Electrophysiological effects of haloperidol on isolated rabbit Purkinje fibers and guinea pigs papillary muscles under normal and simulated ischemia
Introduction
Numerous reports have demonstrated the pro-arrhythmic effects of antipsychotic drugs[1,2], among which, haloperidol, a butyrophenone antipsychotic with established efficacy, exhibits adverse effects on the cardiovascular system, including the alteration of superficial electrocardiogram (ECG) configuration. The most severe forms of ECG abnormalities elicited by haloperidol are shown as Q-T interval prolongation, ST segment depression and torsade de pointes (Tdp) in more severe cases[3−7]. Cardiac arrhythmias under hypoxia are common. It was reported that action potential duration (APD) shortening under myocardial hypoxia might result in cardiac arrhythmias and was one of the major factors that causes Tdp[8,9]. Monique and colleagues reported that cardiac arrhythmias and Tdp were associated with antipsychotic dosage, physiological, and pathological conditions of patients[10]. Nonetheless, no systematic study has been conducted regarding haloperidol-induced arrhythmias with a special focus on hypoxic conditions. Therefore, the aim of this study was to evaluate the pro-arrhythmic risk of haloperidol under ischemia, in order to provide some indication for the clinical use of these antipsychotics.
Materials and methods
Preparation of samples Purkinje fiber samples: Male New Zealand albino rabbits, weighing 2.0±0.3 kg, were stunned by a blow to the back of the neck. The hearts were rapidly removed and placed in high potassium Tyrode’s solution with 99.9% oxygen. The left ventricle was opened and a 5-mm Purkinje fiber with cardiac tissue at both ends was isolated and fixed at the bottom of a thermostatic bath (5 mL). Then the fiber received an excessive high potassium Tyrode’s solution perfusion (velocity about 3 mL·min-1). After 30 min, the fiber was perfused with modified Tyrode’s solution bubbled with 99.9% oxygen at a temperature of 36.0±0.5 oC.
Papillary muscle samples: Male guinea pigs, weighing 200±50 g, were stunned by a blow to the back of the neck. The hearts were rapidly removed and placed in modified Tyrode’s solution with 99.9% oxygen. The papillary muscle of the right ventricle was isolated and fixed at the bottom of a thermostatic bath. The muscle was superfused with modified Tyrode’s solution (about 3 mL·min-1) bubbled with 99.9% oxygen at a temperature of 36.0±0.5 oC.
The solutions included modified Tyrode’s solution (in mmol/L): NaCl 147.0, KCl 4.0, CaCl2 1.8, MgCl2 0.5, Tris 1.2, and glucose 5.5, pH 7.35±0.05; excessive high potassium Tyrode’s solution (in mmol/L): NaCl 147.0, KCl 27.0, CaCl2 1.8, MgCl2 0.5, Tris 1.2, glucose 55.0, and 99.9% O2, pH 7.35±0.05; and simulated ischemic fluid (mmol/L)[11,12]: NaCl 147.0, KCl 4.0, CaCl2 1.8, MgCl2 0.5, Tris 1.2, and sodium lactate 22.0 saturated with 99.0%N2, pH 6.50.
Experimental protocols The samples received a 1 Hz stimulation (stimulation potency was 150% of the threshold potency) and were stabilized in modified Tyrode’s solution for 30 min. Then a glass microelectrode (filled with 3 mol/L KCl and with a resistance of 20±10 MΩ) was inserted in the samples to give the action potential curve. The signals were amplified and recorded with a RM6240B image processing system (Chengdu Instruments Factory, Sichuan, China). The sampling frequency was 100 kHz. The entire experiment was conducted in the same myocyte.
The Purkinje fibers were supervised with simulated ischemic fluid for 50 min. The action potential curves at 0, 10, 20, 30, 40, and 50 min were obtained, respectively, under 1 Hz frequency.
Different concentrations of haloperidol (0.1, 0.3, 1, 3, and 10 µmol/L) were prepared with simulated ischemic fluid. Haloperidol was added to the perfusion chamber from low to high concentrations. The effects of each concentration were observed for 10 min.
The action potential parameters were: the action potential amplitude (APA), phase 0 maximum upstroke velocity (Vmax), action potential amplitude at 90% of repolarization (APD90); and effective refractory period (ERP).
Statistical analysis Data were expressed as mean±SD. Statistical comparisons of data obtained under different testing conditions were obtained by using paired t-tests. Significant differences were fixed at P<0.05.
Results
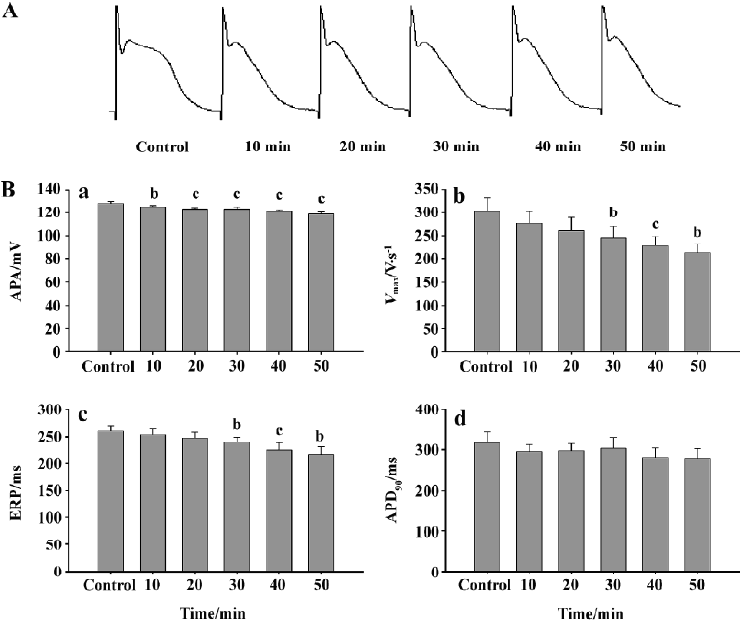
Effects of simulated ischemic fluid on the action potentials of rabbit Purkinje fibers After simulated ischemic fluid perfusion, a time-dependent decrease was observed in the APA of Purkinje fibers with a statistical significance after 10 min. The decrease in the Vmax and ERP also showed time-dependency and became significant at 30 min. Although there was a decrease in APD90, no significant difference was observed (Figure 1).
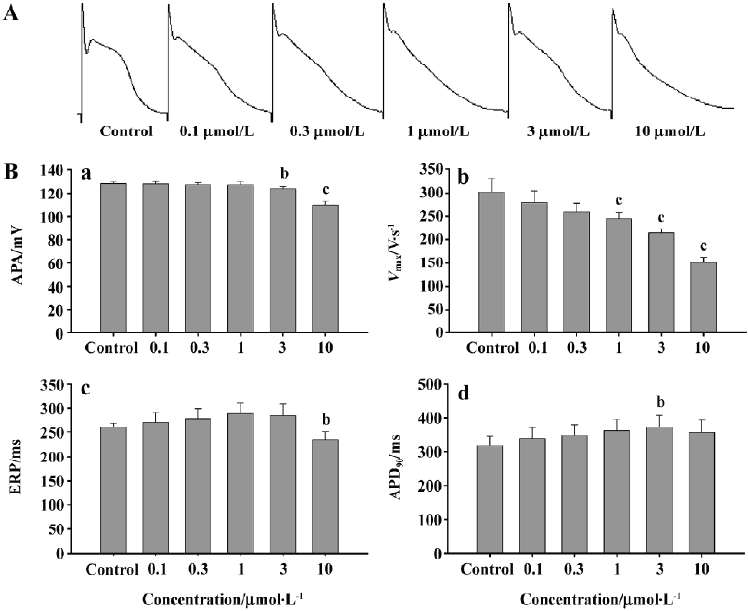
Effects of haloperidol on the action potential properties of rabbit Purkinje fiber The Purkinje fibers were treated with different concentrations of haloperidol and simulated ischemic fluid simultaneously. The results showed that the decrease in the APA revealed a concentration dependency (P<0.05 starting from 3 µmol/L); Vmax also showed a concentration dependency (P<0.01, 1 µmol/L); and there was a tendency of ERP prolongation, although no statistical significance was reached. When the haloperidol concentration was increased to10 µmol/L, a significant decrease in ERP (P<0.05) was observed. In addition, APD90 was prolonged by haloperidol in a concentration-dependent manner with a threshold of significance at 3 µmol/L (Figure 2).
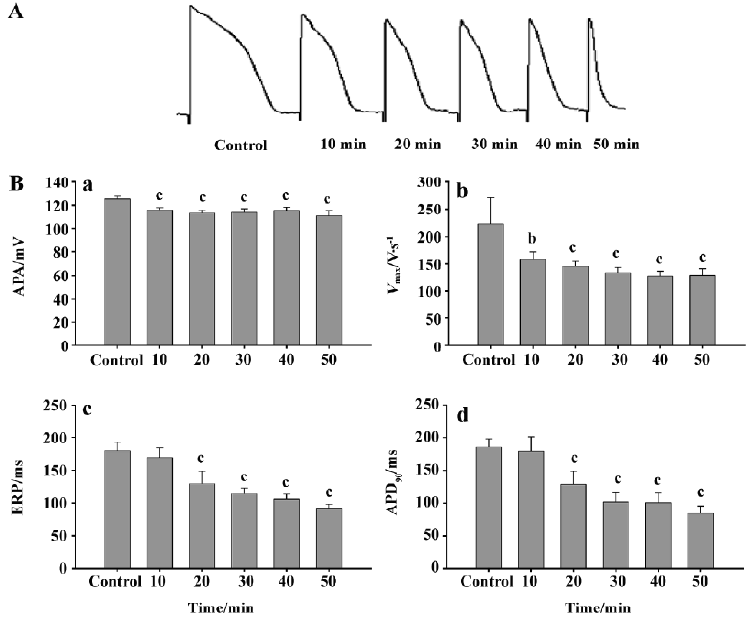
Effects of simulated ischemic fluid on the action potentials of papillary muscles in guinea pigs Ten minutes after simulated ischemic fluid perfusion, the decrease in the APA in papillary muscles showed a time-dependency and had a significant difference (P<0.01). Vmax also showed a time-dependent effect; the effect was significant after 20 min of perfusion (P<0.01). The concentration-dependent shortening of ERP and APD90 also had significance after the cells were exposed to ischemic fluid for 20 min (P<0.01, Figure 3).
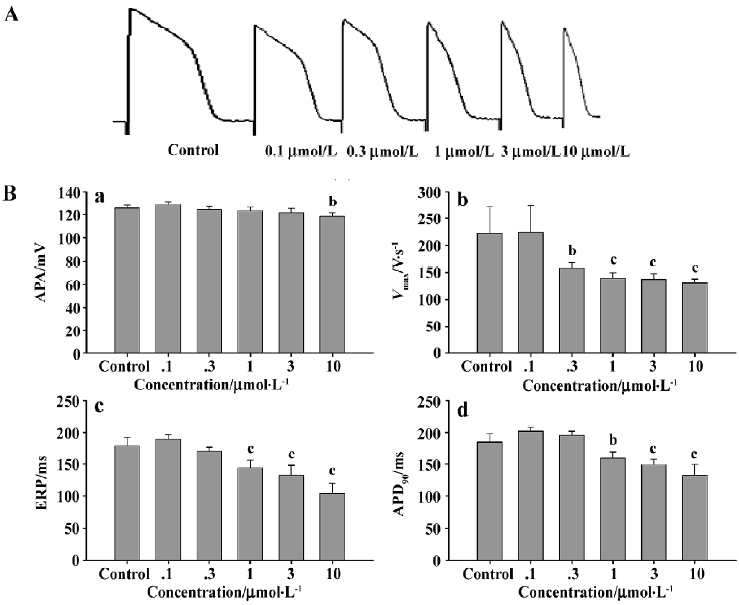
Effects of different concentrations of haloperidol on the action potential characteristics of guinea pig papillary muscles under ischemic conditions Guinea pig papillary muscle cells were perfused with different concentrations of haloperidol solutions prepared with simulated ischemic fluid. The results showed a significant concentration-dependent decrease in the Vmax; this effect was initiated after 0.3 µmol/L haloperidol solution was perfused (P<0.05). Both ERP and APD90 showed a concentration-dependent decrease which were initiated after 1 µmol/L haloperidol solution was added (P<0.05, Figure 4).
Discussion
In recent years, it has been realized that the prolonging or shortening of the Q-T interval would induce arrhythmia and even sudden death. Using the antipsychotic drug, haloperidol, and myocardial ischemia can result in the changing of the Q-T interval. In this study, we observed the effects on the action potential of two kinds of cardiac myocytes superimposed under these two factors and discovered that haloperidol prolonged the shortening of APD produced by supervising simulated ischemic fluid, we presume that the effect of haloperidol may be due to suppression of outflow of K+ induced by ischemia. We will make further studies to verify it.
Simulated ischemia for tissues was produced by supervising Tyrode’s solution containing no glucose, no O2, at low pH levels. The results showed that after 10 min perfusion, the APA of rabbit Purkinje cells was significantly different from the normal group. Then APA further decreased with increasing the duration of ischemia. At the same time, we observed a decrease in both Vmax and ERP, which had significant difference in the normal group after 30 min perfusion with ischemic fluid. Although APD90 tended to decrease, the difference was not significant. These results are in accordance with Arita who reported that ischemia might affect depolarizing Na+ currents and result in a decrease in APA and Vmax, which in turn causes an increase in K+ efflux, and thus shortening ERP and APD90[9]. It has been proven that myocardial ischemia may cause the shortening of APD, which is owed to a lack of ATP caused by ischemia, to open the channel of KATP and to cause an outflow of K+[13]. It has been suggested that inhibition of the inflow of Ca2+ will protect cardiac myocytes from ischemia which caused vasodilatation and depressed the overloads of Ca2+ induced by ischemia.
Our results showed that different concentrations of haloperidol perfusion also caused a decrease in Vmax,; however, there were no significant changes in the APA with 0.1, 0.3, and 1 µmol/L of haloperidol. Significant differences compared to the control were only observed at 3 µmol/L haloperidol. Thus, we presumed that haloperidol may also affect the sodium channels during phase 0 depolarization, and it might be concentration-dependent.
After exposure to the simulated ischemic fluid containing different concentrations of haloperidol, ERP and APD90 levels of the Purkinje fibers were prolonged and showed a concentration-dependency with haloperidol at 0.1, 0.3, 1, and 3 µmol/L. This result indicates that haloperidol prolongs APD shortening induced by ischemia in rabbit Purkinje fibers. KATP channel may be one the target of haloperidol to suppress K+ efflux during ischemia. The site of action of this effect needs further investigation using patch-clamp techno-logy.
We also investigated the effects of different concentrations of haloperidol on the action potential characteristics of papillary muscle cells under simulated ischemic conditions. As described earlier, papillary muscle cells possess higher sensitivity to ischemia than Purkinje fibers for the following reasons: (i) the volume of Purkinje fiber cells is larger, and the storage of ATP is perhaps more than that of papillary muscle cells, so it is not sensitive to ischemia; and (ii) the quantity of the KATP channel protein distributed on these two cell types is different. After 10 min superfusion with ischemic fluid, both the APA and Vmax of papillary cells showed a significant decrease compared with the normal group. All of the parameters of the action potentials were significantly decreased after 20 min perfusion. After adding haloperidol to the simulated ischemic fluid, the parameters tended to decrease, but to a lower scale.
It is necessary to notice that it is not profitable to cardiac myocytes that haloperidol prolonged the shortening of APD induced by ischemia, because the APD prolongation of the drug in the pathological state actually increases the risk of overloads of Ca2+ of cardiac myocytes. Meanwhile, higher dosages of haloperidol also depress Vmax and the APA of phase 0 to inhibit conductivity, and then aggravate the conduction blockade of ischemic regions to increase the probability of re-entry. Thus, patients suffering from myocardial ischemia should be careful when applying haloperidol.
References
- Gury C, Canceil O, Iaria P. Antipsychotic drug and cardiovascular safety: current studies of prolonged Q-T interval and risk of ventricular arrhythmia. Encephale 2000;26:62-72.
- Lathers CM, Lipka LJ. Cardiac arrhythmia, sudden death and psychoactive agents. Clin Pharmacol 1987;27:1-14.
- Franco-Bronson K, Gajwani P. Hypotension associated with intravenous haloperidol and imipenem. J Clin Psychopharmacol 1999;19:480-1.
- Hatta K, Takahashi T, Nakamura H, Yamashiro H, Asukai N, Matsuzaki I, et al. The association between intravenous haloperidol and prolonged QT interval. J Clin Psychopharmacol 2001;21:257-61.
- Kriwisky M, Perry GY, Tarchitsky D, Gutman Y, Kishon Y. Haloperidol-induced torsades de pointes. Chest 1990;98:482-4.
- Douglas PH, Block PC. Corrected QT interval prolongation associated with intravenous haloperidol in acute coronary syndromes. Catheter Cardiovasc Interv 2000;50:352-5.
- Zee-Cheng CS, Mueller CE, Seifert CF, Gibbs HR. Haloperidol and torsades de pointes. Ann Int Med 1985;102:418.
- Wang YG, Lipsius SL. β-adrenergic stimulation induced acely-chline to activate ATP-sensitive K+ channel in cat atrial myocytes. Circ Res 1995;77:565-74.
- Arita M, Sato T, Ishida H, Nakazawa H. Celluar electrophysiological basis of proarrhymic and antiarrhythmic effects of ischemia related lipid metabolites. Cardiovasc Res 1997;35:256-72.
- Adamantidis MM, Kerram P, Caron JF, Dupuis BA. Droperidol exerts dual effects on repolarization and induces early afterdepo-larization and triggered activity in rabbit Purkinje fibers. J Pharmacol Exp Ther 1993;266:844-93.
- Zhang JJ, Hu DY, Liu XL. Effect of ischemic preconditioning on action potential of anoxic reoxygenated papillary muscle of SD rats. Chin J Cardiac Pacing Electrophysiol 1999;13:178-81.
- Qi SY, Yang L, He ZS. Effects of simulated ischemia on the transient outward potassium current heterogeneity of myocytes from rabbit left ventricular free wall. J Bethune Milit Med Coll 2006;4:65-7.
- Hu K, Duan D, Li GR. Protein kinase C activates ATP sensitive K+ current in human and rabbit ventricular myocytes. Circ Res 1996;78:492-8.
