Low-dose carvedilol reduces transmural heterogeneity of ventricular repolarization in congestive heart failure1
Introduction
Congestive heart failure (CHF) is the final stage of various structural cardiovascular, such as dilated cardiomyopathy, coronary heart disease, and hypertension. Patients with CHF have a high incidence of ventricular arrhythmias causing sudden cardiac death (SCD)[1,2]. However, the mechanism of ventricular arrhythmia in CHF is unclear.
On the other hand, recently some researches have proved that there are different electrophysiological characters among epicardial, midmyocardial, and endocardial cells. It is known that the midmyocardium has longer monophasic action potential duration (MAPD) than the epicardium and endocardium[3,4]. The transmural dispersion of repolarization (TDR) provides a basis for the development of malignant ventricular arrhythmia, such as Torsade de pointes[5]. After myocardial infarction in animals, an increase of TDR leads to ventricular transmural re-entrance and re-entrant arrhythmia[6,7], so the increasing TDR may be the main electrophysiological mechanism for malignant ventricular arrhythmia in CHF.
Carvedilol, a nonselective alpha- and beta-adrenergic receptor antagonist, has been reported to reduce ventricular arrhythmias in patients with CHF[8,9]. However, the electrophysiological mechanisms underlying this improvement are not fully understood. We hypothesized that the anti-arrhythmic action of carvedilol may be related to its electrophysiological effects on the midmyocardium.
To verify our hypotheses, we recorded the monophasic action potential (MAP) of the 3 myocardial layers in rabbits with CHF and observed the effects of carvedilol on the transmural heterogeneity of ventricular repolarization. Our final aim was to study the electrophysiological mechanism of ventricular arrhythmia and reduce SCD in patients with CHF.
Materials and methods
Animal models Twenty four adults, New Zealand White rabbits of either sex, weighing 2–3 kg, were randomly divided into 3 groups: control, CHF, and carvedilol treated CHF group. The rabbit model with CHF used in this study was previously described[10]. Adriamycin (1 mg/kg, twice weekly) was intravenously administered via a marginal ear vein for 8 weeks. In the CHF group treated with carvedilol, the rabbits received low-dose carvedilol (0.25 mg/kg, twice daily) orally for 8 weeks at the same time[11]. In both of the groups, the last administration of adriamycin or carvedilol was given 48 h before the experiment. All the rabbit hearts were perfused with Tyrode’s solution in vitro, and the monophasic action potential (MAP) in the 3 myocardial layers was recorded.
Measurement of hemodynamic parameters Aortic catheters were chronically placed into the aorta of all the animals to measure hemodynamic parameters, including mean blood pressure (MBP), cardiac output (CO), and peripheral resistance (PR) by the thermodilution technique using 5% dextrose injected into the right atrium at 25 °C. The thermistor catheter was inserted into the aorta via the left iliolumbar artery under anesthesia and positioned just below the renal arteries[12]. Right atrial and central ear artery thermistor catheters were inserted under local anaesthesia (2% xylocaine) at least 1 h before the study.
Heart perfusion All rabbits were anticoagulated with heparin (1.7 µkat/kg, iv) and anesthetized with urethane (1 g/kg, iv). The chest was opened via a left thoracotomy, and the heart was excised and placed in cold Tyrode’s solution and stored at 4 °C. The aorta was cannulated and the heart was connected to a Longendorff perfusion system (Radnoti Glass Technology, Monrovia, CA, USA). The preparation was then placed in a small tissue bath and arterially perfused with Tyrode’s solution (115.0 mmol/L NaCl, 5.4 mmol/L KCl, 1.0 mmol/L MgCl2, 1.8 mmol/L CaCl2, 1.0 mmol/L NaH2PO4, 5.0 mmol/L HEPES (4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid), and 10.0 mmol/L glucose buffered with 95% O2 and 5% CO2) at 37 °C. The perfusate was delivered by a roller pump (Cole-Parmer Instruments, Chicago, IL, USA). Perfusion pressure was monitored with a pressure transducer (World Precision Instruments, Sarasota, FL, USA) and maintained between 50 and 60 mmHg by adjustment of the perfusion flow rate. The rabbit hearts were allowed to equilibrate in the tissue bath until they were electrically stable, usually for about 1 h[13,14].
Electrophysiological recordings A transmural electrocardiogram (ECG) signal was obtained with 4 silver–silver chloride electrodes and amplified with a standard ECG amplifier. The electrodes for the 3 layers of the myocardium were made from a 0.3 mm diameter silver-silver chloride electrode thread insulated with Teflon except at the tip. The epicardium electrode was placed on the anterior wall of the left ventricle[12,13]. Compared to the left ventricular wall and midmyocardial region thickness in normal rabbits and the left ventricular wall thickness in heart failure rabbits, the electrode was inserted into the midmyocardial region, with the tip of the electrode placed 2.0–2.5 mm up to the epicardial surface (2 mm in the CHF and CHF treated with carvedilol groups and 2.5 mm in the control group, Figure 1)[13]. The endocardium electrode was inserted into the left ventricular cavity and maintained tight contact with the endocardium.
The atrioventricular node was ablated by cautery to slow the intrinsic heart rate and allow the heart to pace at a fixed rate. Right ventricular pacing was set at a cycle length of 1 s for at least 20 min to assure steady parameters. The MAP electrodes of the epicardium, midmyocardium, and endocardium were connected to a high-input impedance amplifier, and the action potentials in the 3 myocardial layers were simultaneously recorded. The MAPD was measured at 90% repolarization (MAPD90). ΔMAPD90 was the difference of MAPD90 fixed between the midmyocardium and endocardium, which was not related to the MAPD90 of the epicardium. The TDR was defined as the difference between the longest and the shortest MAPD90 among the 3 myocardial layers[14].
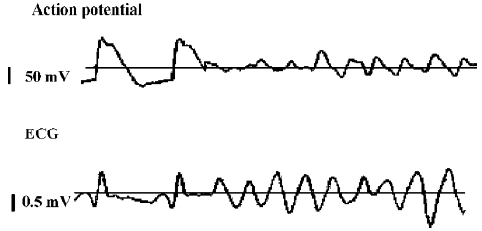
For the next protocol step, heart pacing was continued at a fixed cycle length of 1 s. An approximate ventricular fibrillation threshold (VFT) was measured by increasing the amplitude in 0.3 V steps from 1 V until ventricular fibrillation (VF) and sustained for at least 4 s[15] (Figure 2).
Measurement of ventricles Each rabbit heart was weighed. The ventricular internal dimension (VID) and ventricular wall thickness (VWT) were measured by incising the rabbit hearts along with the lower edger of the coronary artery[16].
Statistical analysis All data were presented as mean±SD. The statistical analysis of data was performed using one-way analysis of variance (ANOVA). Significance was defined as a value of P<0.05.
Results
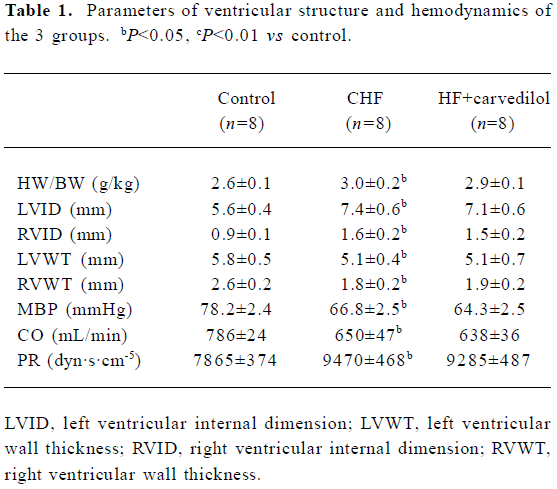
Measurements of ventricles and hemodynamic parameters As shown in Table 1, all the rabbits in the CHF and the carvedilol treated CHF groups had signs of severe CHF after being treated with adriamycin for 8 weeks. Heart weight/body weight (HW/BW) and VID were significantly increased (P<0.05), and VWT was significantly decreased (P<0.05) in the CHF group. Compared with the control group, the MBP fell from 78.2±2.4 to 66.8±2.5 mmHg (P<0.05) and CO decreased from 786±24 to 650±47 mL/min (P<0.01), while PR increased from 7865±374 to 9470±468 dyn·s·cm–5 (P<0.01) in the CHF group. However, there were no differences in the ventricular cavity, ventricular wall, and hemodynamic parameters between the CHF and the the carvedilol treated CHF groups (P>0.05). Short-term treatment with low-dose carvedilol had no effects on ventricular remodeling.
Full table
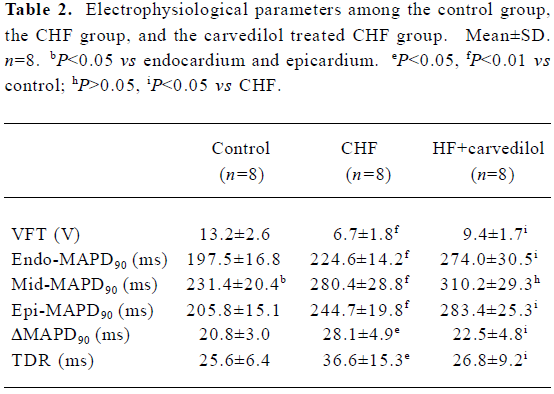
Electrophysiological parameters in the control and the CHF group VFT was remarkably decreased (P<0.01) in the CHF rabbits compared with the controls. MAPD analysis showed that the midmyocardium exhibited a longer MAPD90 than the epicardium and endocardium (P<0.05) in the control group. Compared to the control group, all MAPD90 of the 3 myocardial layers in the CHF group were extended. Furthermore, compared with those in the epicardium and endocardium, the extension of MAPD in the midmyocardium was more obvious in the CHF group (P<0.01). TDR and ΔMAPD90 were significantly increased (P<0.05) in the CHF group (Table 2, Figure 3).
Full table
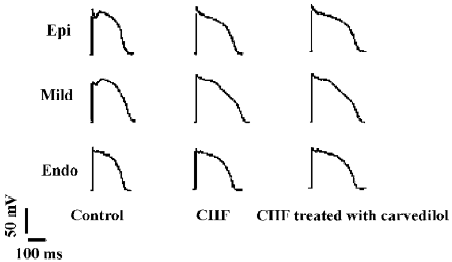
Electrophysiological parameters in the CHF treated with carvedilol group Interestingly, carvedilol treatment significantly increased VFT in the carvedilol treated CHF group. Although the MAPD90 of the 3 myocardial layers were further prolonged in the carvedilol treated CHF group, the prolongation of MAPD90 in the midmyocardium was significant shorter than those in the epicardium and endocardium (P<0.05). Therefore, TDR and ΔMAPD90 were significantly decreased (P<0.05) in the carvedilol treated CHF group (Table 2, Figure 3).
Discussion
In the present study, our results showed that chronic administration of adriamycin in rabbits produced an animal model with low output heart failure. All parameters of ventricular structure and hemodynamics were consistent with the characteristics of CHF in the CHF group. Specifically, although it is reported that carvedilol can improve ventricular remodeling, there were no differences between the CHF and the carvedilol treated CHF groups in the ventricular cavity, ventricular wall, and hemodynamic parameters in our study. We temperately ascribed the results to the low-dose administration of carvedilol. This is in accordance with a previous observation that carvedilol has dose-related effects in the prevention of volume expansion and ventricular hypertrophy[17].
CHF is often accompanied with malignant ventricular arrhythmia, such as ventricular tachycardia and fibrillation[1,2]. Our result showed that VFT was significantly decreased in the CHF group, which provides evidence that VF is more easily induced in CHF. The pathophysiological mechanisms of VF in CHF are probably due to extensive myocardial degeneration, myocardial fibrosis, and increased load, which effect the electrophysiological properties of myocardial cells and promote the occurrence of VF[18,19]. However, the electrophysiological mechanisms of VF in CHF are not well known.
More recently, some researchers have proved that there are different electrophysiological characters among epi-cardial, midmyocardial, and endocardial cells. MAPD90 is significantly longer in the midmyocardium than that in the endocardium and epicardium, which leads to electrophysiological heterogeneity in the ventricular transmural wall[3,4]. Our results are in accordance with this viewpoint.
MAPD dispersion among epicardial, endocardial, and midmyocardial cells provides the electrophysiological basis of ventricular transmural reentrance. In pathophysiological conditions, such as myocardial infarction and hypoxia, ventricular transmural repolarization is increased, which induces early after depolarization and triggers activity[5,20]. Finally, it results in ventricular re-entrant arrhythmia, such as ventricular tachycardia and ventricular fibrillation. The results in our study showed that the prolongation of MAPD90 was more marked in the midmyocardium as compared to the epicardium and endocardium. TDR and ΔMAPD90 were significant increased in the CHF rabbits.
It is reported that ventricular myocytes of the 3 myocardial layers have different electrophysiological responses to pathological changes. The midmyocardial cells are more sensitive to pathological stimulation than epicardial and endocardial cells[21]. In addition, it is shown that gap junctions among midmyocardial cells with CHF are damaged, and coupled conduction is delayed and even off, which weakens the mutual restraint of midmyocardial cells[22]. Therefore, we speculated that midmyocardial cells with CHF manifest more prominent electrophysiological characteristics and display longer MAPD90 than epicardial and endocardial cells.
Interestingly, previous studies have demonstrated that some anti-arrhythmic drugs including quinidine and d-sotalol can extend the 3 myocardial MAPD90. However, the extension of MAPD in the midmyocardium is more obvious, which is bound to result in an increase of myocardial heterogeneity and induce fatal arrhythmia such as ventricular tachycardia and ventricular fibrillation[23,24]. Contrarily, carveldilol, a nonselective alpha- and beta-adrenoceptor antagonist, produces a significant reduction in SCD in CHF patients[1,2]. Further studies also show that carvedilol can prevent ventricular arrhythmia in various animal models[25,26]. However, the detailed mechanisms of the anti-arrhythmic action of carvedilol are not fully understood.
Our study also proved the anti-arrhythmic action in the CHF rabbit model. The results showed that VFT was significantly increased in the carvedilol treated CHF group. Although Keating et al reported that it was an indirect result and related to the beneficial effects of carvedilol on ventricular remodeling[27], our result found that low-dose caveldilol could produce a direct beneficial electrophysiological property and reduce TDR among the 3 myocardial layers in the CHF rabbits. However, the ionic mechanisms of the effects of carvedilol on midmyocardial cells are poorly understood.
In conclusion, the current study shows that the transmural heterogeneity of ventricular repolarization increases in rabbits with CHF, which may be the one mechanism of malignant ventricular arrhythmias in CHF. Low-dose carvedilol can decrease the transmural heterogeneity of ventricular repolarization in CHF rabbits, which may be related to its direct electrophysiological property rather than its effect on ventricular remodeling.
References
- Kjekshus J. Arrhythmias and mortality in congestive heart failure. Am J Cardiol 1990;65:421-81.
- Bigger JT Jr. Why patients with congestive heart failure die: arrhythmias and sudden cardiac death. Circulation 1987;75:IV28-35.
- Antzelevitch C, Fish J. Electrical heterogeneity within the ventricular wall. Basic Res Cardiol 2001;96:517-27.
- Drouin E, Charpentier F, Gauthier C, Laurent K, Le Marec H. Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: evidence for presence of M cells. J Am Coll Cardiol 1995;26:185-92.
- Okazaki O, Wei D, Harumi K. A simulation study of Torsade de Pointes with M cells. J Electrocardiol 1998;31 Suppl:145-51.
- Li Y, Xue Q, Ma J, Zhang CT, Qiu P, Wang L, et al. Effects of imidapril on heterogeneity of action potential and calcium current of ventricular myocytes in infarcted rabbits. Acta Pharmacol Sin 2004;25:1458-63.
- Huang C, Bao M, Jiang H, Liu J, Yang B, Wang T. Differences in the changing trends of monophasic action potential duration and effective refractory period of the ventricular myocardium after myocardial infarction in vivo. Circ J 2004;68:1205-9.
- Fowler MB. Carvedilol prospective randomized cumulative survival (COPERNICUS) trial: carvedilol in severe heart failure. Am J Cardiol 2004;93:35B-9B.
- Kowey PR. A review of carvedilol arrhythmia data in clinical trials. J Cardiovasc Pharmacol Ther 2005;10 Suppl 1:S59-68.
- Bras-Silva C, Fontes-Sousa AP, Moura C, Areias JC, Leite-Moreira AF. Impaired response to ET (B) receptor stimulation in heart failure: functional evidence of endocardial endothelial dys-function? Exp Biol Med (Maywood) 2006;231:893-8.
- Kawai K, Qin F, Shite J, Mao W, Fukuoka S, Liang CS. Importance of antioxidant and antiapoptotic effects of beta-receptor blockers in heart failure therapy. Am J Physiol Heart Circ Physiol 2004;287:H1003-12.
- Korner PI, Oliver JR, Casley DJ. Effect of dietary salt on haemodynamics of established renal hypertension in the rabbit: Implications for the autoregulatory theory of hypertension. Hypertension 1980;2:794-801.
- Ruan YF, Liu N, Zhou Q, Li Y, Wang L. Experimental study on the mechanism of sex difference in the risk of torsade de pointes. Chin Med J (Engl) 2004;117:538-41.
- Zhang C, Li Y, Lu Z, Wu J, Wang C. Relationship between the U wave on electrocardiogram and the midmyocardium of the left ventricular wall. Chin Med J (Engl) 2002;115:509-12.
- Wan YK, Holley L, Einstein R. Ventricular fibrillation and defibrillation thresholds in sheep and dogs. Comp Biochem Physiol A Mol Integr Physiol 1998;121:77-82.
- Qu L, Li F, Huang D. Effects of chronic heart failure on electrical remodeling of failing ventricles in rabbits. Chin J Card Pacing Electrophysiol 2000;14:75-8.
- Yang Y, Tang Y, Ruan Y, Wang Y, Gao R, Chen J, et al. Comparison of metoprolol with low, middle and high doses of carvedilol in prevention of postinfarction left ventricular remodeling in rats. Jpn Heart J 2003;44:979-88.
- Albert NM. Ventricular dysrhythmias in heart failure. J Cardiovasc Nurs 2004;19 Suppl 6:S11-26.
- Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol 2006;48:1977-85.
- Anyukhovsky EP, Sosunov EA, Rosen MR. Regional differences in electrophysiological properties of epicardium, midmyocardium, and endocardium. In vitro and in vivo correlations. Circulation 1996;94:1981-8.
- Sicouri S, Antzelevitch C. A subpopulation of cells with unique electrophysiological properties in the deep subepicardium of the canine ventricle. The M cell. Circ Res 1991;68:1729-41.
- Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res 2004;95:717-25.
- Sosunov EA, Anyukhovsky EP, Rosen MR. Effects of quinidine on repolarization in canine epicardium, midmyocardium, and endocardium: I. In vitro study. Circulation 1997;96:4011-8.
- Sicouri S, Moro S, Elizari MV. D-sotalol induces marked action potential prolongation and early afterdepolarizations in M but not empirical or endocardial cells of the canine ventricle. J Cardiovasc Pharmacol Ther 1997;2:27-38.
- Xie J, Dunn A, Tsikouris JP, Sun Y, Fan C, Kluger J, et al. A placebo controlled evaluation of the antifibrillatory effects of carvedilol. J Electrocardiol 2001;34:25-30.
- Takusagawa M, Komori S, Matsumura K, Osada M, Kohno I, Umetani K, et al. The inhibitory effects of carvedilol against arrhythmias induced by coronary reperfusion in anesthetized rats. J Cardiovasc Pharmacol Ther 2000;5:105-12.
- Keating GM, Jarvis B. Carvedilol: a review of its use in chronic heart failure. Drugs 2003;63:1697-741.
