Changes of multiple biotransformation phase I and phase II enzyme activities in human fetal adrenals during fetal development1
Introduction
During pregnancy, the fetus may be exposed to maternally-administered drugs and other xenobiotics. Significant xenobiotic metabolism is now recognized as occurring in the mammalian fetus, mostly by a range of biotransformation phase I and phase II enzymes. The fetal liver appears to be the most important organ for fetal drug metabolism[1,2]. It is well known that cytochrome (CYP)P450 is a multigene superfamily that plays a critical role in the bio-oxidation of xenobiotics, such as drugs, pesticides, and carcinogens, as well as endogenous agents, including fatty acids and steroid hormones[3]. Of the 17 CYP families in humans, the first 3 families are largely involved in xenobiotic metabolism[4]. The majority of CYP are found in the liver and some are found in extrahepatic tissues, such as kidneys, lungs, small intestines, brain, skin, and placenta. These extrahepatic CYP are likely to participate in tissue-specific biotransformations[5]. Although a great deal of information has been acquired relating to the identification and the characteristics of different CYP isoforms in adult organisms[6], the role of these enzymes in the developing embryo and fetus has received relatively little attention. Phase II drug-metabolizing enzymes are characterized by their abilities to synthesize metabolites that are generally more water soluble than the parent drug. In the adult, this facilitates biliary and renal elimination. In the fetus, however, the production of water-soluble metabolites may paradoxically increase fetal exposure to the metabolite, as the water-soluble compounds are less able to cross the placenta for disposal by the mother. Substances excreted in fetal bile and urine can accumulate in the amniotic fluid and be swallowed and recirculated in the fetus[1,7]. Given the important role of conjugation enzymes in drug and toxicant disposition, the investigation of phase II enzyme (glutathione S-transferase [GST] and uridine diphosphoglucuronyl transferase [UGT]) activities is necessary to understand the risk of fetal xenobiotic exposure.
The adrenal is an important endocrine organ that synthesizes steroid hormones. Adrenal toxicity may have a greater impact on growth, development, and sexual maturation in fetuses than in adults[8]. As an important endocrine organ in fetal development, the adrenal exhibits the most striking differences in structure and function between fetal life and adulthood. Many reports have focused on the morphological and histological changes in the adrenal during the fetal maturation process[9]. However, to the best of our knowledge, about the evolution process of its enzymology, function, and regulation are still unclear. It was found that steroidogenesis of fetal adrenals could be affected by several xenobiotics, such as estrogen[10] and nicotine[11], which are the substrates and inducers of phase I enzymes, but through what mechanism do these xenobiotics affect steroidogenesis? Our recent study investigated the correlations among xenobiotics, xenobiotic-metabolizing enzymes, and steroidogenesis in human fetal adrenal cortical cells during fetal development with respect to possible toxicological significance. The observations showed that 3-methylcholanthrene, phenobarbital, or dexamethasone could interfere with the synthesis of cortisol, aldosterone, and progesterone. This intervention is likely to take effect through the activation of xenobiotic metabolizing-related CYP isozymes[12]. Moreover, our research further demonstrated that prenatal exposure during mid and last gestation to nicotine and caffeine induced typical intrauterine growth retardation (IUGR) in rodents accompanied with a depressed fetal adrenal steroidogenesis function[13]. Xenobiotic metabolizing-related enzymes may exist in fetal adrenals and participate in the toxic mechanism of fetal development. In this study, we examined the changes of activities of several xenobiotic metabolizing-related phase I and phase II enzymes: CYP1A1, CYP2A6, CYP2E1, CYP3A7, GST, and UGT in human fetal adrenals, and compared them with those in fetal livers.
Materials and methods
Chemicals Coumarin, 7-hydroxycoumarin, testosterone, aniline, p-aminophenol, 3-methylcholanthrene (3MC), 7-ethoxyresorufin, resorufin, 6β-hydroxytestosterone (6β-OHT), isocitric acid, isocitric acid dehydrogenase, collagenase I, DMSO, 1-chloro-2,4-dinitrobenzene (CDNB), cumene hydroperoxide (CH), ethacrynic acid (EA), bromosulfophthalein (BSP), reduced glutathione (GSH), glutathione reductase, uridine diphosphate glucuronic acid (UDPGA), p-hydroxy-biphenyl (HBP), 7-hydroxy-4-methylcoumarin (HMC), and a reduced form of NADPH were obtained from Sigma (St Louis, MO, USA). Fetal calf serum and modified McCoy’s 5A medium were purchased from Gibco (Carlsbad, CA, USA). The One-step PCR kit was purchased from TaKaRa Biotechnology (Dalian, China). Oligonucleotide primers were customly synthesized by Sangon Biological Engineering Technology (Shanghai, China). All other chemicals and reagents were of analytical grade.
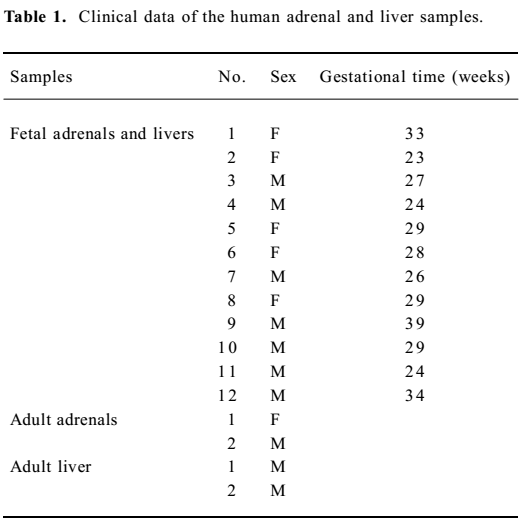
Biological samples and treatment Human fetal tissues, ranging from 23 to 39 weeks of gestation, were obtained from legal abortions and did not exhibit any morphological evidence of defects. The fetal age was obtained from clinical information and confirmed by fetal foot-length measurement. The fetuses were collected within 2 h of abortion, and both the adrenal and liver were obtained from the same fetus. The samples of 2 adult livers and 2 adult adrenals, which acted as positive controls, were taken by biopsies during laparotomy in patients with liver or adrenal disease. Tissues were immediately frozen at -80 °C and used within 3 months. The study was approved by the Ethical and Research Committee of Basic Medical School of Wuhan University (Wuhan, China), and written informed consent was obtained from each adult, pregnant woman. Clinical data of the specimens are shown in Table 1.
Full table
Human fetal specimens were obtained from therapeutic abortion and legal abortion at 23–39 weeks of gestation and did not exhibit any morphological evidence of defects. Both the adrenal and liver are acquired from the same fetus. The samples of 2 adult livers and 2 adult adrenals, which acted as positive controls, were taken by biopsies during laparotomy in patients with liver or adrenal disease.
Subcellular fraction isolation The isolation procedure of the subcellular fractions is described. In brief, the piece of adrenal or liver tissue was homogenized (1:4, w/v) in ice cold Tris-HCl buffer (50 mmol/L, pH 7.4) containing 0.1 mmol/L EDTA. Then the homogenate was spun at 9000×g for 20 min at 4 °C to remove nuclei and mitochondrial pellets. The supernatant (S9) was spun at 105 000×g for 60 min at 4 °C to obtain the pellet (microsome) and supernatant (cytosol). The microsomal pellet was suspended in sucrose (0.25 mol/L) corresponding to 1 g of tissue per mL and stored at –80 °C for further assays. The protein concentrations were determined by the Lowry method[14] using bovine serum albumin (BSA) as standard.
Enzyme assays Due to the lower enzyme activities that existed in the fetal tissues, the enzymatic activities with different incubational time, microsomal protein contents, and substrate concentrations were measured in advance, so as to establish an optimal reaction system.
7-Ethoxyresorufin O-demethylation (EROD) and coumarin 7-hydroxylation (COH) were assayed using the modified fluorescent method[15,16]. In brief, the reaction mixture of EROD included microsomal protein 1–2 mg, Tris·HCl 50 mmol/L (pH 7.8), MgCl2 2.5 mmol/L, KCl 50 mmol/L, BSA 12 g/L, 7-ethoxyresorufin 2 µmol/L, and a NADPH-generating system, including NADP+ 0.4 mmol/L, isocitric acid 10 mmol/L, and isocitric acid dehydrogenase 0.6 units. The reaction was initiated with the NADPH-generating system and stopped by the addition of ice-cold methanol (2.5 mL) at 30 min. The COH reaction mixture included sodium phosphate buffer (pH 7.4) 50 mmol/L, KCl 50 mmol/L, MgCl2 2.5 mmol/L, BSA 1 g/L, coumarin 5 mmol/L, microsomal protein 4 mg, and the NADPH-generating system. The reaction was initiated by the addition of the NADPH-generating system and stopped by 20% (v/v) trichloroacetic acid (0.125 mL) at 120 min.
Aniline hydroxylation was studied by measuring the formation of p-aminophenol[17]. The hydroxylation was carried out under similar conditions with 8 mmol/L aniline. After 120 min incubation at 37 °C, the reaction was terminated by 0.2 mL of 50% (v/v) trichloroacetic acid.
Testosterone 6β-hydroxylation was assayed using the HPLC method[18]. The incubation mixture was incubated for 60 min at 37 °C in a 200 µL volume containing potassium phosphate buffer (pH 7.4) 80 mmol/L, MgCl2 5 mmol/L, testosterone 250 µmol/L, and microsomal protein 0.3 mg. The HPLC system was composed of a pump (LC-10AT, SHIMADZU, Kyoto, Japan) and a UV detector (SPD-10A, SHIMADZU, Kyoto, Japan). The HPLC column (length×diameter: 250×4.6 mm; 5 µm particle size; Dalian, China) was used with a C18 Guard-pack precolumn (10 μm particle size, 20×4.6 mm; SHIMADZU, Kyoto, Japan). A flow rate of 1.0 mL/min was used and the UV detector was set at 245 nm. All chromatography was performed at room temperature. The overall run time for the chromatographic separation was 30 min. The retention time of 6β-OHT was 7.41±0.10 min.
The UGT activities were measured using the fluorescence method with HBP and HMC as substrates[19]. The reaction mixture (0.5 mL) included Tris-HCl buffer (pH 7.4) 0.5 mol/L, MgCl2 5 mmol/L, 0.01% (v/v) Triton X-100, HBP (or HMC) 0.5 mmol/L, microsomal protein 2 mg, and UDPGA 0.6 mmol/L. The reaction was initiated by the addition of UDPGA and stopped by 10% (v/v) perchloric acid (0.5 mL) and chloroform (2 mL) at 60 min.
GSH conjugations with CDNB, CH, EA and BSP as the substrates were measured for determining the activities of total GST, αGST, πGST, and μGST using the spectrophotometric method[19–21].
Preparation of tissue total RNA and RT–PCR Total RNA was isolated from the frozen tissues according to the instructions of the Trizol reagent. The concentration and purity of RNA were determined using a spectrophotometer (UV-1601, Shimadzu, Japan). Total RNA was stored in diethyl pyrocarbonate (DEPC)–H2O at –80 °C until used.
RT–PCR was performed in an attempt to detect expressed CYP1A1 mRNA corresponding to negative enzyme assay results. There was 1 negative control without the template RNA and 2 positive controls performed with adult samples in every series. The primer was designed according to Hakkola et al[22]. The PCR cycling conditions were as follows: 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 50 s for 35 cycles. An aliquot (4 µL) of the RT–PCR production was separated on a 1.5% (w/v) agarose gel and visualized by ethidium bromide with a UV light.
Data analysis Data are presented as mean±standard deviation. We paired the enzyme activities of the adrenal and liver from the same fetus. Statistical Packages for Social Sciences was used for the data analysis (SPSS, Chicago, IL, USA). The difference between the adrenals and livers was analyzed using the unpaired t-test. The correlation was examined by linear regression analysis. The level of significance was set at P<0.05.
Results
Phase I enzymes
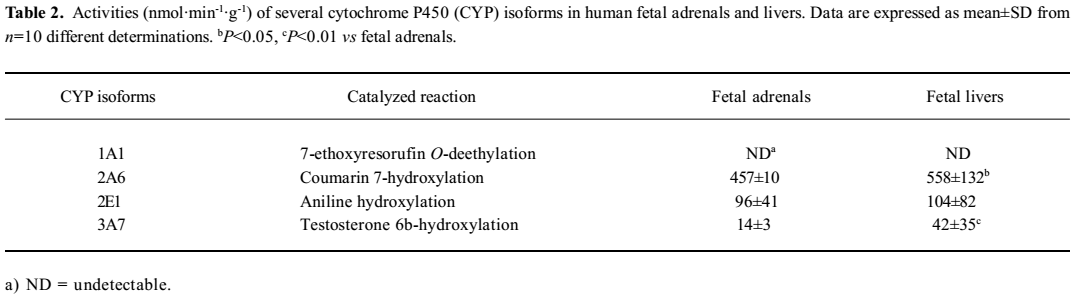
CYP1A1 enzyme activity and mRNA expression EROD is well known as a marker for CYP1A1 enzyme activity[15]. No detectable EROD activity was measured in either adrenal microsomes (Table 2) or the 3MC-treated adrenal cortical cells in vitro[12] in our work.
Full table
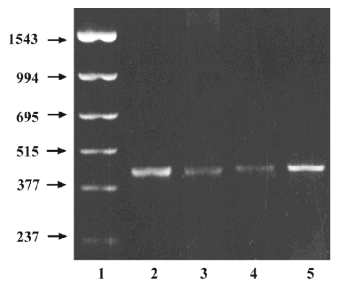
Due to the negative result on CYP1A1 activity, the expression of CYP1A1 mRNA in the adrenals and livers were also investigated using the RT–PCR method and taking 2 adult livers and 2 adult adrenals as positive controls. The representative amplification results by the CYP1A1 primer are shown in Figure 1. The positive expression occurred in 73% of fetal adrenals and 78% of fetal livers. No remarkable gestational time and fetal sex-related differences were observed.
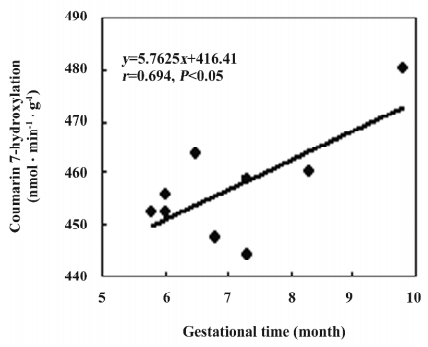
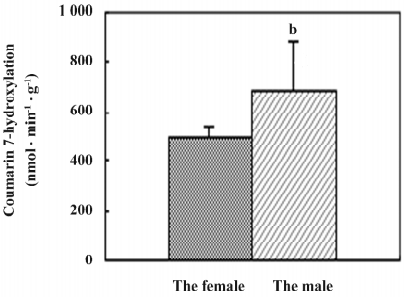
CYP2A6 activity Coumarin was chosen as a selective substrate for the determination of human CYP2A6 activity[16]. The results showed that CYP2A6 enzyme activity occurred in all of the fetal adrenal and liver microsomes, and the average level in adrenals was 457±10 nmol·min–1·g–1, which was 82% of that in the fetal livers (P<0.05; Table 2). The activity in the adrenal showed a gestational time-dependent increase (r=0.694, n=10, P<0.05; Figure 2). Meanwhile, the activities in the fetal liver showed a sex-related difference, and the level of the males (684±198 nmol·min–1·g–1) was higher than that of the females (503±40, P<0.05; Figure 3).
CYP2E1 activity Aniline hydroxylase is a CYP2E1 enzyme marker[17]. Our study found that the activity in the fetal adrenals was 96±41 nmol·min-1·g-1, which was 92% of that in fetal livers (Table 2). There were no significant fetal sex and gestational time-dependent differences of the activity between the adrenals and livers.
CYP3A7 activity Testosterone is always chosen as a selective substrate of CYP3A7 in human fetal experiments[23]. Our results showed that the level of testosterone 6β-hydroxylation in fetal adrenals was 14±3 nmol·min-1·g-1, which was 33% of that in fetal livers (P<0.01; Table 2). No remarkable gestational time and fetal sex-related differences were observed.
Phase II enzymes
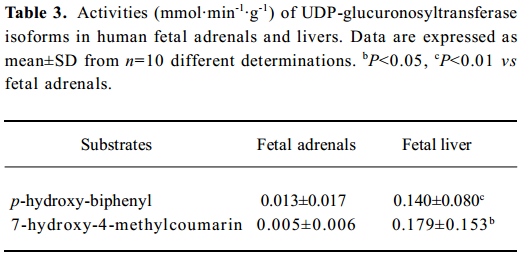
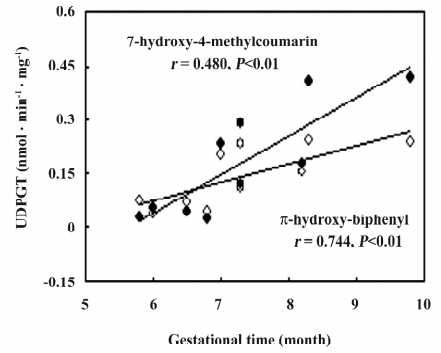
UGT activities UGT activities using HBP and HMC as substrates could be detected in all fetal adrenals and were 9% and 3%, respectively, of those in the fetal livers (Table 3). No gestational time- and sex-related differences were observed in the fetal adrenals. In the fetal livers, there were 6 and 21-fold interindividual variations for HBP- and HMC-UGT activities, respectively, which contributed to the development of UGT enzymes with increasing gestational time (HBP-UGT: r=0.744, n=10, P<0.01; HMC-UGT: r=0.840, n=10, P<0.01; Figure 4).
Full table
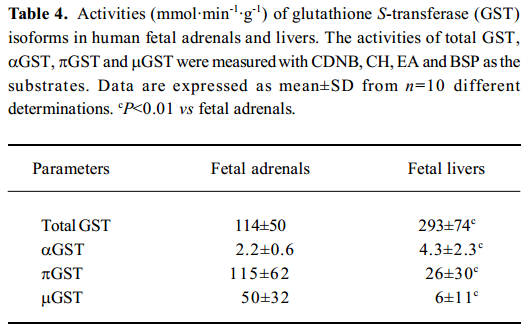
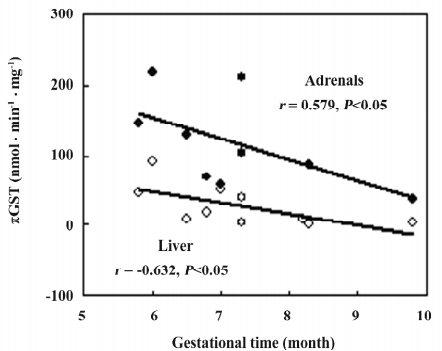
GST isoforms activity In the fetal adrenals, GST isoforms were found to primarily distribute in the cytosols. The activities of the cytosolic total GST, αGST, πGST, and μGST in the fetal adrenals were 0.4, 0.5, 4.4, and 8.3-fold, respectively, of those in the fetal livers (P<0.01; Table 4). There were negative correlations between the gestational time and πGST activity in both the fetal tissues (adrenals: r=–0.579, n=10, P<0.05; liver: r=–0.632, n=10, P<0.05; Figure 5).
Full table
Discussion
Exposure during gestation to many xenobiotics, such as drugs and environmental chemicals, is believed to induce IUGR, bring about morphological defects, or cause in utero death of the embryo or fetus in the surviving offspring[24,25]. The adrenal is very important for the synthesis of steroid hormones and any interference in adrenal development by xenobiotics could have a profound effect on homeostasis. It was reported that the weight of fetal adrenal during mid gestation was 10%–15% of the fetal liver weight, and in newborns, was 20-fold higher than that in adults, although there was a 100-fold difference in adult organ weights between the livers and adrenals[26]. The biotransformation enzymes may be of some importance, particularly when they perform functions in protecting this organ from xenobiotic-induced toxicity. In view of these facts, we investigated the activities of several important xenobiotic activating-related phase I and phase II enzymes in human fetal adrenals.
CYP1A1, which exists primarily in extrahepatic tissues, is implicated in the activation of some xenobiotics with possible deleterious effects. 3MC and other environment carcinogens are substrates and selective inducers of CYP1A1[27]. There was some evidence of CYP1A1 expression in the human fetal liver[22,28], but the expression of this enzyme in fetal adrenals has not been reported until now. In our study, no detectable CYP1A1 activity was measured spectrophotometrically in either fetal adrenal microsomes or 3MC-induced fetal adrenal cells[12], but the expression of the CYP1A1 mRNA in the majority of fetal adrenals and livers were observed using RT–PCR technique. Our recent study also demonstrated the existences of CYP1A1 mRNA as well as arylhydrocarbon receptor mRNA in rodent fetal adrenals during mid and last gestation[13]. These results showed the existence of the CYP1A1 isoform in fetal adrenals, but the activity was lower than the minimal detectable level of the enzyme assay.
Members of the human CYP2A subfamily are known to metabolize several promutagens, procarcinogens, and hepatotoxins. Gu et al[29] found that the CYP2A6 protein can express in extrahepatic tissues, and the level in olfactory mucosa was much higher than that in the liver of the same fetus. In our study, the metabolic activity of CYP2A6 occurred in all of the human fetal adrenals and was comparative to that of the liver. Meanwhile, the activity showed a good gestational time-dependent manner. The results suggested that the adrenal of the human fetus may be a target tissue for the toxicity of chemicals that are activated by CYP2A6.
The catalysis of most substrates by CYP2E1 results in the formation of toxic intermediates, making it one of the most toxicologically-significant CYP isozymes. Roberts et al[30] demonstrated that CYP2E1 is present at relatively high concentrations in the endoplasmic reticulum of the human liver and in lower concentrations in various other extrahepatic tissues. However, CYP2E1 has not been documented in human adrenals. In our study, aniline hydroxylase (a marker for the CYP2E1 isozyme) could be detected in fetal adrenals and was comparative to that of the liver, which suggests that the fetal adrenal may be easily attacked by xenobiotics during development.
Among the members of the CYP3A subfamily, CYP3A7 is the major CYP isoform detected in the human embryonic, fetal, and newborn liver. It plays an important role in the biotransformation of endogenous compounds and is capable of metabolizing potential environmental pollutants (eg aflatoxin B1)[31]. Although CYP3A7 is considered human fetal liver form, our observations showed that it was also expressed in fetal adrenals, and the levels of activity in fetal adrenals was 33% of that in the fetal livers.
In the adult liver, UGT is one of the most predominant biotransformation phase II enzymes. There was some evidence of UGT expression in the fetal liver[32] and extrahepatic tissues, such as the kidney[33]. Chauhan et al[34] found that UGT activity developed during the late fetal period and reached almost 60% of the adult activity at term in the mouse fetal liver; the activity was transplacentally inducible by β-naphthoflavone and 3-methylcholanthrene. In our study, tissue-specific differences for HBP- and HMC-UGT were observed in fetal adrenals and livers, and the activities in adrenals were much lower. This result indicated that UGT was also primarily distributed in the liver during the fetal period.
The family of GST enzymes catalyzes the conjugation of glutathione with a wide variety of nucleophiles and plays an important role in protecting cells from oxidative injury. In humans, there are 4 main classes of GST: α, π, μ, and θ[35]. Immunohistochemical determination in the human embryo at 8 weeks’ gestational age showed that αGST and πGST were present in hepatocytes, gastrointestinal epithelium, adrenal gland medulla, and tela chorioidea in the telencephalon[21]. Furthermore, Mera et al[36] proved immunohistochemically that πGST was the main form of the GST family in the fetal liver, but αGST was the main form in the adult liver. In our study, CH, EA, and BSP were chosen as the selective substrates of the αGST, πGST, and μGST isozymes, respectively. The results showed the existence of αGST, πGST, and μGST activities in human fetal adrenals. The activities of πGST and μGST in fetal adrenals were found to be much higher than those in fetal livers. We also demonstrated that πGST once was the main isozyme by a correlation analysis between gestational time and fetal adrenal πGST activity. The cause of the higher activities of GST isozymes in fetal adrenals might considerably contribute to the local active biotransformation during development.
In summary, our results showed that the human fetal adrenal possessed perfect xenobiotic-metabolizing enzymes, which includes not only phase I enzymes (CYP1A1, CYP2A6, CYP2E1, and CYP3A7 families), but also phase II enzymes (UGT and GST families). The metabolizing capacities of CYP and GST families in fetal adrenals as a whole were close to those in fetal livers. The relatively high activities of xeno-biotic-metabolizing enzymes might participate in the local biotransformation of adrenal. These results suggested that the adrenal could be an important xenobiotic-metabolizing organ in fetal development and may play a potential role in xenobiotic-induced fetal development toxicity.
References
- Syme MR, Paxton JW, Keelan JA. Drug transfer and metabolism by the human placenta. Clin Pharmacokinet 2004;43:487-514.
- Blake MJ, Castro L, Leeder JS, Kearns GL. Ontogeny of drug metabolizing enzymes in the neonate. Semin Fetal Neonatal Med 2005;10:123-38.
- Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome CYP-mediated metabolism of estrogens and its regulation in human. Cancer Lett 2005;227:115-24.
- Choudhary D, Jansson I, Sarfarazi M, Schenkman JB. Xenobiotic-metabolizing cytochromes CYP in ontogeny: evolving perspective. Drug Metab Rev 2004;36:549-68.
- Philpot RM. Characterization of cytochrome CYP in extrahepatic tissues. Methods Enzymol 1991;206:623-31.
- Coon MJ. Cytochrome CYP: nature’s most versatile biological catalyst. Annu Rev Pharmacol Toxicol 2005;45:1-25.
- Briggs GG. Drug effects on the fetus and breast-fed infant. Clin Obstet Gynecol 2002;45:6-21.
- Rainey WE, Rehman KS, Carr BR. The human fetal adrenal: making adrenal androgens for placental estrogens. Semin Reprod Med 2004;22:327-36.
- Langlois D, Li JY, Saez JM. Development and function of the human fetal adrenal cortex. J Pediatr Endocrinol Metab 2002;5:1311-22.
- Albrecht ED, Henson MC, Walker ML, Pepe GJ. Modulation of adrenocorticotropin-treated baboon fetal adrenal dehydroepiandrosterone formation in vitro by estrogen at mid- and late gestation. Endocrinology 1990;126:3083-8.
- Sarasin A, Schlumpf M, Muller M. Adrenal-mediated rather than direct effects of nicotine as a basis of altered sex steroid synthesis in fetal and neonatal rat. Reprod Toxicol 2003;17:153-62.
- Wang H, Huang M, Peng RX, Je J. Influences of 3-methylcholanthrene, phenobarbital and dexamethasone on xenobiotic metabolizing-related cytochrome P450 enzymes and steroidogenesis in human fetal adrenal cortical cells. Acta Pharmacol Sin 2006;27:1093-6.
- Chen M, Wang T, Liao ZX, Pan XL, Feng YH, Wang H. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Exp Toxicol Pathol. 2007. [Epub ahead of print].
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265-75.
- Burke MD, Prough RA, Mayer RT. Characteristics of a microsomal cytochrome P-448-mediated reaction. Ethoxyresorufin O-deethylation. Drug Metab Dispos 1977;5:1-8.
- Denton TT, Zhang X, Cashman JR. Nicotine-related alkaloids and metabolites as inhibitors of human cytochrome P-450 2A6. Biochem Pharmacol 2004;67:751-6.
- Peng RX, Lei SB, Gao P. The capacity of drug metabolism in Chinese fetal livers: metabolism of ethylomorphine, aminopyrine and aniline. Asian Pac J Pharmarcol 1990;5:13-8.
- Sanwald P, Blankson EA, Dulery BD, Schoun J, Huebert ND, Dow J. Isocratic high-performance liquid chromatographic method for the separation of testosterone metabolites. J Chromatogr B 1995;672:207-15.
- Li Y. Study on phase II enzymes of drug metabolism. In: Zhang JT, editor. Modern methods of pharmacological experiment. Peking: The Union Press of Peking Medical University and Chinese Academy of Medical Sciences; 1997. p 1656–7 (in Chinese).
- Ouwerkerk-Mahadevan S, Mulder GJ. Inhibition of glutathione conjugation in the rat in vivo by analogues of glutathione conjugates. Chem Biol Interact 1998;111–112:163-76.
- Van Iersel ML, van Lipzig MM, Rietjens IM, Vervoort J, van Bladeren PJ. GSTP1-1 stereospecifically catalyzes glutathione conjugation of ethacrynic acid. FEBS Lett 1998;441:153-7.
- Hakkola J, Pasanen M, Purkunen R, Saarikoski S, Pelkonen O, Maenpaa J, et al. Expression of xenobiotic-metabolizing cytochrome CYP forms in human adult and fetal liver. Biochem Pharmacol 1994;48:59-64.
- Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver—evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem 1997;247:625-34.
- Young AM, Allen CE, Audus KL. Efflux transporters of the human placenta. Adv Drug Deliv Rev. 2003;55:125-32.
- Yan YE, Wang H, Feng YH. Alterations of placental cytochrome P450 1A1 and P-glycoprotein in tobacco-induced intrauterine growth retardation in rats. Acta Pharmacol Sin 2005;26:1387-94.
- Yan GL. Fetal endocrinology. Peking: People’s Medical Publishing House; 1983. p 24 (in Chinese).
- Shimada T, Fujii-Kuriyama Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes CYP 1A1 and 1B1. Cancer Sci 2004;95:1-6.
- Omiecinski CJ, Redlich CA, Costa P. Induction and developmental expression of cytochrome CYP1A1 messager RNA in rat and human tissues: detection by the polymerase chain reaction. Cancer Res 1990;50:4315-21.
- Gu J, Su T, Chen Y, Zhang QY, Ding X. Expression of biotransformation enzymes in human fetal olfactory mucosa: potential roles in developmental toxicity. Toxicol Appl Pharmacol 2000;165:158-61.
- Roberts BJ, Shoaf SE, Jeong KS, Song BJ. Induction of CYP2E1 in liver, kidney, brain and intestine during chronic ethanol administration and withdrawal: evidence that CYP2E1 possesses a rapid phase half-life of 6 hours or less. Biochem Biophys Res Commun 1994;205:1064-71.
- Wilkening S, Bader A. Differential regulation of CYP3A4 and CYP3A7 by dimethylsulfoxide in primary human hepatocytes. Basic Clin Pharmacol Toxicol 2004;95:92-3.
- Ring JA, Ghabrial H, Ching MS, Smallwood RA, Morgan DJ. Fetal hepatic drug elimination. Pharmacol Ther 1999;84:429-45.
- Hume R, Coughtrie MW, Burchell B. Differential localisation of UDP- glucuronosyltransferase in kidney during human embryonic and fetal development. Arch Toxicol 1995;69:242-7.
- Chauhan DP, Miller MS, Owens IS, Anderson LM. Gene expression, ontogeny and transplacental induction of hepatic UDP-glucuronosyl transferase activity in mice. Dev Pharmacol Ther 1991;16:139-49.
- Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 2004;6:289-300.
- Mera N, Ohmori S, Itahashi K, Kiuchi M, Igarashi T, Rikihisa T, et al. Immunochemical evidence for the occurrence of Mu class glutathione S-transferase in human fetal livers. J Biochem 1994;116:315-20.