Influence of omeprazole on pharmacokinetics of domperidone given as free base and maleate salt in healthy Chinese patients
Introduction
For a drug taken orally in solid dosage preparations, dissolution, a prerequisite to movement across the intestinal wall, is the first step during its gastrointestinal absorption. Factors controlling dissolution include surface exposed to the solvent, solubility of the compound, pH of the medium, stirring rate around the dissolving particle, gastric empty, and intestinal motility[1]. For bases or acids with low aqueous solubility, 2 methods of increasing the dissolution have been considered: use of salt and use of an acidic or a basic environment. Of these, using a salt generally results in faster dissolution. Considering this, many drugs are administered as salts, and domperidone is an example. Domperidone is a potent peripheral dopaminergic antagonist, which has been reported as being effective in functional gastrointestinal disorders, such as dyspepsia, gastroesophageal reflux, nausea, and vomiting. It is usually co-administered with antacids, alginates, and antisecretory compounds, primarily H2 receptor antagonists and proton pump inhibitors in the treatment of gastro-esophageal reflux disease (GERD)[2]. Domperidone has been marketed worldwide since 1978. It is available in 2 formulations: free-base tablets and maleate salt tablets. In vitro dissolution tests have shown that maleate salt dissolves more rapidly than the free base in neutral environment. In vivo studies have shown that in healthy, fasting patients, the free base and maleate salt have demonstrated a good bioequivalence in the extent and the rate of absorption[3], but the absorption of the free base is significantly decreased by the prior administration of 300 mg cimetidine combined with sodium bicarbonate solution (100 mL of 0.5 mol/L). This indicates that reduced gastric acidity impairs the absorption of the base[3,4]. Therefore, domperi-done base is not recommended be taken with antacids and antisecretory agents, at least not simultaneously.
To optimize the therapeutic benefit in GORD patients, proton pump inhibitors are more usually co-administered with domperidone. Jiang et al have reported that antireflux therapy (including 20 mg oral omeprazole once daily and 10 mg domperidone 3 times a day for 6 weeks) may improve pulmonary function and inhibit bronchial hyper-responsiveness in asthmatic patients with GORD[5]. However, their effects on the absorption of domperidone, both as free base and maleate salt, are unclear. In the present study, we compared the influence of omeprazole on the pharmacokinetics of both domperidone base and domperidone maleate salt.
Materials and methods
Patients Ten healthy, male, Chinese patients ranging in age from 20 to 27 years (median age, 22 years), in weight from 60 to 76 kg (67.1 kg±5.6 kg), and in height from 165 to 180 cm (175 cm±6 cm) were enrolled in this study. Before enrolment, each patient was considered to be in good health through medical history, physical examination, electrocardiograms, and routine laboratory tests. No medication was used for at least 2 weeks before the study, and alcohol was forbidden within 72 h prior to drug administration.
Ethics The study was approved by the Independent Ethics Committee of the People’s Hospital of Liaoning Province (Shenyang, China) and was in full compliance with the principles of the ‘Declaration of Helsinki’ (current revision) and the ‘Good Clinical Practice’ guidelines. Written informed consent was obtained from each patient before the study.
Study design This investigation was conducted in an open, randomized, 2-period crossover study with a washout period of 7 d. In each study period, the patients were administered a single oral dose of 10 mg domperidone as free-base tablet (Motilium, Janssen, Xi’an, China) or maleate salt tablet (Domperidone maleate tablet, Hanmi, Tianjin, China) on d 1, and 20 mg of omeprazole (Losec, AstraZeneca, Wuxi, China) twice daily on d 2 and 3 and once on d 4. A single dose of 10 mg domperidone as a free-base tablet or maleate salt tablet was given at 4 h after administration of omeprazole on d 4. On the study days, the drugs were administered in the fasting state, and the patients continued to fast for 2 h after drug administration. Standard meals were provided at 2, 5, and 10 h post-dose.
Sample collection In each study period, blood samples (4 mL) were collected from the forearm vein and placed in heparinized tubes prepared prior to domperidone administration and at 0.25, 0.5, 1.0, 1.5, 2, 3, 4, 6, 8, 12, 24, and 30 h after domperidone administration on d 1 and 4, respectively. The samples were then centrifuged at 1500×g for 10 min. Separated plasma was stored frozen (-20°C) until the assay.
Domperidone assay Plasma concentrations of domperi-done were determined using a validated liquid chromatography–tandem mass spectrometry method[6]. Protein was precipitated by the addition of 300 µL methanol to 100 µL plasma. Following centrifugation, supernatants (20 µL) were injected into a Zorbax XDB C8 column (particle size 5 µm, 150×4.6 mm; Agilent, Wilmington, DE, USA), using a mobile phase of acetonitrile-water-formic acid (75:25:0.2, v:v:v) with a flow rate of 0.50 mL/min. The column temperature was 25 °C. A Thermo Finnigan TSQ triple quadrupole mass spectrometer equipped with an electrospray ionization source (San Jose, CA, USA) was used for the mass analysis and detection. Quantification was performed using selected reaction monitoring of the transitions m/z 426 → m/z 175 for domperidone, and m/z 256 → m/z 167 for the internal standard diphenhydramine, respectively. The retention times of domperidone and the internal standard were 3.12 and 3.27 min, respectively.
The response of domperidone were found linear (r>0.998) over the concentration range of 0.100–100 µg/L, with the lower limit of quantification of 0.100 µg/L. The intra- and inter-run precision values for the concentrations of 0.25, 8.00, and 90.0 µg/L were all less than 5.1%, and the accuracy ranged from 98.9% to 99.4% of the nominal value.
Data analysis Pharmacokinetic parameters were calculated using standard non-compartmental methods. Maximum concentration (Cmax) and the time to reach Cmax (tmax) were determined by the inspection of the plasma concentration-time curves. The elimination rate constant (ke) was determined by liner regression of the terminal linear portion of the ln-concentration-time curve, and the apparent elimination half-life (t1/2) was calculated as 0.693/ke. The area under the plasma concentration-time curve from 0 to the last point (AUC0–t) was calculated by the linear trapezoidal method. The AUC from 0 to infinity (AUC0–∞) was calculated as AUC0–t+Ct/ke, where Ct is the last measurable concentration. The software utilized for the pharmacokinetic analysis was WinNonlin 5.0.1 (Pharsight, Mountain View, CA, USA).
Statistical analysis The parameters AUC and Cmax were logarithmically transformed prior to the statistical analysis. The pharmacokinetic differences (parameters AUC and Cmax) between administration of domperidone alone and pretreatment with omeprazole were assessed using a paired t-test. The differences between free-base and maleate salt domperi-done were assessed by ANOVA, and 90% confidence intervals for ratios were given. A Wilcoxon signed rank test was performed on tmax and t1/2. For all the analyses, P<0.05 was considered statistically significant.
Results
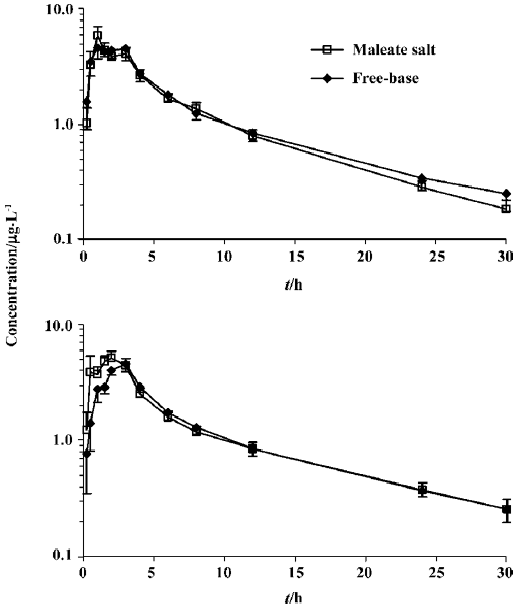
Plasma concentration-time curves of domperidone for the free base and maleate salt tablets administered alone are shown in Figure 1A. Plasma concentration-time curves for these 2 tablets administered in the presence of omeprazole are shown in Figure 1B. The calculated parameters are listed in Table 1.

Full table
For the free-base domperidone, a 16% decrease in Cmax was observed after pretreatment with omeprazole, compared with administration alone (P<0.05). The parameter AUC also decreased, but the change was minor. For maleate salt, with the exception of a prolonged t1/2 from 7.32 to 9.04 h (P<0.05), no pharmacokinetic parameters were significantly changed.
When domperidone base and domperidone maleate salt were administered alone, no differences were found in any parameters between them. In contrast, when they were administered with omeprazole pretreatment, Cmax of domperidone given as the free base was lower (25.9%) than that given as maleate salt (P<0.05), with a 90% confidence interval of 66.1%–86.5%. The statistic results are listed in Table 2.
Safety assessment No adverse effects were found throughout the study. Both domperidone and omeprazole were well tolerated in all the patients.

Full table
Discussion
Increased gastric pH induced by omeprazole administration is a possible mechanism underlying interactions between omeprazole and other drugs. By decreasing gastric acidity, it has the potential to modify the solubility of other drug substances or alter drug release from products with pH-dependent dissolution properties. In the present study, we first compared the relative bioavailability between the free base and maleate salt of domperidone in healthy patients at conditions of administration alone or pretreatment with omeprazole. Similar to the effect of cimetidine combined with sodium bicarbonate[7], pretreatment with omeprazole does not lower the absorption of maleate salt. This result confirms that the absorption of domperidone maleate salt is not affected by gastric pH environment, and this is a superiority of its use in clinical therapy.
In this study, we also found that for domperidone base, in spite of a 16% decrease in Cmax, the effect of omeprazole on the AUC was minor, much less than that of cimetidine combined with sodium bicarbonate (a decrease of >50%)[4,7]. As gastric acidity is a major factor for the absorption of domperidone base, the difference on increasing gastric pH might be an explanation. Although gastric pH determination was not conducted due to the experimental limitation, it has been reported that treatment of omeprazole may increase intragastric pH to a range of 5–6 at most in healthy Helicobacter pylori-negative patients[8–10]. However, sodium bicarbonate can neutralize intragastric acid to a higher pH of 6.7[11], nearly a neutral environment where domperidone base is practically insoluble. That may be a reason for the notable effect of sodium bicarbonate. As we can see in Figure 1B, omeprazole only decreases the rate of absorption of domperidone base, but does not affect the extent of absorption. Apparently, there is no clinical significance.
Since omeprazole is known to inhibit cytochrome P450 3A4[12], which is involved in the metabolism of domperidone[13], it is possible that such an interaction could elevate the bioavailability of the latter when the 2 agents are co-administered. However, the present study showed that for maleate salt, although the elimination half-life was significantly increased when pretreated with omeprazole, the main parameter AUC was not increased significantly, and for the free base, both t1/2 and AUC were unchanged. These results suggested that there may be an effect, but it is only a slight one and without any therapeutic consequences.
In conclusion, the study indicates that the absorption of domperidone as maleate salt is not influenced by the pre-administration of omeprazole, and the rate, but not the extent of absorption of the free base, is affected moderately. In this study, we did not find any clinically relevant interactions between domperidone and omeprazole. Hence, it has justified in pharmacokinetics the combination use of these 2 agents in clinical therapy. Nevertheless, if more effective proton pump inhibitors are used, domperidone maleate would be preferable for its better pharmacokinetic characteristics than that of the free base.
Acknowledgements
We would like to acknowledge Prof Rong-qin LI and the nursing staff of the People’s Hospital of Liaoning Province for their help in performing the clinical studies. We also would like to acknowledge Prof Jiang ZHENG (University of Washington, Seattle, USA) for his help in preparation of the manuscript.
References
- Rowland M, Tozer TN. Clinical pharmacokinetics, concepts and applications. Philadelphia, USA: Lippincott Williams & Wilkins; 1995.
- Hatlebakk JG, Berstad A. Pharmacokinetic optimisation in the treatment of gastro-oesophageal reflux disease. Clin Pharmaco-kinet 1996;31:386-406.
- Huang YC, Colaizzi JL, Bierman RH, Woestenborghs R, Heykants JJ. Pharmacokinetics and dose proportionality of domperidone in healthy volunteers. J Clin Pharmacol 1986;26:628-32.
- Barone JA. Domperidone: a peripherally acting dopamine 2-receptor antagonist. Ann Pharmacother 1999;33:429-40.
- Jiang SP, Liang RY, Zeng ZY, Liu QL, Liang YK, Li JG. Effects of antireflux treatment on bronchial hyper-responsiveness and lung function in asthmatic patients with gastroesophageal reflux disease. World J Gastroenterol 2003;9:1123-5.
- Yu HL, Chen XY, Zhong DF. Determination of domperidone in human plasma by liquid chromatography-tandem spectrometry. J Instrument Anal 2005;24 Suppl 5:149-50. Chinese..
- Brogden RN, Carmine AA, Heel RC, Speight TM, Avery GS. Domperidone, a review of its pharmacological activity, pharmacokinetics and therapeutic efficacy in the symptomatic treatment of chronic dyspepsia and as an antiemetic. Drugs 1982;24:360-400.
- Gan KH, Geus WP, Lamers CB, Heijerman HG. Effect of omeprazole 40 mg once daily on intraduodenal and intragastric pH in H. pylori-negative healthy subjects. Dig Dis Sci 1997;42:2304-9.
- Cederberg C, Rohss K, Lundborg P, Olbe L. Effect of once daily intravenous and oral omeprazole on 24-hour intragastric acidity in healthy subjects. Scand J Gastroenterol 1993;28:179-84.
- Hartmann M, Theiss U, Huber R, Luhmann R, Bliesath H, Wurst W, et al. Twenty-four-hour intragastric pH profiles and pharmacokinetics following single and repeated oral administration of the proton pump inhibitor pantoprazole in comparison to omeprazole. Aliment Pharmacol Ther 1996;10:359-66.
- Graham DY, Opekun AR, Jogi M, Yamaoka Y, Lu H, Reddy R, et al. False negative urea breath tests with H2-receptor antagonists: interactions between Helicobacter pylori density and pH. Helicobacter 2004;9:17-27.
- Li XQ, Andersson TB, Ahlstrom M, Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 2004;32:821-7.
- Simard C, Michaud V, Gibbs B, Masse R, Lessard E, Turgeon J. Identification of the cytochrome P450 enzymes involved in the metabolism of domperidone. Xenobiotica 2004;34:1013-23.
