Effects of long-term, low-dose sex hormone replacement therapy on hippocampus and cognition of postmenopausal women of different apoE genotypes
Introduction
Hormone replacement therapy (HRT) has been widely used in postmenopausal women and is thought to be beneficial in protecting against the development of both cardiovascular disease and osteoporosis. However, there are intense disputes about whether HRT can reduce the incidence of Alzheimer’s disease (AD) in postmenopausal women. AD is a neurodegenerative disease characterized by progressive dementia with very complicated etiology. It is regarded to be as a result of interactions of multiple factors, including genetics, metabolism, and environment. Now it has been confirmed that the apolipoprotein E (apoE) ε4 allelomorphic gene located on chromosome 19, is one of the major genetic risk factors[1-3]. Many studies reported that women with the ε4 allelomorphic gene have greater risk of suffering from dementia than men[4,5]. The relationship between apoE gene type and estrogen is a hot topic in the pathogenesis of AD[6-8]. Many studies have already found that exogenous estrogen has protective effects on the development of dementia and the prevention of cognitive impairment[9,10]. However, not all the estrogen therapies, especially in prospective studies, showed consistent effects. It is thought that HRT can only improve the memory function of non-apoE ε4-carriers[11-14]. An observation from a study with a large sample of 7500 people by the Women’s Health Initiative Memory Study showed that estrogen or progesterone treatment did not effectively prevent the decline of cognitive function in women 65 years old or older. On the contrary, this treatment increased the risk of dementia[12].
Our previous study showed that long-term and low-dose hormone replacement therapy could reduce bone loss and the incidence of bone pain[15]. Meanwhile, the serum levels of total cholesterol, low density lipoprotein 2 cholesterol, apoB, apoCIII, and apoE decreased obviously in the postmenopausal women receiving low-dose and long-term HRT. The decrease of blood lipid levels may be protective for the cardiovascular system and therefore reduces the risk of cardiovascular diseases[16]. This prompted us to conduct this retrospective study of female medical staff at Peking Union Medical College Hospital (PUMCH; Beijing, China) to evaluate the effects of long-term, low-dose HRT treatment on the hippocampus and cognition. We examined the sex hormone level, cognitive function, brain hippocampus volume, and biochemical changes in the anterior cingulate cortex and hippocampus among the apoE carriers.
The reports about the effects of HRT on cognition are controvertible. Part of the reason, as supported by the latest reports[17-20], is due to the different designs of studies, including the initial use of HRT, type and dose of hormones, ratio of estrogen/progestogen, duration of HRT, individual difference, and whether minute cognitive difference can be detected in a dementia screening assay (ie sensitivity of the tests). In this study, besides the cognitive assessment tests, we also detected the effect of HRT on the morphology of the hippocampus based on brain imaging observations provided by proton magnetic resonance spectroscopy.
Materials and methods
Study population and design In this study, 983 (94.6%) of the 1039 female medical staff at PUMCH aged over 40 years old, either still working or retired, were interviewed via telephone. Seven hundred and fourteen of the interviewees were postmenopausal. Among these 714 women, 255 (35.7%) received HRT. According to our study criteria of inclusion and exclusion, only those who never had any of the following diseases, including stroke, AD, breast cancer, or uterine tumors, were included. Eighty three of the 255 women, aged 66.3±8.3 years and had been taking HRT for at least 4 years by the time of interview, were enrolled in the HRT group. Another 99 interviewees, who had matched age (67.1±7.6 years), education level, economic and social status, and had never taken any kind of HRT, were enrolled in the control group. The HRT paradigms of the participants were various and were based on the participants' needs, risk evaluation, and the availability of drugs. Different types of preparation, dosage, route of absorption, and combinations of HRT were used. The dosage of HRT was adjusted according to individuals' symptoms and specific needs during the course of HRT. Furthermore, during the course of HRT, some individuals were allowed to stop from a couple of weeks to a month for different reasons and then resumed their HRT. The hormones used included estradiol valerate, conjugated equine estrogens (Premarin), tibolone, and medroxyprogesterone acetate for women with an intact uterus. However, all of them used a lower dose of HRT. Among the 83 participants, 9 used half a dosage, 52 used one-quarter of a dosage, and 22 used less than one-quarter of a dosage based on the manufacturer’s recommendation. All the participants were at postmenopausal stage at the time of the tests. This study was approved by the institutional review board of the Ethics Committee of PUMCH, and all participants gave written informed consent.
Blood sample preparation Fasting blood (10 mL) was collected from each participant by vein puncture and placed into prepared tubes containing EDTA·K2. The blood samples were centrifuged to separate blood content into the plasma, blood cell pellet (red and white blood cells), and platelet. The separated blood content was frozen immediately and stored at -80 oC until use.
Plasma sex hormone concentration assay The plasma levels of estradiol (E2) and progesterone (P) were assayed by Immulite assay according to the manual of the kit [diagno-stics products corporation (DPC) Immulite-1, chemilumine-scent immunoassay system kit, Genzyme-Techne, Minnea-polis, MI, USA]. The testosterone (T) level was assayed by enzyme immunosorbent assay (Model 550, Bio-Rad, Hercules, CA, USA) using 1 mL plasma.
Genotyping of participants Genomic DNA was isolated from peripheral lymphocytes according to the instructions of the reagent manual (Takara, Dalian, China). ApoE genotyping was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). 10 µL of the PCR product was loaded onto a 1.5% agarose gel containing ethidium bromide (0.3 mg/L) for electrophoresis at 90 V for 30 min; the gels were photographed for the analysis of the apoE alleles.
Assay of the hippocampus volume Magnetic resonance imaging (MRI, GE Signa 3.0 T VHIEXITE3, General Electric Medical Systems, Milwaukee, WI, USA) was performed to determine the volume of the brain hippocampus. The scanning protocol included a T1 weighted image [T1 FLAIR, oblique coronal, vertical to the hippocampus, echo time (TE) 24 ms, repetition time (TR) 2500 ms, TI 2250 ms, slice thickness 3 mm without gaps, and including the whole body of the hippocampus] and a 3-D [fast spoiled GRASS (SPGR), TE 3.3 ms, TR 400 ms, flip angle 15°, slice thickness: 1.6 mm] image of the whole brain. The volume of the bilateral hippocampus and the whole brain were manually measured using the GE workstation (Advanced Workstation 4.2_07, GE Healthcare, Chicago, IL, USA) by first drawing the contour of the hippocampus in each slice of the oblique coronal T1-weighted image, adding all the areas, and then multiplying slice thickness to calculate the volume of the hippocampus. The ratio of the hippocampus volume to the whole brain volume was then calculated.
Assessment of the brain biochemical indexes in apoE ε3 and apoE ε4 carriers The peaks of NAA, tCr, and mI were measured in apoE ε3 and apoE ε4 carriers by 1H MRS respectively. Proton magnetic resonance spectroscopy (1H MRS) was also acquired by 3.0T MRI with the following parameters: single voxel, 20?20?20 mm; TE 35 ms; TR 1500 ms; number of excitations 8; and total acquired times 128. Three locations were examined, including the hippocampus, posterior cingulated cortex (indicating gray matter), and periventricular white matter (indicating white matter). Using post-processing software Sage 11.0 (GE Medical Systems, Chicago, IL, USA), the values of NAA/tC and mI/tCr were then calculated.
Evaluation of the total score of cognition Cognitive ability was assessed by comprehensive neuropsychological tests, including the California verbal learning test, logical memory, figure complex, verbal fluency, constructional praxis, and digit span. The sum of these scores was used to evaluate the overall cognitive ability and was manifested by least squares means (LSMEAN, the LSMEAN of total cognition).
Statistical analysis All data obtained in the study were analyzed by SPSS 13.0 for Windows (SPSS, Chicago, IL, USA). The t-test and variant analysis were used to compare values of normal distribution in the 2 groups. The non-parametric test was used to analyze data of non-normal distribution in the 2 groups. P-values lower than 0.05 were considered to be statistically significant between the 2 groups.
Results
Participants In the HRT group, the mean age of beginning HRT was 54.91±5.75 years and the average time of using HRT was 11.67±5.81 years (4?33 years). Among the 83 participants, 30 (36%) used HRT for 4-9 years, 31 (37%) for 10-14 years, 15 (18%) for 15-19 years, and 7 (9%) exceeded 20 years.
Genotype of apoE After digestion of apoE PCR products, 6 genotypes were obtained, ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4, and ε4/ε4. The distribution of the apoE alleles and the frequency in both the HRT and control groups are shown in Table 1. ApoE ε4 carriers in the HRT and control groups were 15 and 16, respectively. ApoE ε3/ε3 was the most common gene type among all of the participants. There was no significant difference between the 2 groups in the frequency of allele distribution (Table 1).

Full table
Hormone, cognitive ability, and volume of the hippocampus The plasma E2 concentration in the HRT group was significantly higher than that of the control group (P=0.0001, Table 2). Figure 1 depicts the E2 concentration in every age group. No significant difference was observed in plasma P and T concentrations and the total cognitive ability score between the 2 groups (P>0.05, Table 2).

Full table
Furthermore, the brain hippocampus MRI showed a trend of the hippocampus volume in the 2 groups decreasing with age. In the participants older than 60 years of age, the hippocampus volume decreased sharply in the control group and mildly in the HRT group (Figure 2), but the difference was not statistically significant in each age stage between the two groups.
Further analysis in participants carrying different apoE alleles Due to the small size of the study, no statistical analysis could be done with the ApoE ε2 carriers and therefore it was not included in this study. The number of apoE ε4 carriers in the HRT and control groups was 15 and 16, respectively. The number of apoE ε3 carriers in the HRT and control groups was 68 and 72, respectively. However, not all the participants were willing to take MRI detection. As a consequence, only 25 apoE ε4 allele carriers (14 from the HRT group and 11 from the control group) and 126 apoE ε3 allele carriers (54 from the HRT group and 72 from the control group) were further studied by MRI and 1H MRS. There were significant differences in the plasma E2 concentration (P=0.026), but not in P and T (P=0.359, P=0.897, respectively) between the 2 groups in the ε4 allele carriers (Table 3). Similar results were found in the ε3 allele carriers; the E2 level in the HRT group was significantly higher (P=0.001) than that of the control group, while there were no significant differences in the P and T levels (P=0.252, P=0.281, respectively). It is noteworthy that the cognitive scores of the 2 groups in the ε4 allele carriers were comparable (P=0.892, Table 3). We also noticed that all the participants had good educational background, with the average education duration being 14.22?2.63 years in the control group and 14.36?3.11 years in the HRT group (P=0.964). However, for the ε3 allele carriers, the cognition score of the HRT group was also obviously higher than that of the control group.
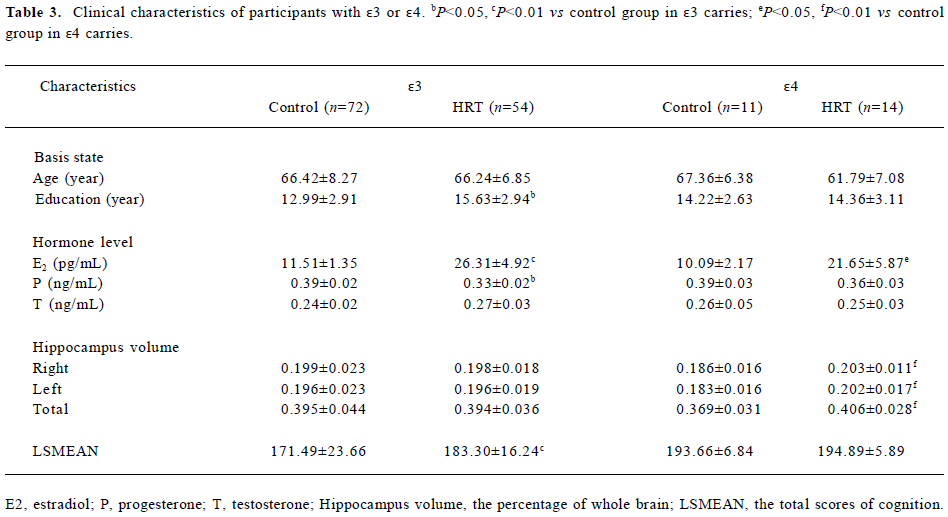
Full table
HRT had a dramatic effect on the volume of the hippocampus of apoE ε4 carriers (Table 3). In the apoE ε4 carriers of the HRT group, the ratio of the left and right hippocampus volume to the left and right brain hemisphere was significantly higher than those of the apoE ε4 carriers of the control group (0.186?0.016, 0.183?0.016, respectively, and P=0.0083, P=0.0097, respectively). Furthermore, the average of the total volume of the hippocampus of the apoE ε4 alleles carriers in the HRT group (0.406?0.028) was significantly larger than that of the apoE ε4 alleles carriers in the control group (0.369?0.031, P=0.0046). A morphological comparison of the hippocampus of 2 participants both aged 65 years —one from the HRT group (Figure 3B) who had been receiving HRT for 12 years and the other from the control group (Figure 3A) who had never received HRT — was made. The 1H MRS analysis in these apoE ε4 carriers showed that there were no differences of NAA/tCr and mI/tCr in either white or gray matters between the 2 groups. However, in the hippocampus, NAA/tCr increased significantly (1.54±0.08, P=0.031) in the HRT group, compared with that of the control group (1.45±0.13). Although mI/tCr decreased in the HRT group, no statistical difference was found (P>0.05; Table 4).


Full table
With the apoE ε3 carriers, there was no significant difference of the volumes of each side of the hippocampus and the whole hippocampus between the 2 groups. The 1H MRS analysis in these apoE ε3 carriers showed that there were no differences of NAA/tCr and mI/tCr in either white or gray matters or the hippocampus between the 2 groups.
Discussion
The present study is a retrospective, cross-sectional study on cognition and brain imaging in postmenopausal women who were medical staff at PUMCH. Our results supported the hypothesis that long-term, low-dose HRT had protective effects on the hippocampus of postmenopausal women. In our study, the mean age of beginning HRT was 54.9 years and the mean HRT duration was over 12 years. No severe HRT-related side effects, such as breast cancer or uterine tumors, were observed before and during this study. The HRT and control groups were well equilibrated and comparable, which enhanced the reliability of the results.
The effects of low-dose HRT on the prevention of postmenopausal osteoporosis were well studied in China in the 1980s. It has been shown that low-dose HRT helped to maintain genital health and postponed age-related atherosclerosis of the cardiovascular system in women receiving HRT for 5-31 years[21]. In this study, we found that despite the diversity of estrogen regimens, all the doses of HRT used were only half does, one-quarter of a dose, or even less, based on the manufacturer’s recommended dosage. All of these doses of HRT increased E2 levels effectively at all ages within the HRT group, although it was still within the postmenopausal range, indicating the reliability of the treatment regimen.
The hippocampus is a critical for retaining memories. Its pathological changes are often used for diagnosis of AD[22,23]. The volume ratio of the hippocampus to the whole brain is closely related to function of learning and memory[24]. There-fore, in this study, we detected the changes of this ratio to evaluate the degree of brain atrophy and found that the mean hippocampus volume in both groups had a trend of decreasing with age, especially after the age of 65 years. In addition, there was a sharp decrease of the hippocampus volume in the control group and a relatively mild decrease in the HRT group, but there was no statistical difference of the ratio between the 2 groups. However, further analysis of the volume ratio of the hippocampus to the whole brain of 25 cases carrying AD risk factor, the apoE ε4 allelic gene, in the 2 groups found that the mean ratio in the HRT group was significantly greater than that of the control group, indicating that long-term, low-dose HRT might be beneficial for reducing the risk of AD development in vulnerable, postmenopausal women.
To some extent, the level of NAA can reflect the metabolism and functions of the neurons. A decrease of NAA indicates damage or reduction of population of the neurons, whereas an increase of NAA implies enhancement of the neuron metabolism and therefore the effectiveness of therapy[25]. Increased mI is often found in neurodege-nerative diseases[26]. tCr is mainly composed of phosphocreatine (PCr) and creatine (Cr)[27]. Under normal conditions, the tCr concentration (Cr+PCr) in the brain is very stable and therefore can be used to normalize other indexes, such as NAA/tCr and mI/tCr. In the hippocampus of apoE ε4 carriers, NAA/tCr was significantly higher in the HRT group than it was in the control group, while mI/tCr decreased in the HRT group compared to the control group. These results indicate that HRT may help to improve brain metabolism, maintain the population of the hippocampus neurons, and reduce glia cell proliferation in apoE ε4 carriers who are vulnerable to AD. These observations were in agreement with the imaging findings from MRI, which showed that the volume of the hippocampus of apoE ε4 carriers in the HRT group was larger than that of the control group.
The total scores of cognition by blind test on the participants in this study showed that there was no significant improvement of cognitive function in postmenopausal women receiving low-dose HRT compared to the women in the control group who had never used HRT. The absence of cognitive changes at the presence of metabolic changes may be explained by high education attainment among the participants in this study, which is known to be able to compensate for mild cognitive impairment[28]. With these apoE ε4 carriers, the discrepancy between imaging and cognition tests might be due to the compensation capability of the brain, which might not enable impairment to cognitive difference when brain atrophy does not reach a critical level. It has been reported that the structural changes in the cortex usually precede cognitive impairment by at least 2 years[29,30]. Among the apoE ε3 carriers, HRT showed little effect on the volume of the hippocampus and the value of NAA/tCr and mI/tCr. However, the cognition score of the apoE ε3 carriers in the HRT group was significantly higher than that of the apoE ε3 carriers in the control group, which is consistent with a previous report that HRT could improve cognition in apoE ε3 carriers[11?14]. The different cognition results in the ε3 and ε4 carriers may be due to the small-scale study; large-scale research will provide a clear answer in the future.
In summary, our data showed that although long-term, low-dose HRT had no effect in brain metabolism and the hippocampus volume in general, it did improve these indexes in apoE ε4 carriers, indicating that long-term, low-dose HRT regimen may be beneficial in reducing the risk of AD development in vulnerable, elderly women. In addition, our results provide a valuable clue for studying the relationship among the apoE gene type, estrogen level, and the delay in the pathogenesis of AD. However, because of limitations due to the small sample size of apoE ε4 carriers in this study, a randomized, double-blind, placebo-controlled longitudinal study needs to be done in the future.
References
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Apolipoprotein E: High-avidity binding to b-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer抯 disease. Proc Natl Acad Sci USA 1993; 90: 1977?81.
- Martins RN, Clarnette R, Fisher C, Bore GA, Brooks WS, Montgomery P, ApoE genotypes in Australia: roles in early and late onset Alzheimer抯 disease and Down抯 syndrome. NeuroReport 1995; 6: 1513?6.
- Farrer LA, Cupples LA, Van Duijn CM, Connor-Lacke L, Kiely DK, Growdon JH. Rate of progression of Alzheimer抯 disease is associated with genetic risk. Arch Neurol 1995;52:918-23.
- Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet 1996;58:803-11.
- Bickeboller H, Campion D, Brice A, Amouyel P, Hannequin D, Didierjean O, et al. Apolipoprotein E and Alzheimer disease: genotype-specific risks by age and sex. Am J Hum Genet 1997;60:439-46.
- Bradbury J. Low brain oestrogen linked to Alzheimer抯 disease risk. Lancet Neurol 2006;5:116-7.
- Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, et al. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer抯 disease animal model. Proc Natl Acad Sci USA 2005;102:19198-203.
- Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer抯 disease. Lancet 1996;348:429-32.
- Costa MM, Reus VI, Wolkowitz OM, Manfredi F, Lieberman M. Estrogen replacement therapy and cognitive decline in memory-impaired post-menopausal women. Biol Psychiatry 1999;46:182-8.
- Steffens DC, Norton MC, Plassman BL, Tschanz JT, Wyse BW, Welsh-Bohmer KA, et al. Enhanced cognitive performance with estrogen use in nondemented community-dwelling older women. J Am Geriatr Soc 1999;47:1171-5.
- Shumaker SA, Legault C, Kuller L, Rapp SR. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women. JAMA 2004;291:2947-58.
- Shumaker SA, Legault C, Rapp SR. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women抯 Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651-62.
- Craig MC, Maki PM, Murphy DG. The Women抯 Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 2005;4:190-4.
- Burkhardt MS, Foster JK, Laws SM, Baker LD, Craft S, Gandy SE, et al. Oestrogen replacement therapy may improve memory functioning in the absence of APOEe4. J Alzheimers Dis 2004;6:221-8.
- Nie M, Sun ML, Ge QS. Effects of long-term and low-dose hormone replacement therapy on bone mineral density in postmenopausal women. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2006;28:421-4. Chinese..
- Song YH, Shen Y, Sun ML, Ge QS. Effect of low-dose long-term hormone replacement therapy on serum lipids in postmenopausal women. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2006;15:157-60. Chinese..
- Sherwin BB. Surgical menopause, estrogen, and cognitive function in women: what do the findings tell us? Ann N Y Acad Sci 2005;1052:3-10.
- Maki PM. Hormone therapy and risk for dementia: where do we go from here? Gynecol Endocrinol 2004;19:354-9.
- Harman SM, Naftolin F, Brinton EA, Judelson DR. Is the estrogen controversy over? Deconstructing the Women抯 Health Initiative study: a critical evaluation of the evidence. Ann N Y Acad Sci 2005;1052:43-56.
- Henderson VW. Hormone therapy and Alzheimer抯 disease: benefit or harm? Expert Opin Pharmacother 2004;5:389-406.
- Ge QS, Tian QJ, Hung TS, Frederick N. Development of low-dose reproductive hormone therapies in China. Gynecological Endocrinol 2006;22:1-10.
- Barnes J, Whitwell JL, Frost C, Josephs KA, Rossor M, Fox NC. Measurements of the amygdala and hippocampus in pathologically confirmed Alzheimer disease and frontotemporal lobar degeneration. Arch Neurol 2006;63:1434-9.
- van de Pol LA, Hensel A, van der Flier WM, Visser PJ, Pijnenburg YA, Barkhof F, et al. Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer抯 disease. J Neurol Neurosurg Psychiatry 2006;77:439-42.
- Gold JJ, Hopkins RO, Squire LR. Single-item memory, associative memory, and the human hippocampus. Learn Mem 2006;77:439-42.
- Nie M, Sun ML, Song AL, Ge QS. The effects of long-tem and low-dose of hormone replacement therapy on blood pressure and blood vessel bioactive factors in postmenopausal women. J Reprod Med 2005;14:321-4.
- Kantarci K, Jack CR Jr, Xu YC, Campeau NG. Regional metabolic patterns in mild cognitive impairment and Alzheimer抯 disease: A 1H-MRS study. Neurology 2000;55:210-7.
- Frey R, Metzler D, Fischer P, Heiden A, Scharfetter J, Moser E, et al. Myo-inositol in depressive and healthy subjects determined by frontal 1H magnetic resonance spectroscopy at 1.5 tesla. J Psychiatr Res 1998;32:411-20.
- Jones RN, Gallo JJ. Education bias in the mini-mental state examination. J Int Psychogeriatr 2001;13:299-310.
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer抯 disease. Ann Neurol 1997;42:85-94.
- Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term outcome. JAMA 2001;286:2120-7.


