Tanshinone IIA inhibits endothelin-1 production in TNF-α-induced brain microvascular endothelial cells through suppression of endothelin-converting enzyme-1 synthesis
Introduction
Endothelin (ET)-1 is a 21-amino-acid peptide isolated from endothelial cells. It has been found to be one of the most potent vasoconstrictor peptides in humans and is involved in the regulation of cerebral blood flow[1]. In addition, ET-1 has been implicated as a mediator of cerebrovascular responses in ischemic stroke and subarachnoid hemorrhage (SAH)[2,3]. ET-1 binds with high affinity to the endothelin-A receptor (ETA), which mediates vasoconstriction by activating the phospholipase C/protein kinase C cascade, decreasing smooth muscle sensitivity to nitric oxide (NO), and increasing cytosolic free calcium levels and superoxide anion production[4]. The endothelin-B receptor (ETB) has a lower affinity for ET-1 and mediates vascular relaxation, although some vasoconstrictor activity of ETB has also been shown[5]. Large ET is a precursor to endothelin with almost no vasoconstrictor activity. ET-1 is formed when large ET-1 is cleaved by endothelin-converting enzyme-1 (ECE-1). ECE-1 has been extensively detected in the human brain[6], and increased serum ECE-1 activity reflects the severity of endothelial injury to cerebral arteries[7]. The use of ECE-1 inhibitors can prevent and reverse cerebral vasospasm following SAH. ECE-1 inhibitors are expected to be efficacious in the treatment of various cerebrovascular disease[8].
TNF-α contributes to the pathology of a broad spectrum of central nervous system diseases and injury via its action on endothelial function. It has been shown that ET-1 can be modulated by TNF-α. ET-1 and ECE-1 mRNA are upregulated in response to TNF-α in endothelial cells[9-11]. To further our understanding on how tanshinone IIA (Tan IIA) could mediate the ET system, we examined the effect of TNF-α on ETA and ETB mRNA, and alterations of ET-1 binding to its receptors in the present study.
The rhizome of Salvia miltiorrhiza Bunge (SM), also known as Tanshen, is an important herb for promoting the circulation of blood and eliminating stasis in Chinese traditional medicine. Previous reports have shown that SM can prevent the postoperative increase of ET-1 after cardiopulmonary bypass in children with congenital heart defects. It can also inhibit ET-1 production and stimulate NO production in human vascular endothelial and mesangial cells[12–15]. However, the mechanisms underlying the therapeutic action of SM are not well understood. Tan IIA is one of the major diterpenes from SM. It can reduce brain infarct volume in transient focal cerebral ischemia, and can markedly inhibit the production of NO, interleukin-1β, and TNF-α, and can suppress the expression of an inducible form of NO synthase in activated mouse leukaemic monocyte macrophage cell line[16]. However, the relationship between ET-1 and Tan IIA has not been well established. As a major lipid and soluble pharmacological constituent of SM, Tan IIA may be involved in the interaction between SM and ET-1 in cerebrovascular diseases. Thus, in the present study, we examined and compared the biochemical and molecular responses of the ET system in cultured rat brain microvascular endothelial cells (BMVEC) to TNF-α and Tan IIA in an attempt to elucidate the possible cerebrovascular effects of Tan IIA.
Materials and methods
Drugs and reagents RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Gibco (Grand Island, NY, USA). TNF-α, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), penicillin, streptomycin, dimethylsulphoxide (Me2SO), the ET-1 immunoassay kit, and 125I-ET-1 were obtained from Sigma (St Louis, MO, USA). All materials used were of analytical grade.
Preparation of Tan IIA extract Tan IIA was isolated from the roots of SM, based on the method described previously[17]. Briefly, SM was extracted with methanol at room temperature, and then partitioned by methylene chloride, ethyl acetate, and n-butanol in turn. The methylene chloride fraction was subjected to column chromatography over a silica gel eluting with a gradient system of hexane, ethyl acetate. The fractions were combined based on their thin-layer chromatography pattern to yield a sub-fraction designated as D1–D11. Sub-fraction D2 was further purified by repeated column chromatography over a silica gel to afford Tan IIA. The purity of Tan IIA used was more than 99%, which was proven by HPLC according to the method for the assay of Tan IIA in Chinese Pharmacopoeia.
Isolation and culture of rat BMVEC Primary rat BMVEC were isolated as previously described[18]. Briefly, fresh rat brains were obtained from 6-week-old Wister rats, placed into ice-cold buffer A (10 mmol/L hydroxyethyl piperazine ethanesulfonic acid, 11.9 mmol/L NaHCO3, 140 mmol/L NaCl, 10 mmol/L KCl, and 0.1% bovine serum albumin). The cortices were cut into 2–3 mm pieces, further digested in a 0.1% collagenase/dispase solution to separate the microvessels from other components, and then centrifuged. The pellet containing crude microvessels was further digested in a second collagenase/dispase solution for 2 h. Microvascular endothelial cells were purified by a Percoll gradient.
The cells were maintained in an atmosphere of 5% CO2 at 37 °C in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 mg/mL streptomycin. The cells of passages 3 and 4 were used for the experiments at >80% confluency. The BMVEC were verified by staining with factor VIII, Von Willebrand factor. The cells were stimulated with TNF-α (5 μg/mL) in the presence or absence of Tan IIA at the indicated concentrations. The stock solutions of Tan IIA were dissolved in Me2SO. The concentration of Me2SO in the final culture media was 0.1% (v/v).
Evaluation of cell viability The cytotoxicity of Tan IIA was evaluated via the reduced activity of MTT. The cells were incubated for 24 h with Tan IIA at the indicated concentrations, and some cells were activated for 8 h with TNF-α (5 μg/mL) after the pre-incubation of Tan IIA for 24 h. MTT (50 μg/mL) was then added to each well. The formazan formed was dissolved in Me2SO; optical density was measured using an ELISA microplate reader at 570 nm to represent cellular viability. The optical density of formazan formed in the control cells (medium alone) was taken as 100% viability.
Extraction and assay of ET-1, including large ET-1 Media samples were collected in ice-cold polypropylene tubes containing a solution of EDTA (1 mg/mL), and aprotinin (500 kIU/mL). The samples were extracted by addition of 1.0 mL trifluoroacetic acid (1%) in 99% distilled water and centrifuged at 6000×g for 20 min at 4 °C. The supernatant was loaded onto a C-18 Sep column (Waters, Milford, MA, USA) that was previously equilibrated by washing with 100% acetonitrile followed by 1% TFA. The peptides were eluted slowly with 60% acetonitrile, 1% TFA, and 39% distilled water. The eluent was collected in a clean polypropylene tube, evaporated to dryness, and reconstituted in assay buffer. ET-1 levels, including large ET-1, were determined using enzyme immunoassay kits. Each kit consisted of a polystyrene 96-well immunoplate pre-coated with a peptide antibody. Aliquots of the reconstituted samples were loaded in duplicate onto the wells, and the assay was carried out according to the manufacturer’s protocol. Absorbance was measured at 450 nm in an automated plate reader. The absorbance was correlated with ET-1 concentrations, including large ET-1, to generate a standard curve that ranged from 0 to 1000 ng/mL. The assay of ET-1, including large ET-1, was carried out in triplicate.
Quantitation of mRNA expression by RT-PCR Whole cell RNA was isolated by guanidine thiocyanate and cesium chloride gradient centrifugation. Total RNA (5 μg, determined spectrophotometrically) was used to generate first-strand cDNA by random priming with reagents and protocols used as recommended by the manufacturers (Pharmacia, Freiburg, Germany; Gibco, USA). The cDNA representing 50 ng input RNA was amplified by PCR using Taq polymerase (Gibco, USA) in a reaction volume of 50 μL. Specific primer pairs, constructed from the reported rat gene sequence for ET-1, ECE-1, ETA, and ETB (shown in Table 1, according to previous reports[19–21]) were applied as described previously. Both primer pairs were added simultaneously to the PCR reaction vials. Each primer pair amplified a single band of the expected size in a total volume of 50 μL for each reaction. The following PCR profile was used: cDNA was denatured initially for 3 min at 94 °C and then cycling started with denaturing at 94 °C for 45 s, annealing at 54 °C for 30 s, and extension at 72 °C for 90 s. The last cycle included a prolonged extension at 72 °C for 7 min. For these experiments, an optimal PCR cycling length was used for each of the primer pairs, such that the PCR product-RNA relationship was kept in the log-linear phase. The number of cycles chosen was 30 for the ETA and ETB receptors and ET-1, and 23 for ECE-1. All RT-PCR experiments were routinely controlled by conducting PCR omitting the reverse transcription. The samples were analyzed by agarose gel electrophoresis, with the agarose gel containing 0.4 μg/mL ethidium bromide and 0.5×Tris-acetate-EDTA buffer. The bands were visualized with 302 nm light and photographed using a video processor (Mitsubishi, Tokyo, Japan). Quantitative data were obtained from a densitometer and analyzed with Quantity One 4.4.0 software (Bio-Rad, Hercules, CA, USA). Each PCR assay was run in triplicate.

Full table
Radioligand binding studies Radiolabeled ET-1 binding was studied in living cells. The binding medium used was minimum essential medium with 50 mmol/L HEPES (pH 7.4), 0.1 mg/mL bacitracin, 0.1 μg/mL aprotinin, 0.48 μg/mL leupeptin, 0.68 μg/mL pepstatin A, 0.2 mmol/L phenyl-methylsulfonyl fluoride, and 2.5 g/L BSA. The cells were washed 3 times with 1.0 mL binding buffer. 125I–ET-1 1×10-11 mol/L and increasing concentrations of unlabeled ET-1 in the presence or absence of TNF-α and Tan IIA at the indicated concentrations were added to each well. Incubations were done at 22 °C and terminated by aspiration of the binding medium and quick washing of the cells 3 times with 2.0 mL ice-cold binding medium. Counts were corrected for non-specific binding or uptake by subtraction of the radioactivity measured in the presence of excess unlabeled ligand. Cell-bound radioactivity was counted with a Wallac 1470 gamma counter (Wallac Inc, Gaithersburg, MD, USA).
Statistical analysis Data were expressed as mean±SD. The statistical differences among the groups were evaluated using one-way ANOVA with Fisher’s protected least significant difference test. P<0.05 was considered significant. Results were analyzed using SPSS 13.0 software (Spss Inc, Chicago, TL, USA).
Results
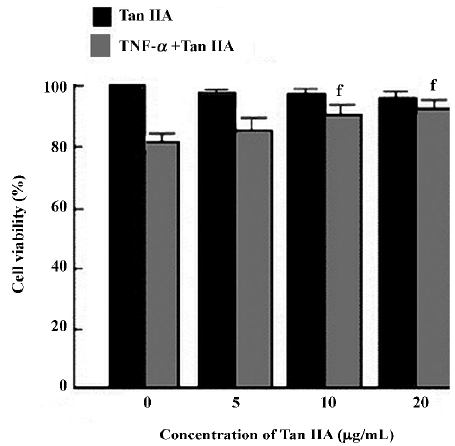
Effects of Tan IIA on cell viability The effect of Tan IIA on cell viability were investigated by MTT; the cells were exposed to Tan IIA for 24 h. As shown in Figure 1, the concentrations (0–20 μg/mL) of Tan IIA used here had no effect on the viability of BMVEC. TNF-α at 5 μg/mL reduced the viability of BMVEC with cell viability at 82%±3%, but Tan IIA significantly reversed the TNF-α-induced reduction of cell viability at concentrations of 10 and 20 μg/mL (90%±3% and 93%±3%, respectively; P<0.05 vs 82%±3%; n=6). Tan IIA (5 μg/mL) increased cell viability, but the difference was not significant (85%±4%; P>0.05 vs 82%±3%; n=6).
Effects of Tan IIA on TNF-α-induced ET-1 production, including large ET-1, in BMVEC As shown in Table 2, TNF-α significantly increased the ET-1 concentration in the media when the BMVEC were treated with Tan IIA. TNF-α-induced ET-1 elevation was suppressed in a dose-dependent manner. Quite a different response pattern became apparent for large ET-1 production. Large ET-1 levels decreased in response to TNF-α. Conversely, large ET-1 levels progressively increased in response to Tan IIA in a dose-dependent manner.

Full table
Effects of Tan IIA on TNF-α-induced ECE-1 activation in BMVEC As shown in Figure 2, the ECE-1 mRNA expression was determined by RT-PCR. The ECE-1 mRNA expression was significantly upregulated by TNF-α (0.97±0.13; P<0.05 vs 0.39±0.10; n=6). TNF-α-induced ECE-1 activation was suppressed significantly by Tan IIA at concentrations of 10 and 20 μg/mL (0.69±0.14 and 0.58±0.06, respectively; P<0.05 vs 0.97±0.13; n=6). Tan IIA (5 μg/mL) downregulated ECE-1 mRNA, but the difference was not significant (0.84±0.09; P>0.05 vs 0.97±0.13, n=6).

Effects of Tan IIA on TNF-α-induced mRNA expression of ET-1 in BMVEC In order to investigate whether the decrease in ET-1 in the media might be related to an decrease in the ET-1 mRNA expression in BMVEC stimulated with TNF-α, RT-PCR analysis was performed. As shown in Figure 3, the expression of ET-1 significantly increased with TNF-α exposure (1.13±0.13; P<0.05 vs 0.50±0.13; n=6). However, no alteration in ET-1 mRNA could be detected after incubation of the cells with Tan IIA at the indicated concentrations (5, 10, and 20 µg/mL) for 24 h prior to TNF-α compared with BMVEC stimulated with TNF-α alone (1.08±0.09, 1.10±0.12, and 1.02±0.10, respectively; P>0.05 vs 1.13±0.13; n=6).

Effects of Tan IIA on TNF-α-induced mRNA expression of ETA or ETB in BMVEC To evaluate the effect of Tan IIA on the mRNA expression of ETA in BMVEC stimulated with TNF-α, we explored the expression of ETA or ETB with RT-PCR. As shown in Figures 4 and 5, TNF-α exposure resulted in a significantly increased mRNA expression of ETA (1.27±0.09; P<0.05 vs 0.50±0.13; n=6). However, there was no appreciative effect on the ETB receptor mRNA expression (0.33±0.05; P>0.05 vs 0.34±0.04; n=6). Compared with TNF-α alone, Tan IIA exposure resulted in a significant decrease in the ETA mRNA expression at concentrations of 10 and 20 μg/mL (1.00±0.12 and 0.75±0.09, respectively; P<0.05 vs 1.27±0.09; n=6). Tan IIA (5 μg/mL) downregulated ETA mRNA, but the difference was not significant (1.13±0.18; P>0.05 vs 1.27±0.09; n=6). Tan IIA (5, 10, and 20 μg/mL) caused a significant increase of ETB mRNA (0.42±0.04, 0.48±0.04, and 0.78±0.06, respectively; P<0.05 vs 0.33±0.05; n=6).


Endothelin receptor binding Receptor binding studies were conducted to characterize the effects of Tan IIA on the interaction of ET-1 with endothelial receptor binding. The cells were incubated for 2 h at 37 °C with 125I-ET-1 and increasing concentrations of unlabeled ET-1 in the presence or absence of TNF-α and Tan IIA at the indicated concentrations. As shown in Figure 6, binding for 125I-ET-1 was measured. Endothelial receptor binding was unaltered in BMVEC stimulated with TNF-α alone or with a combination of TNF-α and Tan IIA.

Discussion
Increased circulating concentrations of TNF-α are seen in several pathological conditions associated with vascular disease. The effect of TNF-α was evident in endothelial cells derived from a variety of sources. The release of ET-1 can be modulated by TNF-α[9–11]. Our findings show that TNF-α exposure led to increased levels of ET-1 and increased ETA receptor mRNA. The increase in ET-1 secretion was accompanied by a corresponding increase in the ET-1 gene resulting in augmented prepro ET-1 mRNA transcription levels. Following TNF-α exposure, the ECE-1 mRNA expression was increased, which may directly result in the elevation of ET-1 levels with a reduction of large ET-1 levels. These responses of BMVEC to TNF-α exposure would be expected to exert multiple biological effects in several neurological and pathological conditions. It has been shown that ET-1 is produced by BMVEC in response to TNF-α to mediate blood-brain barrier (BBB) disruption[22]. Following both stroke and trauma, despite the fact that TNF-α has vasodilatory action achieved through the production of NO[23,24], TNF-α can acutely reduces regional cerebral blood volume, followed by breakdown of the BBB and a reduction in tissue water diffusion mediated via the action of ET on its receptors[25]. TNF-α-stimulated vasoconstriction in cerebral vessels suggests that there are regional differences in vascular sensitivity to ET-1. The interaction between local TNF-α and ET-1 and the resulting regional hemodynamic actions need to be further investigated.
In pathological conditions, such as SAH and traumatic brain injury, stimulation of the cerebral ET system seems to play an important pathophysiological role[1,7,26]. Accordingly, interference with the actions of ET-1 may be a worthwhile therapeutic approach, and receptor antagonists have been proven effective in animal experiments. However, although preliminary clinical data has indicated the safety, there are still limited effects of the ETA or ETB receptor antagonists in treating cerebral vasospasm after SAH in humans. It may provoke a drastic elevation of intracerebral ET-1 concentrations as the result of antagonists competing for ET binding sites[27,28]. Therefore, reducing the synthesis and release of ET-1 from endothelial or parenchymal cells may prove a worthwhile alternative or adjunctive therapeutic option.
The results of our study, show for the first time that Tan IIA may be involved in the processing of large ET-1 to ET-1.This study demonstrates the inhibitory action of Tan IIA in elevating ET-1 levels in cell culture media under stimulated conditions. Theoretically, the decrease in ET-1 caused by Tan IIA may be due to a decrease in production or an increase in cellular binding/uptake or in degradation. However, an increase in ET-1 mRNA was not detectable by RT-PCR under these conditions. Although Tan IIA-induced decreases in ET-1 were not accompanied by a rise in protein synthesis, our data showed a considerable increase in the precursor peptide of large ET-1 as well as a decrease in ET-1. The effects of Tan IIA on ET receptor expression may lead to changes in ET-1 levels and its biological effects. In our study, Tan IIA exposure led to decreased ETA receptor mRNA and increased ETB receptor mRNA. These responses of BMVEC to Tan IIA exposure would be expected to lead to vasodilatation of cerebral vasculature in vivo. Moreover, an upregulation of ETB receptors may lead to an increase in ET-1 binding which will decrease ET-1 levels[29]. To determine whether the decreased ET-1 level in BMVEC was due to enhanced endothelial receptor binding, receptor binding studies were conducted. We found that endothelial receptor binding was unaltered in BMVEC stimulated with TNF-α alone or in the combination of TNF-α and Tan IIA. Taken together, the effect of Tan IIA may be partially attributable to a decrease in ET-1 synthesis. The synthesis of ET-1 starts with the generation of a prepropeptide processed by enzymes of the constitutive secretory pathway to the immediate precursor, large ET-1.
Large ET-1 by itself does not bind to any of the known ET receptors; it is cleaved enzymatically, resulting in the release of the so-called C-terminal fragment and mature ET-1. The latter exclusively mediates the biological effects. Enzymatic processing of large ET-1 occurs at an unusual scission site and is the critical step in ET-1 formation. To date, 2 different specific ECE (ECE-1 and ECE-2) have been identified. In most tissues, the expression of the ECE-1 subtype seems to exceed that of ECE-2[30]. Accordingly, large ET-1 released from endothelial and parenchymal cells seems to be processed predominantly by ECE-1 activity. Therefore, our study lends evidence to the existence of Tan IIA with influence upon ECE-1 activity. The decreased level of ECE-1 assessed semiquantitatively in the present study confirmed our hypothesis; it is possible that Tan IIA inhibits the cleavage of large ET into ET-1, which would explain why Tan IIA decreases ET-1 concentration while large ET concentrations show a parallel increase.
The inhibition of ECE-1 by Tan IIA shown in the present study represents a novel finding. We demonstrate for the first time that Tan IIA decreases TNF-α-induced ET-1 expression in BMVEC through the suppression of ECE-1 synthesis. These results may at least partially explain the cerebral vessel benefits of SM. Although suggestive, further studies need to be carried out to elucidate the extent to which ECE-1 is affected by Tan IIA in vitro and in vivo, and the modulatory mechanism needs to be elucidated.
References
- Andresen J, Shafi NI, Bryan RM. Endothelial influences on cerebrovascular tone. J Appl Physiol 2006;100:318-27.
- Yakubu MA, Shibata M, Leffler CW. Hematoma-induced enhanced cerebral vasoconstrictions to leukotriene C4 and endothelin-1 in piglets: role of prostanoids. Pediatric Re 1995;38:119-23.
- Yakubu MA, Leffler CW. Role of endothelin-1 in cerebral hematoma-induced modification of cerebral vascular reactivity in piglets. Brain Res 1996;734:149-56.
- Yakubu MA, Leffler CW. L-type voltage-dependent Ca2+ channels in cerebral microvascular endothelial cells and ET-1 biosyn-thesis. Am J Physiol Cell Physiol 2002;283:C1687-95.
- Clozel M, Gray GA, Breu V, Loffler BM, Osterwalder R. The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun 1995;186:867-73.
- Naidoo V, Naidoo S, Mahabeer R, Raidoo DM. Cellular distribution of the endothelin system in the human brain. J Chem Neuroanat 2004;27:87-98.
- Juvela S. Plasma endothelin and big endothelin concentrations and serum endothelin-converting enzyme activity following aneurysmal subarachnoid hemorrhage. J Neurosurg 2002;97:1287-93.
- Kwan AL, Lin CL, Chang CZ, Wu SC, Howng SL, Jeng AY. Attenuation of SAH-induced cerebral vasospasm by a selective ECE inhibitor. Neuroreport 2002;13:197-9.
- Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. J Physiol Cell Physiol 1992;262:854-61.
- Zhao RZ, Chen X, Yao Q, Chen C. TNF-alpha induces interleukin-8 and endothelin-1 expression in human endothelial cells with different redox pathways. Biochem Biophys Res Commun 2005;327:985-92.
- Molet S, Furukawa K, Maghazechi A, Hamid Q, Giaid A. Chemokine- and cytokine-induced expression of endothelin-1 and endothelin-converting enzyme-1 in endothelial cells. J Allergy Clin Immunol 2000;105:333-8.
- Ji XY, Tan BKH, Zhu YZ. Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin 2000;21:1089-94.
- Chan K, Chui SH, Wong DY, Ha WY, Chan CL, Wong RN. Protective effects of Danshensu from the aqueous extract of Salvia miltiorrhiza (Danshen) against homocysteine-induced endothelial dysfunction. Life Sci 2004;75:3157-71.
- Xu M, Wang YP, Luo WB, Xuan LJ. Salvianolate inhibits proliferation and endothelin release in cultured rat mesangial cells. Acta Pharmacol Sin 2001;22:629-33.
- Xia ZY, Gu JZ, Ansley DM, Xia F, Yu JF. Antioxidant therapy with Salvia miltiorrhiza decreases plasma endothelin-1 and thromboxane B2 after cardiopulmonary bypass in patients with congenital heart disease. J Thorac Cardiovasc Surg 2003;126:1404-10.
- Jang SI, Jeong SI, Kim KJ, Kim HJ, Yu HH, Park R, et al. Tanshinone IIA from Salvia miltiorrhiza inhibits expression of inducible nitric oxide synthase and production of TNF-α, IL-1β and IL-6 in activated RAW 264.7 cells. Planta Med 2003;69:1057-9.
- Kim HH, Kim JH, Kwak HB, Huang H, Han SH, Ha H, et al. Inhibition of osteoclast differentiation and bone resorption by tanshinone IIA isolated from Salvia miltiorrhiza. Bunge Biochem Pharmacol 2004;67:1647-56.
- Abbott NJ, Hughes CC, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci 1992;103:23-37.
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Coto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelia receptor. Nature 1990;348:732-5.
- Sakurai T, Yanagisawa M, Inoue A, Ryan US, Kimura S, Mitsui Y, et al. cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin-1 mRNA. Biochem Biophys Res Commun 1991;175:44-7.
- Shimada K, Takahashi M, Tanzawa K. Cloning and functional expression of endothelial-converting enzyme from rat BMVEC. J Biol Chem 1994; 269: 18 275–8.
- Didier N, Romero IA, Creminon C, Wijkhuisen A, Grassi J, Mabondzo A. Secretion of interleukin-1β by astrocytes mediates endothelin-1 and tumour necrosis factor-α effects on human brain microvascular endothelial cell permeability. J Neurochem 2003;86:246-54.
- Macnaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun 1993;196:1330-4.
- Hollenberg SM, Cunnion RE, Parrillo JE. The effect of tumor necrosis factor on vascular smooth muscle. In vitro studies using rat aortic rings. Chest 1991;100:1133-7.
- Sibson NR, Blamire AM, Perry VH, Gauldie J, Styles P, Anthony DC. TNF-alpha reduces cerebral blood volume and disrupts tissue homeostasis via an endothelin- and NFR2-dependent pathway. Brain 2002;125:2446-59.
- Armstead WM. Endothelin-induced cyclooxygenase-dependent superoxide generation contributes to K+ channel functional impairment after brain injury. J Neurotrauma 2001;18:1039-48.
- Plumpton C, Ferro CJ, Haynes WG, Webb DJ, Davenport AP. The increase in human plasma immunoreactive endothelin but not big endothelin-1 or its C-terminal fragment induced by systemic administration of the endothelin antagonist TAK-044. Br J Pharmacol 1996;119:311-4.
- Chuquet J, Benchenane K, Toutain J, MacKenzie ET, Roussel S, Touzani O. Selective blockade of endothelin-B receptors exacerbates ischemic brain damage in the rat. Stroke 2002;33:3019-24.
- Pollock DM, Allcock GH, Krishnan A, Dayton BD, Pollock JS. Upregulation of endothelin B receptors in kidneys of DOCA-salt hypertensive rats. Am J Physiol Renal Physiol 2000;278:279-86.
- Parnot C, Le Moullec JM, Cousin MA, Guedin D, Corvol P, Pinet F. A live-cell assay for studying extracellular and intracellular endothelin-converting enzyme activity. Hypertension 1997;31:837-44.