Small interfering RNA of cyclooxygenase-2 induces growth inhibition and apoptosis independently of Bcl-2 in human myeloma RPMI8226 cells
Introduction
Multiple myeloma (MM) is characterized by a clonal proliferation of neoplastic plasma cells in the bone marrow and is the second most common hematological malignancy[1,2]. At present, a cure for multiple myeloma has not been achieved with chemotherapeutic regimen, and many patients die of drug-resistant diseases. High-dose chemotherapy with stem cell support has achieved higher response rates than conventional therapy, but few patients remain in long-term remission[3]. Thus, the development of new effective anticancer targets to treat both early and advanced MM seems to be an attractive perspective.
Prostaglandins are important mediators implicated in inflammation and angiogenesis, and support the growth of several solid tumors[4,5]. Cyclooxygenase 2 (COX-2) is the key enzyme of prostaglandin synthesis in inflamed tissues[6]. In most normal tissues, its expression is at a low level, but is induced by cytokines and growth factors[7]. Overexpression of COX-2 is related to tumorigenesis by mechanisms of chronic inflammation, immunosuppression, apoptosis resistance, and angiogenesis[8]. Overexpression of COX-2 has been reported in various cancers such as breast, stomach, colon, and lung[5,9].
MM is known to involve a deregulated cytokine network with the secretion of inflammatory mediators[10], and it has been recently reported that COX-2 is frequently expressed in MM[11,12]. In the present study, we investigated the function of COX-2 by siRNA-mediated gene silencing in human myeloma RPMI8226 cells. Silencing of COX-2 leads to an inhibited cell proliferation and induced apoptosis, which is independent of the Bcl-2 family.
Materials and methods
siRNA for COX-2 RNA interference mediated by duplexes of 21-nucleotide RNA was performed in RPMI8226 cells. The siRNA sequence was used to target exon 5 of the COX-2 gene (GenBank accession N
Cell culture and transfection of siRNA The RPMI8226 human myeloma cell line and one colorectal cell line (HT-29) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI1640 containing 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 units/mL penicillin, and 100 µg/mL streptomycin at 37 oC with 5% CO2. The cells were passaged 2–3d before nucleofec-tion, and the cells for nucleofection were in their logarithmic growth phase.
The transfection of siRNA used the Amaxa cell optimization kit V (Amaxa, Koeln, Germany) and followed the Amaxa guidelines. Briefly, the cells were resuspended in the nucleofector V solution. 100 µL of cell suspension at a density of 2×106–5×106/mL mixed with 2 µg of pmax green fluorescent protein (GFP) vector plus 1 µL of 100 µmol/L siRNA were transferred to a cuvette and nucleofected with an Amaxa nucleofector apparatus. The cells (RPMI8226siRNA) were transfected using the G-16 pulsing parameter and were immediately transferred into wells containing 37 oC pre-warmed culture medium in 12-well plates. Untreated cells (RPMI8226Untreated) and cells (RPMI8226Blank) in nucleofector solution, without siRNA and with the application of the electroporation program, were both used as negative controls. Twenty four hours after the electroporation, the cells were monitored with an Olympus BH-2 fluorescence microscope (Tokyo, Japan).
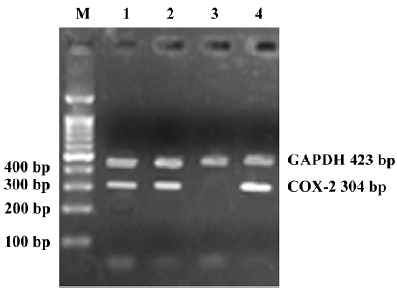
RT-PCR analysis Total RNA was isolated from the RPMI8226 cells using TRIzol Reagent (Gibco-BRL, Gaithersburg, MD, USA). 4 µg RNA was reverse transcribed to cDNA by the Thermoscript RT-PCR System reagent (Gibco-BRL, USA). Primers for PCR reaction were designed specially to human COX-2 gene. The sequences was 5'-CCGA-GGTGTATGTATGAGTG-3' for the sense and 3'-GGAAGA-GATTGTAGAGAGGA-5' for the antisense. Primers for control were designed according to the GAPDH gene. The sequences were as follows: 5'-GTCATCATCTCTGCCCCCTC-TGCT-3' for the sense and 3'-GACGCCTGCTTCACCACCT-TCTTG-5' for the antisense primer. PCR amplification consisted of 35 cycles: 15 s at 94 oC for denaturing, 30 s at 58 oC for annealing, and 45 s at 72 oC for elongation.
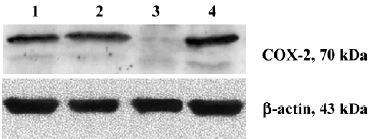
Western blot analysis Treated cells (1×106) were washed twice with phosphate-buffered saline (PBS) and lysed in ice-cold cell lysis buffer [50 mmol/L Tris (pH 7.2), 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L sodium orthovanadate, and 1 µg/mL leupeptin] containing 1 mmol/L phenylmethylsulfonyl fluoride. Equivalent amounts of proteins (20 µg) were boiled in Laemmli sample buffers and fractionated on 10% SDS-PAGE. The separated proteins in the gel were transferred onto polyvinylidine difluoride (PVDF) membranes using an electroblot apparatus (Bio-Rad Inc, Hercules, CA, USA). The filters were blocked for 2 h in PBS containing 0.1% Tween 20 and 5% low fat milk, and the filters were then incubated with specific primary Abs (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at dilution of 1:200 in TBS/T, 5% low fat milk at 4 oC for 12 h. The membranes were then washed with PBS-T and incubated with horseradish peroxidase (HRP) conjugated anti-goat Abs or anti-rabbit Abs (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at dilution of 1:1000 in TBS/T, 5% low fat milk for in room temperature for 1 h. Specific signals were detected on X-ray films using an enhanced chemiluminescence detection system (SuperSignal, Pierce, Rockford, IL, USA). The blots were then stripped and reblotted with the antibody against β-actin to ensure that equivalent levels of proteins were present in each lane.
3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT) assay RPMI8226siRNA, RPMI8226Untreated, or RPMI8226Blank cells were seeded onto 96-well plates at a density of 5×104/mL after being treated or untreated (200 µL/well) and cultured for 12, 24, 48, and 72 h, respectively. Sub-sequently, 20 µL MTT (Janssen Chimica Co, New Brunswick, NJ, USA) at a concentration of 5 g/L was added to each well, and the cells were incubated for an additional 4 h at 37 oC. After a brief centrifugation, supernatants were carefully removed and 200 µL DMSO was added to the cells. After the insoluble crystals were completely dissolved, absorbance at 490 nm was measured using an EL×800 reader (Bio-Tek Instruments, Winooski, VT, USA).
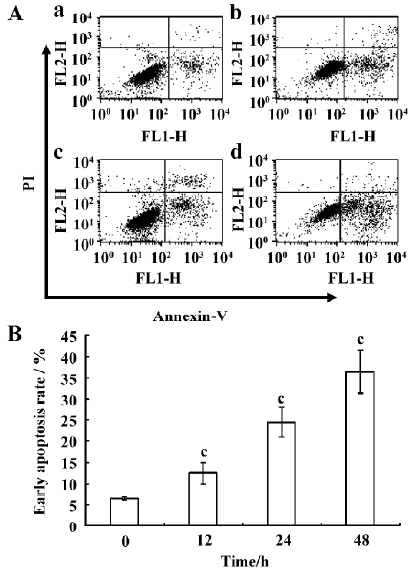
Annexin-V staining Annexin-V staining was performed based on the Annexin-V- fluorescein isothiocyanate (FITC) apoptosis assay kit (Bender MedSystems, Vienna, Austria). In this assay, Annexin-V-FITC (1:250) and propidium iodide (PI, 1 µg/mL) were used. FITC can specifically bind to the phosphatidyl serine (PS) residues on the cell membrane, while PI can bind to DNA once the cell membrane has become permeable. The cells were stained and analyzed by FACScan (Becton Dickinson, Franklin Lakes, NJ, USA). The data were analyzed using the CellQuest (Becton Dickinson, USA) software. For each analysis, 10 000 events were recorded.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining Silencing of COX-2 induced apoptosis was also confirmed by TUNEL by TACS (Total Access Cellular System) TdT Kit (R&D Systems, Inc, Minneapolis, MN, USA). Briefly, the slides were rinsed with Ca2+, Mg2+, and DNase-free PBS (10 mmol/L PBS, pH7.4) and permeabilized with proteinase K at room temperature to make the DNA accessible to the labeling enzyme. For the positive control, the cells were incubated with TACS nuclease for 30 min, which generated DNA strand breaks in virtually every cell. Endogenous peroxidase activity was quenched using 5% H2O2 (in methanol, v/v) for 5 min, and the cells were incubated with TdT labeling buffer for 5 min before starting the labeling reaction. Then the cells were incubated with the TdT enzyme and biotinylated nucleotides (for negative control, labeling buffer was used instead of TdT enzyme) for 1 h at 37 oC in a humidified chamber. The reaction was stopped by adding TdT stop buffer for 5 min. The slides were incubated with streptavidin-conjugated horseradish peroxidase for 10 min. A brown color was developed with incubation in the diaminobenzidine (DAB) solution (Sigma, St Louis, MO, USA) for 7 min at room temperature. The slides were counterstained in 1% methyl green for 1.5 min and visualized and scored under a light microscope. The apoptosis was evaluated by calculating the rate of positive cells (brown-stained cells) at 10 arbitrarily-selected fields at a magnification of 400 in a double-blinded manner.
Statistical analysis Statistical analysis was performed using the F and q tests by SPSS10.0 (SPSS Inc, Chicago, IL, USA), and statistical significance was defined as P<0.05. Data were expressed as mean± SD for at least 3 experiments.
Results
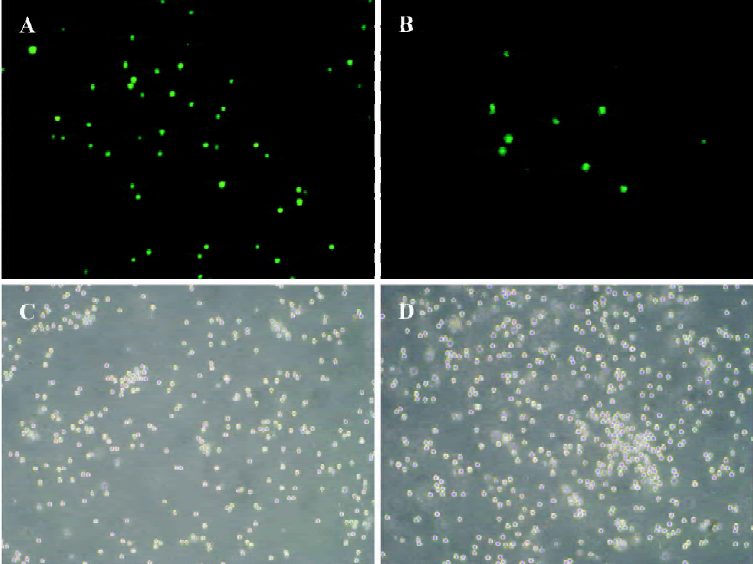
Silencing COX-2 gene with siRNA duplexes To estimate transfection efficacy, 2 µg pmaxGFP vector was co-transfected into RPMI8226 cells with a siRNA duplex designed against maxGFP. More than 300 cells were detected for fluorescence (Figure 1). The same system was used as for the silencing of the COX-2 gene. RT-PCR (Figure 2) and Western blot (Figure 3) analysis indicated that the transcription and expression of the COX-2 gene was extremely attenuated in the cells transfected with the specific COX-2 siRNA. Thus, we demonstrated that COX-2 specific siRNA can efficiently suppress COX-2 expression.
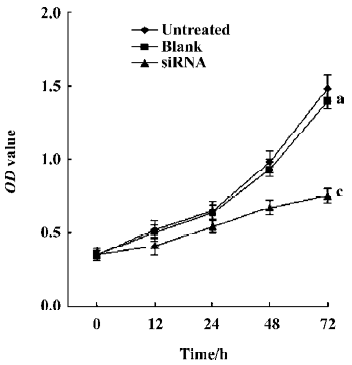
siRNA mediated silencing of COX-2 inhibits proliferation of RPMI8226 cells As shown in Figure 4, silencing of COX-2 led to the inhibition of cell proliferation in RPMI8226 cells. The value of OD (optical density) doubling time of RPMI8226siRNA was significantly longer than that of RPMI8226Untreated (P<0.001) and RPMI8226Blank (P<0.001).
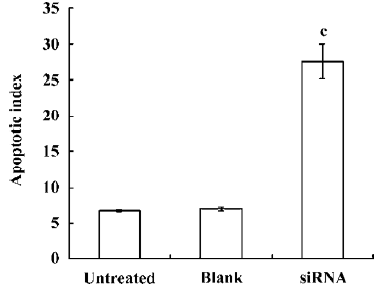
Induced apoptosis of RPMI8226 cells by the silencing of COX-2 gene Twenty four hours after transfection, RPMI8226siRNA, RPMI8226Untreated, and RPMI8226Blank cells were stained with Annexin-V to investigate whether apoptosis would occur. The silencing of COX-2 via siRNA led to significant apoptosis changes in the RPMI8226 cells (Figures 5, 6). Meanwhile, TUNEL assay was used to confirm whether apoptosis was elevated and draw the same result. This demonstrated that the silencing of COX-2 via siRNA brought obvious apoptosis to the RPMI8226siRNA cells.
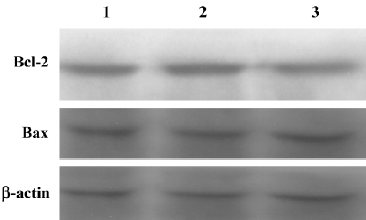
Silencing the COX-2 gene has no effect on the expression of Bcl-2 family proteins in RPMI8226 cells We examined the expression of Bcl-2 family proteins, Bcl-2 and Bax, which play vital roles in programmed death in various types of cells. As shown in Figure 7, the result of the Western blot analysis showed that no significant differences could be found in the expression of both the Bcl-2 and Bax proteins in RPMI8226siRNA cells compared with the RPMI8226Untreated or RPMI8226Blank cells, which demonstrated that silencing the COX-2 gene inhibited proliferation and induced apoptosis in RPMI8226 cells via a Bcl-2 independent way.
Discussion
The nucleofector technique is a new non-viral transfection method especially designed for hard-to-transfect cell lines[13]. COX-2 gene silencing has been reported in various tumor cells[14–16], which suggests that siRNA is an effective technique for tumor study and treatment. However, there have been no reports of silencing COX-2 in myeloma cells until recently. Here, we successfully silenced the COX-2 gene in human RPMI8226 myeloma cells by RNA interfering. For this study, 3 different pairs of siRNA fragments were provided and the most effective one was chosen for this study. Co-transfection of pmaxGFP, which encodes the GFP from copepod Pontellina p. with an siRNA directed against maxGFP into RPMI8226 cells helps us to understand the transfection efficiency. Successful gene silencing is monitored as a decrease of green fluorescence compared to the control sample using fluorescence microscopy.
COX and lipoxygenase (LOX) are 2 important enzyme classes that metabolize polyunsaturated fatty acids and affect carcinogenesis[17,18]. We have previously shown that 12-LOX, an important arachidonic acid-metabolizing enzyme, expressed in RPMI8226 cells and its specific inhibitor, baicalein, plays an important role in the growth and apoptosis of RPMI8226 cells[19]. COX-2 is essential for the survival and proliferation of malignant cells[5]. Recently, COX-2 has been reported to be frequently expressed in MM and is an independent predictor of poor outcome[11]. However, the function of COX-2 in the development of human MM remains less clear even though its inhibitor celecoxib has been involved in clinical trials combined with thalidomide for refractory and relapsed myeloma therapy[20].
In this study, we demonstrate that successfully silencing the COX-2 gene using siRNA duplexes can lead to the inhibition of COX-2 expression in transfected RPMI8226 cells. Furthermore, significant growth inhibition and apoptosis induction have been detected in these transfected cells (Figures 4–6). Interleukin-6, a pleiotropic cytokine known to play a critical role in the survival and growth of multiple myeloma cells[6], participates in a cytokine network and can be induced by prostaglandin E2 (PGE2), a major product of COX-2[21]. Therefore, COX-2 seems to play an important role in MM via the cytokine network[22]. However, the direct effects of COX-2 on MM cells have not been elucidated [23].
There are 2 main apoptosis signaling pathways: death receptor-dependent apoptosis and mitochondria-dependent apoptosis[24]. As anti-apoptosis proteins, Bcl-2 and Bax have been demonstrated to prevent the release of cytochrome C into the cytosol and inhibit the subsequent activation of caspase 9[25]. The mitochondrial-mediated pathway of apoptosis is regulated by the Bcl-2 family of anti-apoptotic (Bcl-2, Bcl-xL, and Mcl-1) and pro-apoptotic proteins (Bax, Bad, and Bak), and Bcl-2 inhibits apoptosis by interacting and forming inactivating heterodimers with Bax/Bak[26]. PGE2 had been found to inhibit apoptosis via a Bcl-2-dependent pathway in a human colonic cancer cell line[27]. It was found that some antimultiple myeloma agents, such as dexamethasone, thalidomide, and proteasome inhibitors (PS-341), induce apoptosis in myeloma cell lines or patient cells associated with the downregulation of Bcl-2 or/and upregulation of Bax[3]; however, in this study, we found that although COX-2 was knocked down in RPMI8226 cells and obvious apoptosis was observed, the expression of Bcl-2 and Bax did not decrease. This demonstrates that apoptosis in RPMI8226 cells, induced by the silencing of COX-2, is independent of modulation of Bcl-2 and Bax, which is consistent with the results of Zhang’s report[23].
In summary, our study suggests that COX-2 takes considerable part in the regulation of cellular proliferation and apoptosis of human myeloma cells, which is independent of the Bcl-2 pathway. COX-2 could be an important target for MM treatment, and small interfering RNA of COX-2 could serve as an effective tool.
References
- Hellek M, Bergsagel PL, Anderson KC. Multiple myeloma: increasing evidence for a multistep transformation process. Blood 1998;91:3-21.
- Hideshima T, Chauhan D, Ishitsuka K, Yasui H, Raje N, Kumar S, et al. Molecular characterization of PS-341 (bortezomib) resistance: implications for overcoming resistance using lysophos-phatidic acid acyltransferase (LPAAT)-β inhibitors. Oncogene 2005;24:3121-9.
- Mitsiades N, Mitsiades C, Poulaki V, Chauhan D, Richardson P, Hideshima T, et al. Biologic sequelae of nuclear factor-κB blockade in multiple myeloma: therapeutic applications. Blood 2002;99:4079-86.
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004;431:461-6.
- Zhang DY, Wu J, Ye F, Xue L, Jiang S, Yi J, et al. Inhibition of cancer cell proliferation and prostaglandin E2 synthesis by Scutel-laria baicalensis. Cancer Res 2003;63:4037-43.
- Anderson GD, Hauser SD, McGarity KL, Bremer ME, Isakson PC, Gregory SA. Selective inhibition of cyclooxygenase (COX)-2 reverses inflammation and expression of COX-2 and interleukin 6 in rat adjuvant arthritis. J Clin Invest 1996;97:2672-9.
- Morita I. Distinct function of COX-1 and COX-2. Prostaglandins Other Lipid Mediat 2002;68–69:165-75.
- Pai R, Soreghan B, Szabo IL, Pavelka M, Baatar D, Tarnawski AS. Prostaglandin E2 transactivates EGF receptor: a novel mechanism for promoting colon cancer growth and gastrointestinal hypertrophy. Nat Med 2002;8:289-93.
- Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res 1999;59:991-4.
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, et al. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature 1988;332:83-5.
- Ladetto M, Vallet S, Trojan A, Maria D, Monitillo L, Rosato R, et al. Cyclooxygenase-2 (COX-2) is frequently expressed in multiple myeloma and is an independent predictor of poor outcome. Blood 2005;105:4784-91.
- Trojan A, Tinguely M, Vallet S, Seifert B, Jenni B, Zippelius A, et al. Clinical significance of cyclooxygenase-2 (COX-2) in multiple myeloma. Swiss Med Wkly 2006;136:400-3.
- Aluigi M, Fogli M, Curti A, Isidori A, Gruppioni E, Chiodoni C, et al. Nucleofection is an efficient nonviral transfection technique for human bone marrow-derived mesenchymal stem cells. Stem Cells 2006;24:454-61.
- Zhi HY, Wang L, Zhang J, Zhou C, Ding F, Luo A, et al. Significance of COX-2 expression in human esophageal squamous cell carcinoma. Carcinogenesis 2006;27:1214-21.
- Yan Y, Li JX, Ouyang WM, Ma Q, Hu Y, Zhang DY, et al. NFAT3 is specifically required for TNF-α-induced cyclooxy-genase-2 (COX-2) expression and transformation of Cl41 cells. J Cell Sci 2006;119:2985-94.
- Shin YK, Park JS, Kim HS, Jun HJ, Kim GE, Suh CO, et al. Radiosensitivity enhancement by celecoxib, a cyclooxygenase (COX)-2 selective inhibitor, via COX-2-dependent cell cycle regulation on human cancer cells expressing differential COX-2 levels. Cancer Res 2005;65:9501-9.
- Tsujii M, DuBois R. Alterations in cellular adhesion and apptosis in epithelial cells overexpressing prostaglandin endoperoxide syntherase 2. Cell 1995;83:493-501.
- Wu J, Xia XH, Tu PS, Fan DM, Lin CM, Kung HF, et al. 15-Lipoxygenase-1 mediates cyclooxygenase-2 inhibitor-induced apoptosis in gastric cancer. Carcinogenesis 2003;24:243-47.
- Li QB, You Y, Chen ZC, Lv J, Shao J, You Y, et al. Role of Baica-lein in the regulation of proliferation and apoptosis in human myeloma RPMI8226 cells. Chin Med J 2006;119:948-52.
- Prince HM, Mileshkin L, Roberts A, Ganju V, Underhill C, Catalano J, et al. A Multicenter Phase II Trial of Thalidomide and Celecoxib for Patients with Relapsed and Refractory Multiple Myeloma. Clin Cancer Res 2005;11:5504-14.
- Lacroix S, Rivest S. Effect of acute systemic inflammatory response and cytokines on the transcription of the genes encoding cyclooxygenase enzyme (COX-1 and COX-2) in the rat brain. J Neurochem 1998;70:452-66.
- Costes V, Portier M, Lu ZY, Rossi JF, Bataille R, Klein B. Interleukin-1 in multiple myeloma: producer cells and their role in the control of IL-6 production. Br J Haematol 1998;103:1152-60.
- Zhang M, Abe Y, Matsushima T, Nishimura J, Nawata H, Muta K. Selective cyclooxygenase-2 inhibitor NS-398 induces apoptosis in myeloma cells via a Bcl-2 independent pathway. Leuk Lymphoma 2005;46:425-33.
- Kawane K, Fukuyama H, Yoshida H, Nagase H, Ohsawa Y, Uchiyama Y, et al. Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat Immunol 2003;4:138-44.
- Panaretakis T, Pokrovskaja K, Shoshan MC, Grander D. Interferon-alpha-induced apoptosis in U266 cells is associated with activation of the proapoptotic Bcl-2 family members Bak and Bax. Oncogene 2003;22:4543-56.
- Oltvai ZN, Milliman CL, Korsmeyer K. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993;74:609-19.
- Sheng H, Shao J, Morrow JD, Beauchamp RD, Dubois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res 1998;58:362-6.
