Role of hypothalamus nociceptin/orphanin FQ in pre-ovulatory luteini-zing hormone surge of estrogen and progesterone-primed, ovariectomized rats
Introduction
Nociceptin/orphanin FQ (OFQ) is a heptadecapeptide that exhibits high amino acid sequence homology to the endogenous opioid peptides, especially dynorphin A[1]. OFQ is the putative endogenous ligand for the opioid receptor-like 1 receptor (ORL1 receptor)[2]. The ORL1 receptor has structural and functional homology with the δ, κ, and μ classic opioid receptors. Despite its close similarity to opioid receptors, the ORL1 receptor does not selectively bind opioids and opioid antagonists[2]. A number of physiological effects of OFQ have been reported, including nociceptive modulation, and cardiovascular and renal physiological functions[3].
The accumulated investigations suggest OFQ, like the opiate, may be involved in the regulation of anterior pituitary hormone secretion. It has been demonstrated that OFQ activates the G-protein coupled, inwardly-rectifying K+ channel and hyperpolarizes many hypothalamic neurosecretion cells, especially in the arcuate nucleus (ARC)[4]. OFQ administration stimulates prolactin and growth hormone release in a dose- and time-related manner[5], and OFQ plays a physiologically significant role in the regulation of prolactin secretion[6]. A recent study has revealed that OFQ potently and dose-dependently inhibits forskolin-induced, gonadotropin-releasing hormone (GnRH) release from rat hypothalamic fragments[7]. Our previous work demonstrates that the central administration of OFQ might inhibit the release of hypothalamic GnRH and decrease the level of plasma luteinizing hormone (LH) via the ORL1 receptor in ovariectomized (OVX) rats[8]. Such an OFQ-induced alteration of anterior pituitary hormone secretion would implicate OFQ as an important modulator of reproductive function.
GnRH, as an important hypothalamic decapeptide, plays a key role in the functions of the hypothalamic-pituitary-ovary axis (HPOA) by modulating the secretion of LH and the follicle-stimulating hormone (FSH) from the anterior pituitary over the estrus cycle[9]. The most marked of these modulations is the LH surge at mid cycle, a critical event responsible for initiating ovulation[10]. Evidence in published studies has demonstrated that the neurotransmission of afferent neuronal systems that are sensitive to steroids is necessary to stimulate GnRH neurons to induce the mid-cycle LH surge[10,11]. There is general agreement that endogenous opioid peptides (EOP) mediate the negative feedback action of steroid hormones on GnRH pulse frequency during the estrus cycle[12]. The precise mechanisms underlying the opioid neural system mediating the steroid regulation of GnRH secretion remains to be determined.
OFQ and the ORL1 receptor are densely localized in the pre-optic area (POA), ventromedial hypothalamus (VMH) and ARC, all of which have been identified as the chief hypothalamic nuclei regulating GnRH release[9,13]. As far as the inhibitory effect of OFQ on GnRH and LH release are concerned, we postulate that hypothalamus OFQ might be involved in the neuroendocrinological regulation of the LH surge during the estrus cycle. The estrogen and progesterone primed (EBP), ovariectomized (OVX) rats have always been adopted for studying the neuroendocrine mechanisms underlying the LH surge during the estrus cycle[14,15]. To test the hypothesis, we also observed the effects of the central administration of OFQ on the LH surge of EBP-primed, OVX rats. Moreover, the changes in the mRNA and protein levels of OFQ coupled with the ORL1 receptor in the related hypothalamic area are also investigated at different stages during the estrus cycle of female rats.
Materials and methods
Animals and drugs Female Sprague-Dawley rats weighing 160–200 g were obtained from the Animal Center of Shanghai, Fudan University (Shanghai, China). They were kept in an air-conditioned room with controlled lighting (12 h light/12 h dark) and given free access to laboratory chow and tap water. Nociceptin/orphanin OFQ (MW 1810) and [Nphe1]NC(1–13)NH2 (NC13; MW=1382) were purchased from Phoenix Pharmaceutical Company (St Joseph, MD, USA). The compounds were dissolved in artificial cerebrospinal fluid (ACSF, 128 mmol/L NaCl, 2.5 mmol/L KCl, 1.4 mmol/L CaCl2, 1.0 mmol/L MgCl2, and 1.2 mmol/L Na2HPO4; pH 7.4). All other reagents and solvents were of analytical grade.
EBP-primed, OVX rats and the induction of the LH surge The female rats were subjected to the surgical removal of both ovaries (OVX) under aseptic conditions. The experiments were carried out 4–6 weeks after the ovariectomy. The rats were given 7.5 μg estradiol benzoate (EB) subcutaneously in corn oil at 10.00 hours. Progesterone (5 mg) was administered subcutaneously in corn oil 48 h after the EB treatment. The studies were performed from 12.00 to 20.00 hours, when the steroid-induced LH surge release took place between 13.30 and 14.30 hours[14,15].
Intracerebroventricular injection The implantation of the cannula was performed stereotaxically under anesthesia with sodium pentobarbital (40 mg/kg, ip). Stereotaxic surgical procedures were used to implant 1 22 gauge, stainless steel guide cannula with a removable 28 gauge, inner stylette to the left lateral ventricle (bregma AP: 1.0 mm; length: 1.5 mm; height: 3.0 mm)[16]. Experiments with intracerebroven-tricular (icv) injection were performed at least 7 d after the operation. The ACSF was added with the protease inhibitor (1 g/L) for preventing proteolysis after the injection of OFQ and NC13. OFQ dissolved in 10 µL ACSF was infused through a 28 gauge cannula, extending 0.5 mm beyond the guide cannula. The needle was connected to a 10 µL syringe by a polyethylene tube and the drug solutions were delivered by an infusion pump at a flow rate of 5 µL/min. The injection needle was maintained in place for an extensive period of 10 min after the injection. Without any restraint, the EBP-primed, OVX rats were icv injected with ACSF (10 µL, as the control), OFQ (20 or 200 nmol OFQ in 10 µL ACSF), NC13+OFQ (20 nmol NC13 in 2 µL ACSF, followed by 20 nmol OFQ in 8 µL ACSF) or NC13 (20 nmol NC13 in 10 µL ACSF) at 14.00 hours. Sequential blood samples, each 200 µL, were taken through the indwelling cannula from the tail veins of EBP-primed, OVX rats at 2 h intervals between 12.00 to 20.00 hours. The same volume of physiological saline was replaced at each bleeding. Blood plasma (50 µL) was separated from each blood sample by centrifugation and stored at -20 °C until the LH radioimmunoassay (RIA).
LH RIA Plasma LH was measured in duplicate samples (n=6) by double antibody RIA using an LH RIA kit (Shanghai Institute of Biological Products, Shanghai, China) according to the manufacturer’s instructions. Cross-reactivities to the related compound were less than 3.0%, and the sensitivity of the assay was 0.4 ng/mL. The intra-assay and the interassay coefficient of variation was 3.5% and 4.8%, respectively, over the range of 2.0–20 ng/mL.
RT-PCR analysis Daily vaginal smears were obtained and only the rats having 2 consecutive 4 d estrus cycles were used for the experiments. The stage of the estrus cycle was monitored by the collection of vaginal smears at 6 h intervals and each of the 6 animals was decapitated on diestrus, pro-estrus, and estrus, respectively. The total cytoplasmic RNA was isolated from the POA of the hypothalamus as described by Lamar et al[17] using Trizol reagent (Life Technologies, Rockville, MD, USA). The total RNA (4 µg) was digested with DNase RNase-free enzyme to eliminate genomic DNA, and then converted to cDNA using 200 U Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA) in 20 µL buffer containing 0.4 mmol/L deoxynucleotide triphosphates, 20 U RNase inhibitor, and 0.8 µg oligo (deoxythymidine)15 (Sino-American Biotechnology Company, Shanghai, China). The specific primers for the ORL1 receptor were 5'-CAGGC-TGTTAATGTGGCCATATG-3' and 5'-GAGCCTGAAAGCA-GACGGACACC-3' and the primers for amplifying the OFQ were 5'-GTGACTCTGAGCAGCTCAGC-3' and 5'-TTCTGG-TTGGCCAACTTCCG-3' (synthesized at the Shanghai Sangon Biological Engineering Technology and Service Company, Shanghai, China)[18]. The housekeeping gene β-actin expression was used as an internal control since it is widely utilized for PCR reaction and did not change in the rat hypothalamus during the estrus cycle (data not shown). The PCR conditions were carried out as follows: denaturation at 94 °C for 45 s, annealing at 60 °C for 45 s, and elongation at 72 °C for 60 s with Thermus aquaticus DNA polymerase (Promega, USA; 28 cycles for the ORL1 receptor and prepronociceptin, and 18 cycles for β-actin). The RT-PCR products (5 µL) were electrophoresed in a 1.6% agarose ethidium bromide gel and visualized using the SYNGENE imaging system (GeneSnape Software, London, UK).
Immunohistochemistry The stage of the estrus cycle was monitored by the collection of vaginal smears at 6 h intervals and each of the 6 animals were deeply anesthetized with 75 mg/kg sodium pentobarbital ip on diestrus, pro-estrus, and estrus, respectively. They were transcardially perfused with 200 mL of 0.9% NaCl, followed by 300 mL fixative containing 4% paraformaldehyde (PF) in 0.1 mol/L phosphate buffer (PB; pH 7.4). The brains were removed and postfixed for 48 h in 4% PF with 10% sucrose and sectioned coronally on cryostat (Leica CM1800, Nussloch, Germany) at 30 µm and stored in cryoprotectant (sodium PB, pH 7.4, with 0.9% saline, 30% sucrose, and 30% ethylene glycol) at -20 °C until use. The floating rat brain sections were washed in 0.01 mol/L phosphate-buffered saline (PBS) to remove the cryoprotectant. The sections were incubated for 60 min in bovine serum albumin (BSA) diluent (0.01 mol/L PBS with 0.9% NaCl, 5% BSA, and 0.3% Triton X-100), then transferred to a solution containing rabbit anti-rat OFQ antibody (1:1000, Phoenix Pharmaceutical Company, St Joseph, MO, USA) or a rabbit anti-rat GnRH antibody (1:2000, Chemicon Interna-tional, Temecula, California, USA) in BSA diluent, and incubated at 24 °C for 48 h. After the primary antibody incubation, the sections were washed in 0.01 mol/L PBS and incubated with biotinylated goat anti-rabbit immunoglobulin G (IgG; 1:1000) for 60 min at room temperature, followed by an avidin biotin complex coupled to horseradish peroxidase (1:1000, Vector Elite Kit, Vector Laboratories, Burlingame, CA, USA) for 60 min, also at room temperature. Immuno-staining was visualized with a 0.04% solution of 3,38-diaminobenzidine tetrahydrochloride (DAB) containing 2.5% nickel chloride and 0.01% H2O2 dissolved in 0.1 mol/L sodium acetate. After 5 min in DAB, the reaction was terminated by 2 consecutive washes in 0.9% NaCl. The sections were mounted from 0.9% NaCl onto polylysine-subbed microscope slides, dehydrated with graded alcohol, followed by xylene, and coverslipped with Permount (Fisher Scientific Company, Pittsburgh, PA, USA). Immunohistochemical bright field staining was viewed with a microscope (Nikon, Hasunuma, Tokyo, Japan) with camera attachments, and the number and the mean optical density of the immunoactive neurons and fibers were observed and analyzed under the support of Image Master VDS (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Western blotting The dissection of the medial basal hypothalamus (MBH) fragments and protein isolation of the above samples were performed as described by Lamar et al[17]. The protein samples (100 µg/sample) were obtained with the assistance of a BCA-100 protein quantitative analysis kit (Shenergy Biocolor BioScience Technology Company, Shanghai, China) and were separated by 10% SDS-PAGE. Molecular weight standards were loaded onto each gel to verify the stained proteins. The proteins were then electro-blotted onto polyvinylidene difluoride membranes (BioRad, Hercules, CA, USA). The polyclonal antibodies against the ORL1 receptor (1:500, Santa Cruz Bio-technology, Santa Cruz, CA, USA) and β-actin (1:500, Boster Biotechnology Company, Wuhan, China) were used to detect changes of the ORL1 receptor protein and β-actin (as the internal control) according to the manufacturer’s protocols. The secondary goat anti-rabbit IgG (1:200, Sino-American Biotechnology Company, China) was conjugated to horseradish peroxidase in conjunction with the enhanced chemiluminescence system (ECI, Amersham Biosciences, Little Chalfont, UK) which allowed for the visualization of proteins. Protein determination was made using the SYNGENE imaging system (Gene-Snape Software, UK).
Statistical analysis All results were expressed as mean± SD and were analyzed by SPSS statistical software (SPSS Inc, Chicago, Illinois, USA). The data in Figure 1 were analyzed by using a 2-way repeated measures ANOVA with time as the repeated measure. The other results were analyzed by 1-way ANOVA, and the significance of difference was determined by the Newman-Keuls test. When only 2 treatment groups were compared, Student’s t-test was used. A probability of P<0.05 was considered to be statistically significant.
Results
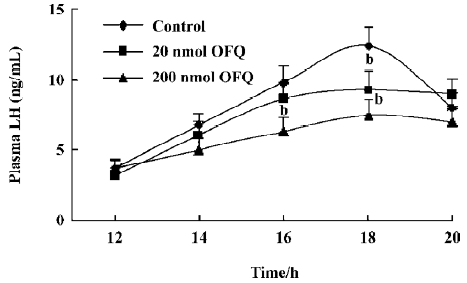
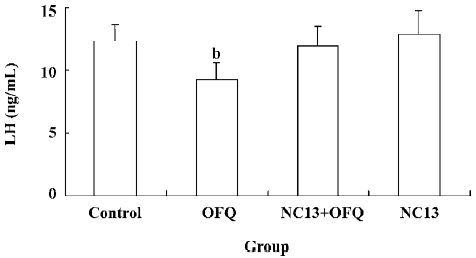
Effect of OFQ and NC13 icv injection on EBP-primed OVX rats As reported in previous studies[14,15], it was apparent that the LH level in the control group began to increase at 14.00 hours (6.72±0.85 ng/mL), but with no statistical significance, as compared with the base LH level at 12.00 hours (3.68±0.54 ng/mL). The significant steroid-induced plasma LH surge in EBP-primed, OVX rats took place at 16.00 hours (9.79±1.18 ng/mL) and reached its peak at about 18.00 hours (12.38±1.32 ng/mL). In the animals receiving icv injections of 20 and 200 nmol OFQ at 14.00 hours, the steroid-induced LH surges also occurred in the same period, but with lower amplitudes. The LH levels of the 20 and 200 nmol OFQ groups at 16.00 and 18.00 hours decreased significantly in comparison with the control group at the same time (P<0.05). OFQ showed a dose-related inhibitory effect on the LH surge of EBP-primed, OVX rats (Figure 1). The significantly decreased LH surge at 18.00 hours in the 20 nmol OFQ group (9.27±1.33 ng/mL) was abolished by pretreatment with 20 nmol NC13, a competitive antagonist of the ORL1 receptor (11.98±1.57 ng/mL). No change in the LH surge at 18.00 hours was observed with a single icv injection of 20 nmol NC13 (Figure 2).
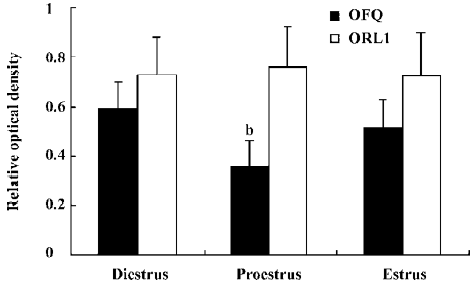
Change of OFQ and ORL1 mRNA levels in the POA during the estrus cycle The relative mRNA level of OFQ in the POA on pro-estrus (0.36±0.09) was observed to decrease significantly than those on diestrus and estrus (0.59±0.11 and 0.51±0.12; P<0.05), and the discrepancy between diestrus and estrus did not reach statistical significance (Figure 3 and 4). The relative levels of the ORL1 receptor mRNA expression in the POA were not significantly different among the 3 stages of the estrus cycle (Figure 4).
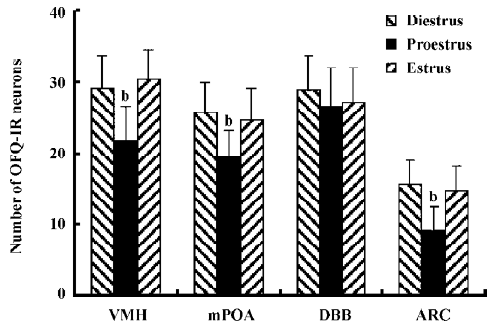
Change of OFQ-immunoreactive neurons and GnRH-IR fibers in related areas of the hypothalamus during the estrus cycle Throughout the rostral half of the hypothalamus, some anterior and lateral hypothalamic areas such as the diagonal band of Broca (DBB), paraventricular nucleus, supraoptic nuclei, and the medial POA (mPOA) contained sparse, moderately stained OFQ-immunoreactive (OFQ-IR) neurons immediately adjacent to the third ventricle, with moderate fiber and terminal labeling. The ARC also contained moderately stained cells and fibers, with numerous puncta throughout its rostral to caudal extent. Lateral to the ARC, lightly stained neurons were present in the VMH nucleus VMH (Figure 5). The number of OFQ-IR neurons in the mPOA (19.6±3.6) and VMH (21.8±4.8) in the hypothalamus on pro-estrus decreased significantly than those on diestrus (25.8±4.2 and 29.2±4.6) and estrus (24.8±4.4 and 30.4±4.2; P<0.05). The site-specific downregulation of OFQ-IR neurons on pro-estrus was also observed in ARC, but not in DBB (Figure 6). There were no statistical significances detected between diestrus and estrus in those hypothalamic nucleus mentioned earlier.
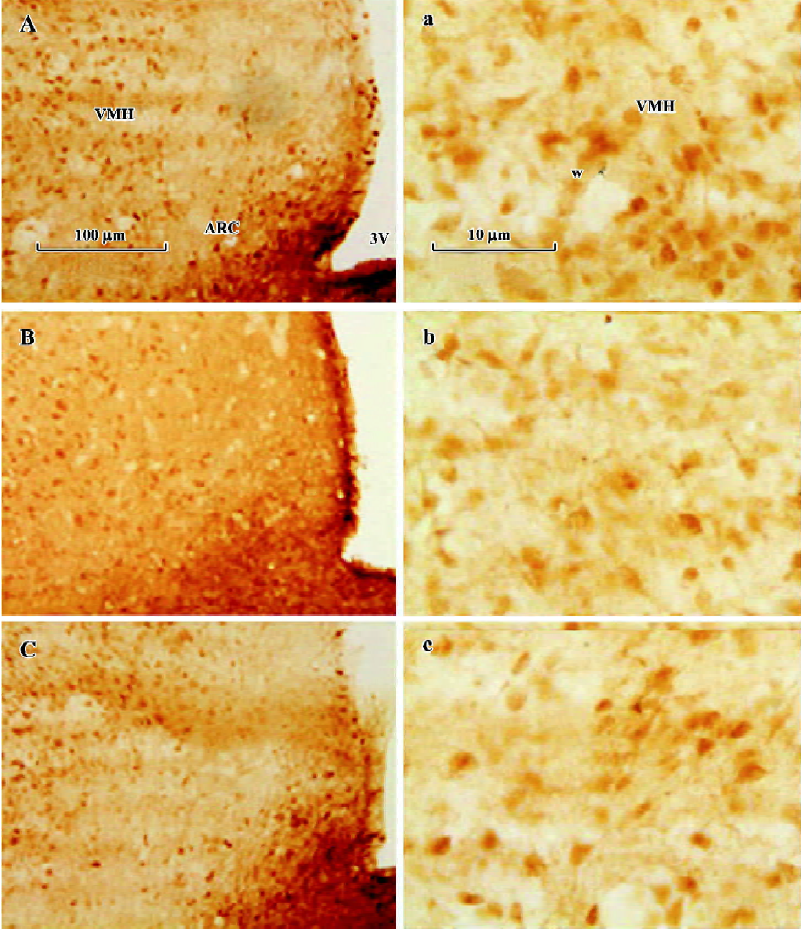
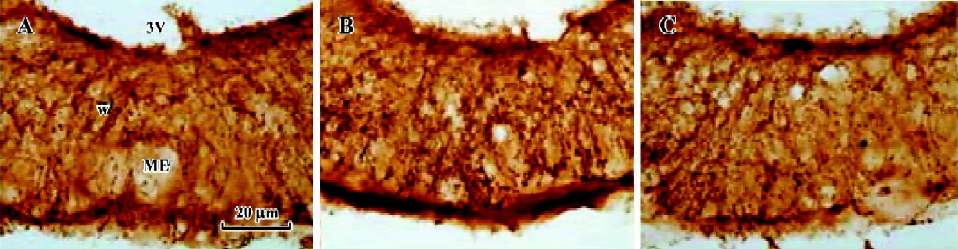
The median eminence (ME) contained an intense GnRH immunoreactive (GnRH-IR) fiber plexus and numerous puncta. Large, densely stained fibers filled the more medial aspect of ME in its rostral part (Figure 7). As expected, a significant increase in the mean optic density of GnRH-IR fibers in ME was observed on pro-estrus (27.6±4.4), as compared with those on diestrus and estrus (19.4±2.7 and 20.4±3.1; Figure 8).
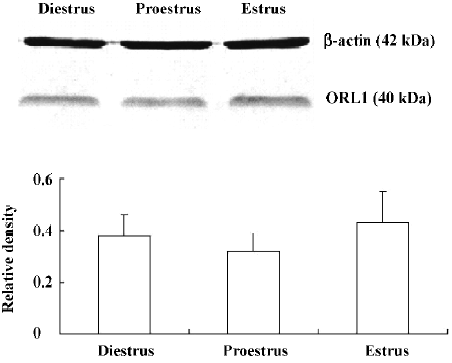
Western blot analysis There were no statistically significant differences detected in the relative ORL1 receptor protein levels in the MBH among the diestrus (0.38±0.08), pro-estrus (0.32±0.07), and estrus (0.43±0.12; Figure 8). The Western blot finding was consistent with the change of the ORL1 receptor mRNA expression in the POA.
Discussion
GnRH is the primary brain signal responsible for the release of LH and FSH from the anterior pituitary gland. Many classical neurotransmitters and neuropeptides alter GnRH neuronal activity through direct and sometimes indirect actions[19]. EOP constitutes an important inhibitory component of the neural circuitry that regulates GnRH secretion, and a significant decrease in the inhibitory opioid tone is critical for the generation of a LH surge during the estrus cycle[11]. Immunohistochemical staining and an in situ hybridization study have indicated that OFQ and the ORL1 receptor are abundant in the hypothalamus, especially in the mPOA, VMH, supraoptic nuclei, ARC, DBB, and ME, many of which are closely relevant for the regulation of GnRH release[20]. The homology between the opiate peptides and OFQ, as well as between their receptors, coupled with the localization of OFQ and its receptor in the hypothalamus, suggest a neuroendocrine role for OFQ in reproduction[13,21].
Previous studies have shown that OFQ can potently inhibit GnRH release from MBH fragments in vitro and from the POA of OVX rats in vivo[7,8]. These studies offer important clues about a possible participation of OFQ in the regulation of the HPOA. Our initial experiments confirmed that OFQ icv injections of 20 or 200 nmol can significantly decrease plasma LH levels of OVX rats, and the strongest effects occurred 2 h after administration[8]. Based on the results, we hypothesize that OFQ is most likely a predominant regulator of GnRH and LH secretion, at least in rats. In addition, the present study reveals that the central administration of 20 or 200 nmol OFQ at 14:00 h results in significant decreases of pre-ovulatory LH surges induced in the EBP-primed, OVX rats. Commonly accepted as the ideal animal model adopted for reproduction research, the EBP-primed, OVX rats may help us to elucidate the neuroendocrine mechanisms underlying the LH surge during the estrus cycle[14,15]. Due to the limits of observing periods and sampling intervals in our experiments, we can not exclude the possibility that OFQ is not only able to decrease, but can also delay pre-ovulatory LH surges in the EBP-primed, OVX rats.
As the first identified selective ORL1 receptor antagonist, [Nphe1]NC(1–13)NH2, that is, NC13, has allowed us to characterize biological effects that are clearly mediated through the ORL1 receptor in vitro and in vivo[3]. The inhibitory effect of 20 nmol OFQ on the LH surge in the EBP-primed, OVX rats can be reversed by pretreatment with 20 nmol selective ORL1 receptor antagonist NC13. In our preliminary experiments, it was found that an icv injection of 20 nmol NC13 alone has no marked influence on plasma LH levels in OVX rats. These results further indicate that the decrease of pre-ovulatory LH secretion induced by OFQ administration is mediated by the ORL1 receptor in the brain. There has been no evidence of OFQ receptors on the anterior pituitary gland until now, implying that OFQ might have no direct effect on pituitary hormone secretion. Taken together, it is strongly suggested that by interacting with its own receptor, the ORL1 receptor, OFQ elicits its inhibitory effect on hypothalamus GnRH release, thus decreasing the plasma LH surge in the estrus cycle.
The secretion of LH, which is assumed to reflect the GnRH secretion from the hypothalamus into the portal vein, shows 2 remarkably different patterns. One is pulsatile LH secretion and the other is pre-ovulatory LH surge secretion[16]. The ovarian steroid hormone estradiol feeds back at the hypothalamus to regulate the patterns of release of GnRH and the gonadotropins. Estradiol exerts an inhibitory effect on the GnRH secretion on diestrus and estrus of the estrus cycle of female rats, while the rising circulating estradiol levels cause an increase in GnRH release only during pro-estrus[12,22]. Recent evidence has demonstrated that the neurotransmission of afferent neuronal systems that are receptive to estradiol is necessary to drive reproductive cyclicity[10]. Using the double in situ histochemistry method in female rats, it has been observed that the estrogen receptor beta mRNA co-expressed with OFQ mRNA in some nucleus of the hypothalamus, indicating that estrogen may hold a direct effect on OFQ neurons[23]. Quite recently, it was reported that OFQ mRNA levels in the hypothalamus were also regulated by steroids in EBP-primed, OVX rats[24]. Taken together, it is strongly suggested that OFQ is likely to play a role in mediating the negative feedback effects of estrogen on GnRH release.
Studies in the past few decades have demonstrated that the neuronal component responsible for inducing the GnRH and LH surges is located in the POA and MBH of the hypothalamus[20]. In our study, we observed OFQ mRNA expression in the POA across the estrus cycle and found that the levels were reduced in the POA on pro-estrus. The higher level of OFQ mRNA expression on diestrus and estrus is consistent with an inhibitory role for OFQ because GnRH and LH secretion are restrained at this time by the combined negative feedback effects of estrogen and progesterone. The number of OFQ-IR neurons in the mPOA, VMH, and ARC was also found to decrease more significantly on pro-estrus than those on diestrus and estrus. This finding suggests the pre-ovulatory decline of OFQ mRNA and the protein levels in those chief nucleus governing GnRH surge secretion is likely to be associated with the onset of the pre-ovulatory LH surge in cyclic female rats. Undoubtedly, it needs more strong evidence to confirm that a decrease in OFQ tone in the POA is involved in the activation of GnRH neurons. Our next study will focus on determining the OFQ release in the POA during the estrus cycle by microdialysis.
GnRH is synthesized in neuronal cell bodies diffusely distributed across the basal forebrain and is secreted from neuronal terminals in ME[21]. Because of the difficulties in studying the diffusely distributed GnRH neurons and measuring the arbitrary volume of GnRH secretion in hypophyseal portal blood, we examined GnRH neuronal terminals in ME during the estrus cycle using immunohistochemistry and computer-assisted techniques. To some degree, the number and mean optic density of GnRH-IR fibers in ME reflect the content of immunoassayable GnRH released into the fenestrated capillaries in ME, which is commensurate with the quantities of GnRH transported from hypothalamic GnRH neurons to the pituitary portal system. Therefore, the significant increase in the mean optic density of GnRH-IR fibers observed in our experiments in ME on pro-estrus is parallel with the GnRH and the LH surge that occurred at the same time. These results also confirm the feasibility and accuracy of ascertaining the different stages in the estrus cycle by vaginal smear investigations. Admittedly, an obvious question that remains to be answered is whether the down regulation of OFQ mRNA and protein level in POA occurs just before the pre-ovulatory LH surge on pro-estrus in cycling rats.
We have also measured the mRNA and protein levels of the ORL1 receptor in the POA and MBH in the 3 stages during the estrus cycle. The high expression level of the ORL1 receptor mRNA and protein was detected in the POA and VMH, which is consistent with the localization of the ORL-1 receptor in the hypothalamus of rats[21]. The results presented in our study show that the mRNA level of the ORL1 receptor in the POA on pro-estrus has no evident difference with those on diestrus and estrus. Accordingly, the Western blot analysis indicated that there are no statistical significant changes in the protein levels of the ORL1 receptor during the estrus cycle. These data indicate that periodic steroid hormone fluctuations in the estrus cycle have no influence on the ORL1 receptor mRNA level, which is inconsistent with what has been documented by other investigators who found that sex-related differences in the ORL1 receptor gene expression appear to be determined in part by estrogen levels[25].
Although the precise neuroendocrine mechanisms for the LH surge in cycling rats remain to be elucidated, the current findings support our hypothesis that the hypothalamic endogenous OFQ might participate in the regulation of GnRH and LH secretion. Taken together with earlier studies, these results suggest that OFQ may be a novel hypothalamic modulator of reproduction.
References
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsem RA, Bunzow JR, et al. Orphanin FQ: a neuropeptide that activates an opioid-like G protein-coupled receptor. Science 1995;270:792-4.
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, et al. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 1995;377:532-5.
- Calo G, Guerrini R, Rizzi A, Salvadori S, Regoli D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol 2000;129:1261-83.
- Wanger EJ, Ronnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits beta-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology 1998;67:73-82.
- Bryant W, Janik J, Baumann M, Callahan P. Orphanin FQ stimulates prolactin and growth hormone secretion in male and female rats. Brain Res 1998;807:228-33.
- Chesterfield M, Janik J, Murphree E, Lynn C, Schmidt E, Callahan P. Orphanin FQ/nociceptin is a physiological regulator of prolactin secretion in female rats. Endocrinology 2006;147:5087-93.
- Dhandapani KM, Brann DW. Orphanin FQ inhibits GnRH secretion from rat hypothalamic fragments but not GT1-7 neurons. Neuroreport 2002;13:1247-9.
- An XF, Chen HP, Ma SL, Feng Y, Hao JW, Chen BY. Involvement of nociceptin/orphanin FQ in release of hypothalamic GnRH mediated by ORL1 receptor in ovariectomized rats. Acta Pharmacol Sin 2005;26:1039-44.
- Herbison AE. Multimodal influence of estrogen upon gonadotropin releasing hormone. Endocr Rev 1998;19:302-30.
- Smith MJ, Jennes L. Neural signals that regulate GnRH neurones directly during the oestrous cycle. Reproduction 2001;122:1-10.
- Kalra SP. Mandatory neuropeptide-steroid signaling for the preovulatory luteinizing hormone-releasing hormone discharge. Endocr Rev 1993;14:507-37.
- Goodman RL, Gibson M, Skinner DC, Lehman MN. Neuroendocrine control of pulsatile GnRH secretion during the ovarian cycle: evidence from the ewe. Reprod Suppl 2002;59:41-56.
- Neal CR, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ. Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system. J Comp Neurol 1999;406:503-47.
- Sharma P, Kaur G, Bhardwaj SK, Kaur G. Role of opioidergic and monoaminergic neurotransmission in the GnRH release mechanism of EBP-primed OVX rats. Brain Res Bull 1998;47:81-6.
- Kaur G, Kaur G. Role of cholinergic and GABAergic neurotransmission in the opioids-mediated GnRH release mechanism of EBP-primed OVX rats. Mol Cell Biochem 2001;219:13-9.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 4th ed. New York: Academic Press; 1998.
- Lamar CA, Bhat GK, Mahesh VB, Brann DW. Evidence that neuronal oxide synthase but not heme oxygenase increases in the hypothalamus on proestrous afternoon. Neuroendocrinology 1999;70:360-7.
- Briscini L, Corradini L, Ongini E, Bertorelli R. Up-regulation of ORL1 receptors in spinal tissue of allodynic rats after sciatic nerve injury. Eur J Pharmacol 2002;447:59-65.
- Pau KY, Spies HG. Neuroendocrine signals in the regulation of gonadotropin-releasing hormone secretion. Chin J Physiol 1997;40:181-96.
- Funabashi T, Mitsushima D, Nakamura TJ, Uemura T, Hirahara F, Shinohara K, et al. Gonadotropin-releasing hormone (GnRH) surge generator in female rats. Prog Brain Res 2002;141:165-73.
- Neal CR, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, et al. Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: Comparison of ORL1 receptor mRNA expression with 125I-[14Tyr]-orphanin FQ binding. J Comp Neurol 1999;412:563-605.
- Smith MJ, Jennes L. Neural signals that regulate GnRH neurons directly during the oestrous cycle. Reproduction 2001;122:1-10.
- Isgor C, Shieh KR, Akil H, Watson SJ. Colocalization of estrogen beta-receptor messenger RNA with orphanin FQ, vasopressin and oxytocin in the rat hypothalamic paraventricular and supraoptic nuclei. Anat Embryol (Berl) 2003;206:461-9.
- Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol 2006;496:252-68.
- Flores CA, Shughrue P, Petersen SL, Mokha SS. Sex-related differences in the distribution of opioid receptor-like 1 receptor mRNA and colocalization with estrogen receptor mRNA in neurons of the spinal trigeminal nucleus caudalis in the rat. Neuroscience 2003;118:769-78.
