Reversal of hyperglycemia by protein transduction of NeuroD in vivo
Introduction
The discovery of novel therapeutical protein or peptide has become one of the main trends in the development of a new drug in recent times. However, a natural barrier, the cell plasma membrane, makes direct intracellular delivery of most macromolecules impossible to achieve. Until the discovery of the protein transduction domain (PTD)[1,2], this problem could not be addressed[3]. PTD are small cationic peptides which allow proteins to penetrate the plasma membrane into nuclei directly and more efficiently[4]. The protein transduction system also improves the safety of the process by avoiding the latent risk of gene insertion or mutation. Until recently, several researchers have applied this novel technology to the treatment of diseases, for instance, tumors[5–9], ischemia[10,11], and inflammation[12]. There is growing focus on applying protein transduction as a potentially valuable tool to clinics by transducing the therapeutic protein or peptide into patients.
Diabetes mellitus is the most prevalent metabolic disease characterized by hyperglycemia. Type 1 diabetes results from insulin deficiency caused by autoimmune destruction of insulin-producing pancreatic β cells[13–15]. Although islet transplantation has been tested and found to be effective in restoring normoglycemia, the scarcity of the tissue supply and the dependency on immunosuppressive therapy hinder the clinical application of the treatment[16,17]. Therefore, there is a great medical need to search for substitute insulin-producing cells.
The intestine has close relations with the pancreas. There are several developmental similarities between endocrine cells in the gut and those of the endocrine pancreas. Both of them are derived from the endoderm, with the embryonic pancreas originating from a dorsal and ventral protrusion of the primitive gut epithelium[18]. The plasticity between the intestine and pancreas has been reported. Although the expression of the insulin gene is restricted to the β cell in mature mammals, the intestinal cells have been induced to express insulin in vivo by transfecting the intestine with Ad-MafA or Ad-PDX-1[19,20] (recombinant adenovirus containing MafA or Pdx-1 gene). In vitro, the intestinal cells have been induced to express insulin by either transfection with PDX-1 and exposure to the growth factor, betacellulin[21], or transfection with PDX-1 and the LIM homeodomain transcription factor, Islet-1[22].
BETA2/neurogenic differentiation (NeuroD), a member of the basic helix-loop-helix (bHLH) family, plays an essential role in pancreatic islet morphogenesis at early stages of development and maintenance of adult β cell function by positively regulating insulin expression[23]. The adenovirus-mediated introduction of BETA2/NeuroD could induce ectopic insulin expression in the liver[24,25]. Our previous research and others found that BETA2/NeuroD contains its own protein transduction domain which allows it to cross the membrane of several kinds of mammalian cells. Furthermore, the internalized BETA2/NeuroD protein still preserves insulin transcription activity[26,27], which opens up the possibility of applying the novel approach of protein therapy to benefit diabetic patients.
Our present study aims at evaluating whether the BETA2/NeuroD protein has transduction activity in vivo and alleviates symptoms of diabetes mellitus by inducing enteric insulin expression.
Materials and methods
Plasmid construction and protein purification The method of constructing the plasmids is described in our previous report[26]. BL21 (DE3) cells containing the expression plasmids of the NeuroD–EGFP (Enhanced Green Fluorescent Protein) protein and the EGFP protein were cultured at 37 °C so that the OD600 (Optical Density at 600 nm) reached 0.8. Isopropyl-β-D-thiogalactopyranoside was added to a final concentration of 1 mmol/L, and the cells were then incubated at 24 °C for 12 h. The cells were sonicated, the supernatants were recovered and applied to a column of Ni-NTA agarose (medium for affinity chromatography used to bind recombinant proteins with a 6×His tag) (Novagen, San Diego, CA, USA), and the target proteins were eluted with an elution buffer containing 300 mmol/L NaCl, 50 mmol/L NaH2PO4, and 150 mmol/L imidazole (pH 8.0). The protein purification procedure was then repeated in order to enhance the purity of the target proteins. Finally, Amicon Ultra-4 centrifugal filter devices (Millipore, Billerica, MA,USA) were used to remove the salt and other contaminating microsolutes. The fusion proteins were either used immediately after purification or stored at 4 °C.
Cell culture and protein internalization STC-1 (entero-endocrine cell line) cells were obtained from American Type Culture Collection (Manassas, VA, USA), and cultured in high glucose Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum. All cells were cultured at 37 °C in a humidified atmosphere with 5% CO2. The cells were seeded on a 24-well plate at a density of 1×105 cells/well. After 24 h incubation, the medium was replaced with fresh medium containing 1 μmol/L EGFP or the NeuroD–EGFP protein. The cells were incubated with the protein-containing medium for 4 h, washed 3 times with phosphate-buffered solution (PBS), fixed in 4% paraformaldehyde (PFA), and then observed using a fluorescence microscope.
Diabetic mice preparation and treatment with protein ICR male mice(derived from Institute of Cancer Research) (8 weeks of age, weighing 22±2 g, provided by and bred at the Laboratory Animal Center of the Second Military Medical University, Shanghai, China; Certificate N
RT-PCR and quantitative PCR analysis Total RNA were isolated from the small intestine using Trizol agent (Ambion, Austin, TX, USA). Reverse transcription of RNA was performed with a M-MLV (Moloney Murine Leukemia Virus) reverse transcriptase (Promega, Madison, WI). Two pairs of primers were used for the amplification of insulin (5'-TGGCTTCTTCTACACCCAAG-3' and 5'-ACAA-TGCCACGCTTCTGCC-3') and GAPDH (5'-TGGCAAA-GTGGAGATTGTTGCC-3' and 5'-AAGATGGTGATGGG-CTTCCCG-3').
The expression levels of the insulin gene were quantified by real-time quantitative PCR, using SYBR(Synergy Brands, Inc) green I dye (Invitrogen, Carlsbad, California, USA) and the ABI (Applied Biosystems) Prism 7900 Real Time PCR System (ABI, Foster City, CA, USA). The amount of copy numbers of the target genes in each sample was calculated and expressed as a ratio to the GAPDH gene. Primers are described as above.
Immunohistochemistry The mice were killed by cervical dislocation, and after a midline abdominal incision was made, the small intestine tissues were removed from the mice and fixed overnight with 4% PFA. The fixed tissues were routinely processed for paraffin embedding and approximately 4 μm sections were prepared and mounted onto slides. To detect insulin, the Streptavidin-biotin complex (SABC) method was performed using the SABC Kit (Boster, Wuhan, Hubei, China). The sections were incubated overnight with a rabbit anti-insulin antibody (H-86; Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted at 1:100 in PBS containing 1% BSA (Bovine Serum Albumin), followed by incubation with biotinylated anti-rabbit IgG (Immunoglobulin G) (Boster, Wuhan, Hubei, China), SABC reagent incubation, and 3,3'-diaminobenzidine tetrahydro-chloride (Boster, Wuhan, Hubei, China) staining.
Statistics The results of the quantitative data were expressed as mean±SD. Statistical differences between the groups were analyzed with the t-test, and the significant level was defined as a P<0.05. The data were analyzed by SPSS statistical software (SPSS, Chicago, IL, USA).
Results
Transduction of BETA2/NeuroD protein in intestine To examine whether the BETA2/NeuroD protein can be transduced into small intestine epithelium cells in vitro, we purified the NeuroD protein with EGFP fused at the C-terminal (Figure 1D) and used the fluorescence activity of EGFP to track the location of the NeuroD protein. Intestine epithelium-derived STC-1 cells were treated with the purified NeuroD–EGFP protein. After 4 h incubation, the STC-1 cells incubated with NeuroD–EGFP were nearly 100% EGFP positive as determined by a fluorescence microscope (Figure 1A, left panel). In contrast, hardly any EGFP-positive cells could be observed when they were treated with EGFP proteins (Figure 1A, right panel). These results demonstrate that the BETA2/NeuroD protein can efficiently permeate intestine epithelium cells in vitro.
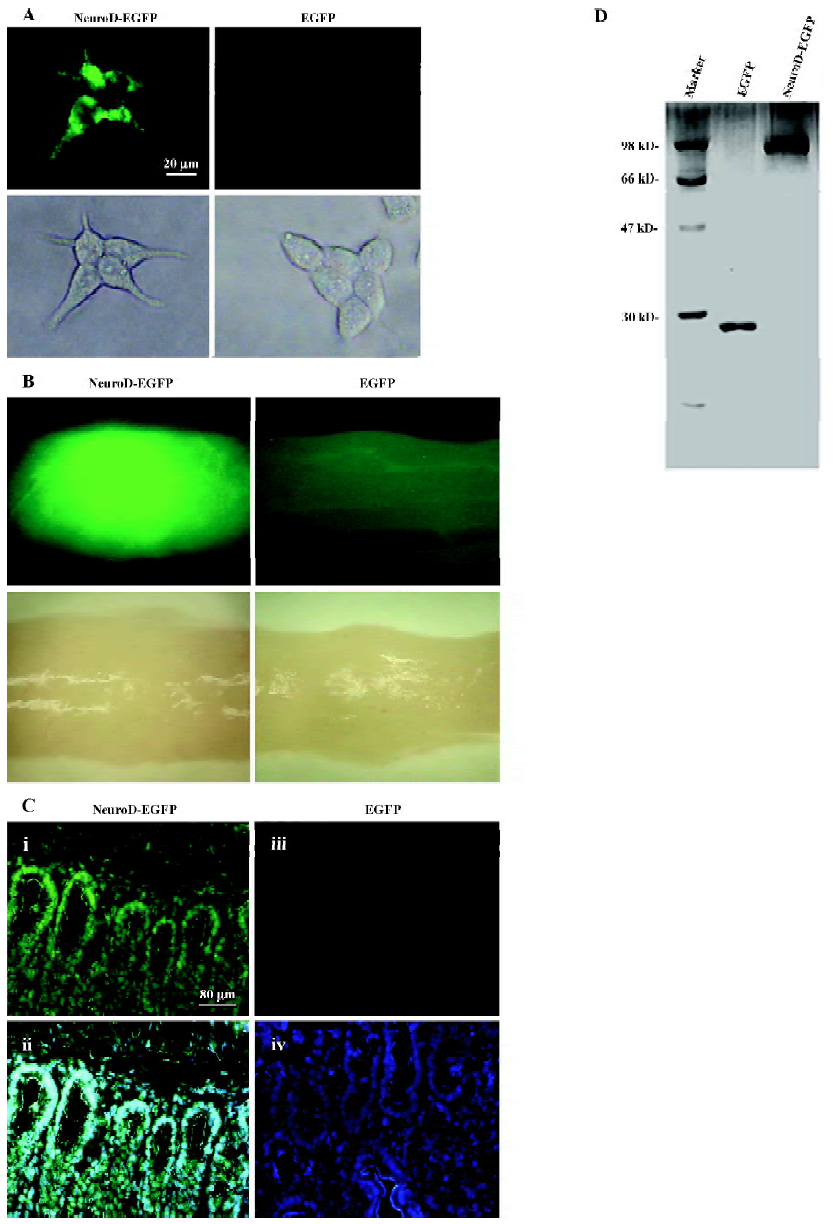
We further tested whether the BETA2/NeuroD protein can preserve its transduction activity and be internalized into the small intestine epithelium cells in vivo. The 8-week-old ICR mice were intravenously injected with NeuroD–EGFP protein (9 mg/kg) while the same dosage of EGFP protein was used as the negative control. Two hours later, the ICR mice were killed and tissue samples were isolated from the liver, pancreas, brain, small intestine, and heart. The mice were perfused with PBS to avoid possible contamination of EGFP remnants in body fluid. The tissue samples were then analyzed for the intensity of fluorescence. The NeuroD–EGFP protein was found to accumulate at the small intestine (Figure 1B) and the liver (data not shown). However, no NeuroD–EGFP protein was clearly detected in the brain, pancreas, and heart (data not shown). To confirm the distribution of the NeuroD–EGFP protein in the small intestine tissue, a technical procedure of frozen section was then conducted and analyzed. The 5 µm sections of the small intestine were prepared and treated with DAPI(4',6-diamidino-2-phenylindole) to stain the nuclei. As observed by the fluorescent microscope, the small intestine cells of the NeuroD–EGFP protein-treated mice were almost 100% EGFP positive (Figure 1C). In contrast, no EGFP-positive cell was detected in the control intestine (Figure 1C). These results indicate that NeuroD–EGFP can be transduced into and accumulate at the small intestine epithelium cells in vivo.
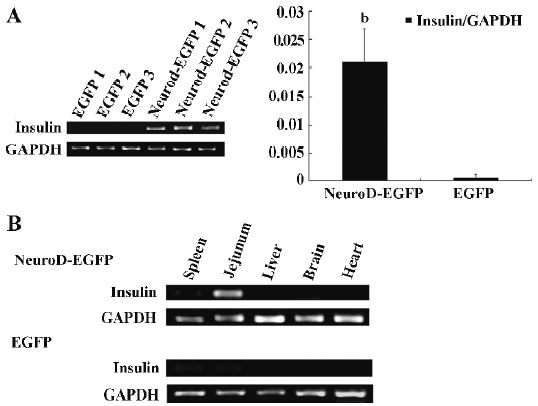
Effect of the internalized BETA2/NeuroD protein on insulin expression BETA2/NeuroD is known to activate insulin expression in pancreatic β cells. To test whether BETA2/NeuroD can stimulate the ectopic expression of the insulin gene in the small intestine of diabetic mice where the BETA2/NeuroD protein accumulated, RT-PCR and real-time quantitative PCR were performed. The 8-week-old ICR mice were treated with 150 mg/kg STZ. One week later, blood glucose concentrations were measured and hyperglycemia was verified. The STZ-induced diabetic mice were intravenously injected with the NeuroD–EGFP protein (5 mg/kg) or the EGFP protein (5 mg/kg) as the negative control. After 16 h, the tissue samples for the jejunum were separated and total tissue RNA was extracted. Semiquantitative RT-PCR revealed that the transcription of the insulin gene greatly increased in the jejunum of NeuroD–EGFP-treated mice (Figure 2A, left panel). However, no obvious insulin gene transcription was detected in the jejunum of EGFP-treated mice. Real-time quantitative PCR further demonstrated that treatment with the NeuroD–EGFP protein gave a 38-fold increase in the transcription level of the insulin gene in terms of increase in mean insulin/GAPDH ratio. For the experiment group, the ratio reached (2.109±1.019)×10-2, which was significantly different from that of the negative control equaling (5.4±0.9)×10-4 (Figure 2A, right panel, t-test, n=3, P<0.05). To test whether the NeuroD–EGFP protein could only stimulate insulin ectopic expression in the small intestine in vivo, we examined insulin expression levels in various tissues (spleen, jejunum, liver, brain, and heart). As shown by semiquantitative RT-PCR, the increasing expression levels of insulin was only clearly detected in the jejunum, but not in any other tissues (Figure 2B).
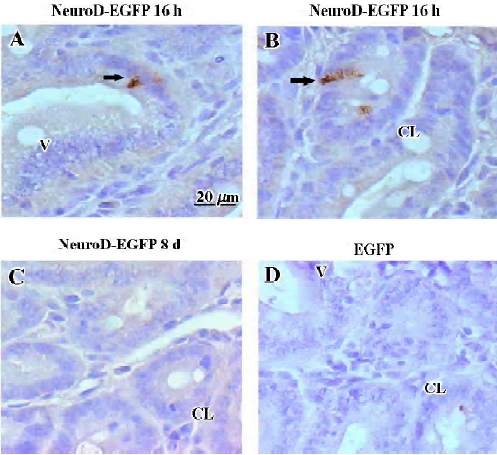
To examine whether the insulin protein was synthesized and stored in the jejunum, we used immunohistochemistry to test the insulin expression after treatment with NeuroD–EGFP and estimated the number of insulin-producing cells in the diabetic mice after different treatments. Immunostaining for insulin 16 h after treatment with the NeuroD–EGFP protein revealed several insulin-positive cells in the cytoplasm. These cells were located both in the crypts of Lieberkuhn (Figure 3B) and the intervillus epithelia (Figure 3A) of the jejunum. The density of insulin-positive cells 16 h after the NeuroD–EGFP protein treatment was 1.53±0.28 cell/mm2 (mean±SD, n=5). However, by examining 30 sections derived from 3 NeuroD–EGFP-treated mice, no insulin-positive cells were observed 8 d after NeuroD–EGFP treatment (Figure 3C). The insulin-producing cells were also not detectable in the EGFP-treated jejunum (Figure 3D) or by immunostaining without a primary antibody (data not shown).
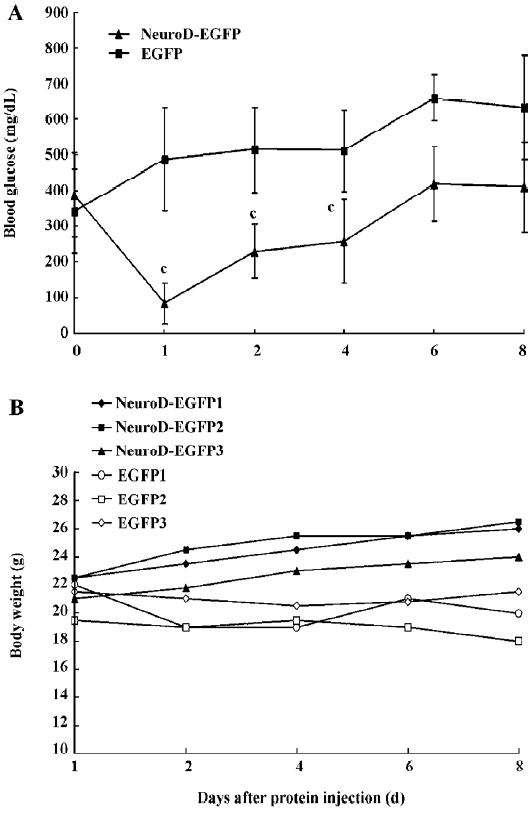
Effect of hyperglycemia alleviation by BETA2/NeuroD protein treatment To examine whether enteric insulin production induced by the NeuroD–EGFP protein is capable of controlling blood glucose levels in diabetic mice, we injected 150 mg/kg STZ into ICR mice, and 1 week later treated the mice with 5 mg/kg of the NeuroD–EGFP protein or the same dosage of the EGFP protein as the negative control. Sixteen hours later, fasting serum insulin levels were determined. The blood glucose levels and the body weight of the mice were then measured regularly. The fasting serum insulin level of the control diabetic mice was 84±23 pg/mL. In contrast, that of the NeuroD–EGFP-treated diabetic mice was 337±39 pg/mL (t-test, n=5–6, P<0.01 vs control). The shape of the blood glucose level curve demonstrated that the administration of the NeuroD–EGFP protein could significantly reverse hyperglycemia and the effect could last for about 2–3 d (t-test, n=5−6, P<0.01). After that, however, blood glucose levels gradually increased. We did not detect any significant decrease in blood glucose levels of the control diabetic mice (Figure 4A). Moreover, we noticed that, compared with the control mice, the body weights of the diabetic mice treated with NeuroD–EGFP gradually increased (Figure 4B).
Discussion
Protein transduction technology has been considered to be an alternative tool for clinical therapy. Efforts have been made to alleviate many kinds of diseases in animal models. In our study, the results revealed that the NeuroD protein with innate transduction activity could obviously alleviate diabetic symptoms. NeuroD–EGFP-treated intestinal cells were as almost as high as 100% EGFP positive, and displayed rapid biological activity. In addition, since no extraneous gene has been introduced, the protein transduction system improves the safety of the process by avoiding the latent risk of gene insertion or mutation.
In our study, we found that the BETA2/NeuroD protein could be efficiently transduced into small intestine and liver (data not shown) tissues in vivo, implying tissue preference to a certain extent. This result is interesting because both the small intestine and liver are potential target organs to be induced to produce insulin. Several researchers have trans-differentiated liver cells into insulin-expressing cells by adenovirus-mediated gene transduction[23,24,26,27]. Never-theless, we did not detect any obvious increase of insulin gene transcription in the liver by delivery of the BETA2/NeuroD protein. Therefore, we speculate that some other factors might be involved in the process of transdifferentiation from liver cells. For instance, recently, other researchers found that a host response to an adenovirus was required for the correction of diabetes using Pdx-1 or neurogenin-3 in the liver[30]. The transdifferentiation of liver cells may be much more complicated than we expected.
In our research, the NeuroD–EGFP protein successfully stimulated intestinal cells to express insulin. This result further confirms that small intestine can serve as a target organ in diabetes treatment. Furthermore, we found that most insulin-positive cells were located at the crypts of Lieber-kuhn. It is believed that several stem cells lie in the crypt under a normal, steady-state condition[31]. Therefore, we propose a hypothesis that the internalized BETA2/NeuroD protein could bind to an insulin promoter and activate its expression in the immature intestinal cells. Other researchers have reported that IEC-6 rat intestinal epithelial crypt cell line, which have the characteristics of immature intestinal crypt cells, could be induced to differentiate into insulin-producing cells in vitro[22]. This result could prove our hypothesis to a certain extent from another aspect.
In our study, many cells in the small intestine were permeated by the NeuroD–EGFP protein, but not all cells expressed substantial amounts of insulin; in fact, 1.53±0.28 cell/mm2 in the jejunum were detected to express substantial amounts of insulin in the small intestine. Taken into account the large surface area of the jejunum, we suppose that the total insulin production in it is sufficient to correct hyperglycemia.
The BETA2/NeuroD protein could stimulate its own transcription by binding to its own E box[32]. Thus, once BETA2/NeuroD is transduced into cells that still have differentiation potential, it may induce endogenous BETA2/NeuroD trans-cription, stimulate insulin expression, and facilitate their differentiation into insulin-producing cells[3,33]. However, the BETA2/NeuroD protein-treated diabetic mice did not restore normoglycemia for a long time. Our data suggest that the effect of reversing hyperglycemia merely lasted 2–3 d. It is also believed that intestinal epithelial cells turn over every 3–5 d by dissociating from the villi tips into the gut lumen or by undergoing apoptosis at the tip of the villi[34]. Therefore, the short-lasting effect may be due to the quick turnover of intestinal epithelium cells. Moreover, we noticed that although the NeuroD–EGFP protein could reverse hyperglycemia in diabetic mice, it could not significantly ameliorate glucose tolerance (data not shown). We have to admit that there are still a few disadvantages in our technology, such as high dosage, immune activity, and unknown long-term effects. Further research should be conducted to address these problems, such as aiming at improving target specificity to reduce the protein dosage. In any case, we have to overcome a significant obstacle for clinical application of the protein transduction in diabetes treatment. Nevertheless, our research provides a new perspective of protein transduction in handling diabetes and has proven its effectiveness in correcting hyperglycemia. Put simply, the value of the research can not be outweighed by its shortcomings.
In conclusion, the protein transduction of the BETA2/NeuroD protein induces ectopic insulin expression in the small intestine, which results in improved serum insulin levels and ameliorates non-fasting glucose levels in STZ-treated mice. Here we reported an alternative strategy for diabetes treatment other than insulin injection, which provided relatively longer maintenance of euglycemia, although further research is still required for its clinical application.
References
- Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988;55:1179-88.
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988;55:1189-93.
- Noguchi H, Matsumoto S. Protein transduction technology offers a novel therapeutic approach for diabetes. J Hepatobiliary Pancreat Surg 2006;13:306-13.
- Snyder EL, Dowdy SF. Recent advances in the use of protein transduction domains for the delivery of peptides, proteins and nucleic acids in vivo. Expert Opin Drug Deliv 2005;2:43-51.
- Harbour JW, Worley L, Ma D, Cohen M. Transducible peptide therapy for uveal melanoma and retinoblastoma. Arch Ophthalmol 2002;120:1341-6.
- Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 . J Biol Chem 2002; 277: 44 236–43.
- Vucic D, Deshayes K, Ackerly H, Pisabarro MT, Kadkhodayan S, Fairbrother WJ, . SMAC negatively regulates the anti-apoptotic activity of melanoma inhibitor of apoptosis (ML-IAP). J Biol Chem 2002; 277: 12 275–9.
- Mai JC, Mi Z, Kim SH, Ng B, Robbins PD. A proapoptotic peptide for the treatment of solid tumors. Cancer Res 2001;61:7709-12.
- Hosotani R, Miyamoto Y, Fujimoto K, Doi R, Otaka A, Fujii N, et al. Trojan p16 peptide suppresses pancreatic cancer growth and prolongs survival in mice. Clin Cancer Res 2002;8:1271-6.
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, et al. Treatment of ischemic brain damage by perturbing NMDA receptor–PSD-95 protein interactions. Science 2002;298:846-50.
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, et al. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain Injury and neuronal apoptosis. J Neurosci 2002;22:5423-31.
- Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 2000;6:1362-7.
- Schranz DB, Lernmark A. Immunology in diabetes: an update. Diabetes Metab Rev 1998;14:3-29.
- Adorini L, Gregori S, Harrison LC. Understanding autoimmune diabetes: insights from mouse models. Trends Mol Med 2002;8:31-8.
- Notkins AL. Immunologic and genetic factors in type 1 diabetes. J Biol Chem 2002; 277: 43 545–8.
- Jun HS, Yoon JW. Approaches for the cure of type 1 diabetes by cellular and gene therapy. Curr Gene Ther 2005;5:249-62.
- Weir GC, Bonner-Weir S. Scientific and political impediments to successful islet transplantation. Diabetes 1997;46:1247-56.
- Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mech Dev 2003;120:65-80.
- Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci USA 2003;100:5034-9.
- Koizumi M, Nagai K, Kida A, Kami K, Ito D, Fujimoto K, et al. Forced expression of PDX-1 induces insulin production in intestinal epithelia. Surgery 2006;140:273-80.
- Yoshida S, Kajimoto Y, Yasuda T, Watada H, Fujitani Y, Kosaka H, et al. PDX-1 induces differentiation of intestinal epithelioid IEC-6 into insulin-producing cells. Diabetes 2002;51:2505-13.
- Kojima H, Nakamura T, Fujita Y, Kishi A, Fujimiya M, Yamada S, et al. Combined expression of pancreatic duodenal homeobox 1 and islet factor 1 induces immature enterocytes to produce insulin. Diabetes 2002;51:1398-408.
- Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, et al. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev 1997;11:2323-34.
- Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, et al. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes 2005;54:1009-22.
- Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med 2003;9:596-603.
- Chen J, Li G, Lu J, Chen L, Huang Y, Wu H, et al. A novel type of PTD, common helix-loop-helix motif, could efficiently mediate protein transduction into mammalian cells. Biochem Biophys Res Commun 2006;347:931-40.
- Noguchi H, Bonner-Weir S, Wei FY, Matsushita M, Matsumoto S. BETA2/NeuroD protein can be transduced into cells due to an arginine- and lysine-rich sequence. Diabetes 2005;54:2859-66.
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 2000;6:568-72.
- Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, . Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem 2003; 278: 31 950–7.
- Wang AY, Ehrhardt A, Xu H, Kay MA. Adenovirus transduction is required for the correction of diabetes using Pdx-1 or neurogenin-3 in the liver. Mol Ther 2007;15:255-63.
- Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol 1997;78:219-43.
- Miyachi T, Maruyama H, Kitamura T, Nakamura S, Kawakami H. Structure and regulation of the human NeuroD (BETA2/BHF1) gene. Brain Res Mol Brain Res 1999;69:223-31.
- Noguchi H, Matsumoto S. Protein transduction technology: a novel therapeutic perspective. Acta Med Okayama 2006;60:1-11.
- Kaur P, Potten CS. Circadian variation in migration velocity in small intestinal epithelium. Cell Tissue Kinet 1986;19:591-9.
