risk factors, which are considered to be involved in the etiology of NAFL, such as obesity, dyslipidemia and diabetes, and the another is intervention in the pathogenetic mechanism of the disease itself, which remains unclear. Therefore, in clinical hypocaloric diets and physical exercise, treatment of associated insulin resistance, lipid-lowering medications, anti-oxidants and so on are used for the disease[18], but no treatment has definitely evidence-based NAFL lesion-ameliorating or progression-avoiding effects. There are also no ideal drugs that can prevent or reverse the disease[19].
Our experimental results show that after treatment with osthole for 6 weeks, the levels of serum TC, TG, LDL-C, hepatic weight and its coefficient, and the hepatic tissue contents of TC and TG decreased significantly in fatty milk-induced fatty liver rats. The histological evaluation of rat liver demonstrated osthole dramatically decreased lipid accumulation. More importantly, we found that osthole might enhance the activities of LPL and HL and decrease the content of MDA. These results suggest a new function of osthole that protects the liver from fat accumulation, and its mechanism might be associated with the increment of LPL, HL activities and anti-oxidation.
LPL and HL are essential in the hydrolysis of TG-rich lipoproteins[20]. By proteoheparan sulfate, active LPL is attached to capillary endothelium and active HL is attached to sinusoids and capillaries, respectively, and they can be released to the blood stream by an injection of heparin. NAFL is caused by the excessive accumulation of TG droplets within hepatocytes. Sources of hepatic TG are transported via CM from the intestines, and then secreted into blood as VLDL. Accordingly, LPL plays a major role in lipid metabolism by regulating the catabolism of TG-rich lipoprotein particles that convert lipoprotein TG to free fatty acid. So LPL is a rate-limiting enzyme for the hydrolysis and removal of CM and VLDL-TG from the circulation. LPL can also act as a structural cofactor facilitating the cellular uptake of whole lipoprotein particles and selective cholesterol ester[21]. HL activity is present mainly in the liver. It plays a major role in lipoprotein metabolism as a lipolytic enzyme that catalyzes the hydrolysis of TG and phospholipids in CM remnants, intermediate-density lipoprotein, and HDL[22]. HL also serves as a ligand that facilitates the uptake of lipoproteins and lipoprotein lipids by cell surface receptors or proteoglycans, thereby directly affecting cellular lipid delivery[23].
Our experimental results demonstrated that in fatty milk-induced fatty liver rats, osthole significantly decreased the levels of serum TC, TG, LDL-C, and the hepatic tissue contents of TC and TG. In fatty milk-induced hyperlipidemic mice, osthole dramatically increased the post-heparin plasma activities of LPL, HL, and TL. High LPL and HL activities lead to more TG and phospholipids hydrolyzed, which may contribute to the reduction of serum TG, TC, and LDL-C concentrations and improvement of hepatocyte lipid accum-ulation. From these results, we assumed that the therapeutic effect of osthole on fatty milk-induced fatty liver was probably due to its lipid-modifying effects.
In very recent studies, it was proved that oxidative stress and subsequent lipid peroxidation played a pivotal role in the development of fatty liver. Mitochondria are thought to be the source of the reactive oxygen species (ROS); overproduction of ROS triggered steatohepatitis by oxidizing the accumulated hepatic lipid and causing lipid peroxidation, contributed significantly to hepatocyte injury[24,25]. The present results demonstrate that in the fatty milk-induced fatty liver rats, osthole significantly depressed MDA production and reduced the degree of hepatocellular steatosis in the liver tissue. These results suggested that osthole possessed a therapeutic effect on fatty milk-induced fatty liver, which was partly attributable to its anti-oxidation against lipid peroxidation.
In summary, our present study demonstrated that osthole was effective in alleviating fatty milk-induced fatty liver in rats. Notably, it also elevated the activities of LPL and HL, and the effect, together with its anti-oxidation, might be the main mechanisms for its therapeutic effects on NAFL.
Acknowledgment
We thank Associate Professor Min DENG for her technical assistance.
References
- Younossi ZM, Gramlich T, Liu YC, Matteoni C, Petrelli M, Goldblum J, et al. Nonalcoholic fatty liver disease: assessment of variability in pathologic interpretations. Mod Pathol 1998;11:560-5.
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology 1999;116:1413-9.
- Bugianesi E, Leone N, Vanni E, Marchesini G, Brunello F, Carucci P, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002;123:134-40.
- Ryan MC, Wilson AM, Slavin J, Best JD, Jenkins AJ, Desmond PV. Associations between liver histology and severity of the metabolic syndrome in subjects with nonalcoholic fatty liver disease. Diabetes Care 2005;28:1222-4.
- Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. Can Med Assoc J 2005;172:899-905.
- Yang XH, Li SM, Ma FG. Study on the sterilization of Cnidium monnieri solution. Guangzhou Med J 1997;28:14-5. Chinese..
- Basnet P, Yasuda I, Kumagai N, Tohda C, Nojima H, Kuraishi Y, et al. Inhibition of itch-scratch response by fruits of Cnidium monnieri in mice. Biol Pharm Bull 2001;24:1012-5.
- Liu JX, Zhang WP, Zhou L, Wang XR, Lian QS. Anti-inflammatory effect and mechanism of osthole in rats. J Chin Med Mater 2005;28:1002-6. Chinese..
- Wang SH, An F, Zhang DS, Zhang L. Experiment study on anti-oxidation effect of osthole. Chin Tradit Patent Med 2005;27:488-9. Chinese..
- Zhou J, Cheng WX, Xu YH. Experimental study on anti-tumour effect of osthole extracted from the fruits of Cnidium monnieri (L.) Cusson. Zhejiang J Integr Tradit West Med 2002;12:76-8. Chinese..
- Okamoto T, Kawasaki T, Hino O. Osthole prevents anti-Fas antibody-induced hepatitis in mice by affecting the caspase-3-mediated apoptotic pathway. Biochem Pharmacol 2003;65:677-81.
- Yan M, Lu RJ, Jia XQ, Meng FL, Zhao XC. An experimental study on treating hyperlipidemic fatty liver with simvastain. Chin J Dig 2002;22:223-5. Chinese..
- Ni HC, Li J, Jin Y, Zang HM, Peng L. The experimental animal model of hyperlipidemia and hyperlipidemic fatty live in rats. Chin Pharmacol Bull 2004;20:703-8. Chinese..
- Tang Y, Tang ZZ, Yang L, Wang XK, Qiu W. Experimental study on treatment of fatty liver with hyperlipemia by xiao-zhi potion in rat model. Pharm J Chin PLA 2003;6:429-31. Chinese..
- Cai BX, Xie ML, Gu ZL. The interactions of effective site clusters from Danshen and Shanzha on blood lipid of hyperlipidemic mice by orthogonal experimental design. Chin Tradit Herb Drugs 2004;35:192-4. Chinese..
- Huang ZS, Wang ZW, Liu MP, Wu QG, Zhou L. Pharmacodynamic study on anti-fatty liver of hujin pills. Chin Tradit Patent Med 1998;20:27-8. Chinese..
- Zhong L, Fan JG, Wang GL, Wu WQ, Jiang JM, Shi Q, et al. The establishment of nonalcoholic steatohepatitis (NASH) animal model. J Chin Prac Med 2000;2:3-6. Chinese..
- Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol 2005;100:1072-81.
- Angulo P. Non-alcoholic fatty liver disease. N Engl J Med 2002;346:1221-31.
- Bos G, Snijder MB, Nijpels G, Dekker JM, Stehouwer CD, Bouter LM, et al. Opposite contributions of trunk and leg fat mass with plasma lipase activities: the hoorn study. Obes Res 2005;13:1817-23.
- Merkel M, Heeren J, Dudeck W, Rinninger F, Radner H, Breslow JL, et al. Inactive lipoprotein lipase (LPL) alone increases selective cholesterol ester uptake in vivo, whereas in the presence of active LPL it also increases triglyceride hydrolysis and whole particle lipoprotein uptake. J Biol Chem 2002;277:7405-11.
- Schneider JG, von Eynatten M, Schiekofer S, Nawroth PP, Dugi KA. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care 2005;28:2181-6.
- Santamarina-Fojo S, Gonzalez-Navarro H, Freeman L, Wagner E, Nong Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscl Throm Vas 2004;24:1750-4.
- Pessayre D, Berson A, Fromenty B, Mansouri A. Mitochondria in steatohepatitis. Semin Liver Dis 2001;21:57-69.
- Zeng MD. Pathogenesis and two “hits” hypothesis of fatty liver. Chin J Dig 2002;22:167-8. Chinese..
