Ouabain induces cardiac remodeling in rats independent of blood pressure1
Introduction
Ouabain is a steroid hormone which is released from the hypothalamus and the adrenal gland. Ouabain and other cardiac glycosides are known as specific inhibitors of sodium pumps ever since the very first observations of the inhibition of sodium pumps of red blood cells. As a sodium pump is an intrinsic plasma membrane enzyme that hydrolyses ATP to maintain the transmembrane gradients of Na+ and K+ found in most mammalian cells, as an inhibitor of sodium pumps, ouabain is implicated in sodium homeostasis and exerts direct actions on the vasculature, the heart[1], and tubular sodium reabsorption[2], thus playing an important role in the pathogenesis of hypertension and some other cardiovascular disorders.
There are many data suggesting that ouabain is associated with both high blood pressure and altered cardiac morphology in hypertensive patients[3,4]. For patients with more advanced hypertension, circulating levels of ouabain were directly related to both blood pressure and total peripheral resistance, and inversely related to cardiac index[4]. The presence of left ventricular hypertrophy with high plasma ouabain levels has been found to be associated with alterations in cardiac function[5]. The young offspring of hypertensive patients had higher plasma levels of ouabain than the offspring of normotensive parents, which were correlated with diastolic dysfunction[6]. Moreover, circulating ouabain is a novel, independent, and incremental marker that predicts the progression of heart failure according to Pitzalis’s report[7].
Although ouabain might have a primary role in causing cardiac dysfunction and failure, its precise role and mechanism remains unclear. Our research group proposed that ouabain at pathological concentrations might affect the structure and function of the vascular endothelium and trigger vascular remodeling in hypertension[8]. Due to the demonstrated effects of ouabain on vascular areas, an effect of ouabain on cardiac remodeling and function could be predicted, so it prompted us to investigate ouabain’s contributions to the heart, on the assumption that ouabain might have a primary role in the progression of both vascular remodeling and cardiac remodeling in hypertension. This study investigated ouabain’s effects on the rat heart in order to assess whether the steroid hormone affects the structure and function of the heart and if it is involved in cardiac remodeling.
Materials and methods
Reagents and drugs Ouabain was purchased from Sigma (St Louis, MO, USA). Kits for RNA extraction and reverse transcription were purchased from Invitrogen (San Diego, CA, USA). The primers and probes were synthesized by Sangon Biological Engineering Technology and Service Co (Shanghai, China). All other reagents were of analytical grade.
Established animal model The rats used to establish the animal model were Grade II (Certificate N
Echocardiographic examination Transthoracic echo-cardiography was performed on the anaesthetized rats using a Sonos 5500 echocardiographic system (Philips Medical Systems, Andover, MA, USA). The system was equipped with a 7−12 MHz transducer that was placed on the shaved left hemithorax. The parasternal long-axis views of the left ventricle at or just below the tip of the mitral valve leaflets were used to obtain targeted M-mode recordings. The left ventricular end-diastolic diameter (LVEDd) and end-systolic diameter (LVEDs), septal thickness (IVST) and posterior wall thickness (PWT) were measured. The pulsed Doppler recordings were made from the standard apical 4 chamber view. Some indexes of left ventricular filling by Doppler flow velocity were measured: the isovolumic relaxation time (IVRT), the peak velocity of early left ventricular filling (E), the peak velocity of late ventricular filling (A), and the ratio between the early and late peak flow velocity (E/A). All data given are the means for 5 consecutive cardiac cycles and all measurements were manually obtained by the same observer. Midwall fractional shortening (FS) was calculated as describ-ed by Shimizu et al[10]. The ejection fraction (EF) was measured using a modified version of Simpson’s monoplane analysis[11].
Hemodynamic measurements Hemodynamics data were obtained by catheterization of the right common carotid artery as described previously[12]. The carotid artery was isolated and cannulated with a 3-F high-fidelity microtip catheter connected through a data acquisition unit (PowerLab 4.12, AD Instruments Inc, Castle Hill, NSW, Australia) to a computer running MacLab software (AD Instruments Inc, Australia). The catheter was advanced into the left ventricle. After an equilibration period of 20 min, the left ventricular systolic pressures (LVSP) and end-diastolic pressures (LVEDP), and the left ventricular dp/dtmax were monitored. All hemodynamic parameters were recorded continuously for 15 min. Then the rats were sacrificed and the hearts were isolated rapidly. The left ventricles were cut into 2 pieces; one piece was fixed with 4% paraformaldehyde for histo-chemistry, and the other was stored in liquid nitrogen for real-time quantitative RT-PCR.
Myocardial ultrastructure examination The animals were anesthetized with 20% urethane ip, and the apical part of the ventricle was removed. The samples were chipped into tissue blocks of 1 mm3 on ice and immediately put into a 2.5% glutaraldehyde fixation solution at 4 oC for 2 h. Electron microscope slices were produced according to routine methods for the preparation of transmission electron microscope specimens. Ultrathin sections were stained with uranyl acetate and lead citrate for examination on a transmission electron microscope (Hitachi H600, Tokyo, Japan).
Morphological examination The samples of the left ventricle were fixed in 4% paraformaldehyde for 24 h, dehydrated in ethanol, cleared in dimethylbenzene, and embedded in paraffin. The sections were prepared and stained with Picrosirius red[13]. The slides were observed with a Nikon Microphot FXA light microscope (Tokyo, Japan) equipped with a polarized set. The collagen fraction of the left ventricle (CFLV) was assessed using Picrosirius red stain and calculated as described previously[14]. The CFLV was expressed as the mean percentage of connective tissue areas divided by the total tissue area in the same field. The operator was blinded to the experimental group during the analysis.
RT-PCR primers and probes design Primers and probes for quantitative RT-PCR were designed and synthesized by Sangon Biological Engineering Technology and Service Co (Shanghai, China). The melting temperature for the primers was set at 50−60 oC, but the probe’s melting temperature was at least 10 oC higher. The minimum GC content for the primers and probes was 20%−80%, and runs of identical nucleotides were avoided. All the probes were labeled at the 5' end with the reporter dye molecule, FAM (6-carboxy-fluorescein), and at the 3' end with the quencher dye, TAMRA (6-carboxytetramethylrodamine; Table 1).
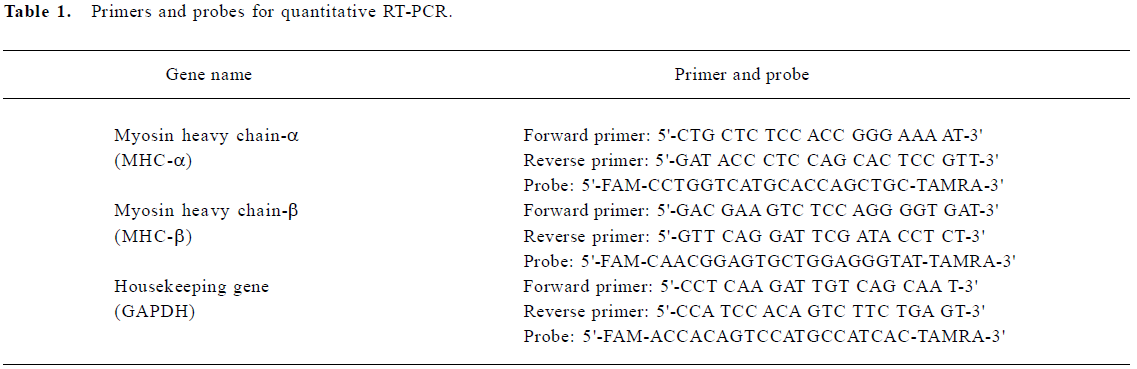
Full table
Quantitative RT-PCR The total RNA in the left ventricles was extracted using the Trizol Max kit (Invitrogen, USA). The expression of MHC-α and MHC-β was examined by fluorescent real-time quantitative RT-PCR. The quantitative RT-PCR was performed using SuperScript one-step RT-PCR kit with the Platinum Taq system (Invitrogen, USA), following the manufacturer’s instructions (with modifica-tions). The cDNA amplification product was predicted to be a 164 bp fragment for MHC-α, and a 148 bp fragment for MHC-β. A total reaction volume consisting of 1 µL forward primer, 1 µL reverse primer, 1 µL fluorescent probe, and 1 µL RT/Platinum Taq Mix, and 4 µL total RNA was made up to 50 µL with RNase-free water. One-step RT-PCR amplification was performed in the ABI Prism 7700 Sequence Detection System (Perkin-Elmer, Foster City, CA, USA). Thermal cycling conditions were as follows: 94 oC for 5 min, followed by 50 cycles of 94 oC for 30 s, 56 oC for 30 s, and 72 oC for 30 s, then 72 oC for 5 min, and finally the reaction was held at 4 oC. The housekeeping gene GAPDH was used to normalize samples to remove the impact of any variations in RNA loading. The 2—ΔΔCT method was used to calculate relative changes in gene expression determined from the real-time quantitative RT-PCR experiment[15].
Statistical analysis Data are expressed as mean±SD. Statistical differences were determined by ANOVA with the SPSS software package (version 10.0). Student’s t-test was employed to compare the data between the 2 groups. A probability value of P<0.05 was considered statistically significant.
Results
Effects of ouabain on SBP In the 4 weeks, there was no significant difference in the mean SBP between the ouabain group and the control group (P>0.05). After 4 weeks, the mean SBP in the ouabain group began to increase and was significantly higher than that in control group after 6 weeks (P<0.01; Figure 1).
Echocardiographic findings Four weeks after treatment with ouabain, the rats in the ouabain group showed clear signs of left ventricle dysfunction, whereas the rats in the control group displayed normal echocardiographic parameters. Systolic performance worsened in the ouabain group, which is indicated by the reduced EF and FS. Diastolic performance was affected also, as shown by the decreased E/A and increased IVRT. LVEDs, LVEDd, PWT and IVST were significantly greater in the rats of the ouabain group, indicating that left ventricle enlargement and left ventricle wall thickening were induced by ouabain treatment (Table 2; Figure 2). Six weeks after treatment with ouabain, echocardiographic parameters of the rats were more severe than that of the rats treated by ouabain for 4 weeks.
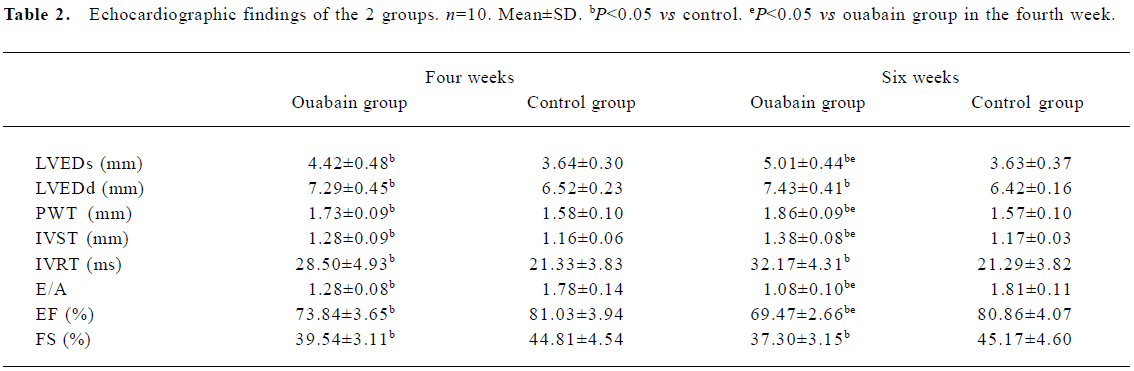
Full table
Hemodynamic data After treatment with ouabain for 4 weeks, there were no significant differences in the carotid blood pressure (CBP) of the 2 groups. However, compared with the normal control animals, LVSP and ±dp/dt significantly decreased and LVEDP increased in the rats of the ouabain group. After treatment with ouabain for 6 weeks, the CBP in the ouabain group was significantly higher than that in the control group, and all hemodynamic results further deteriorated (Table 3).
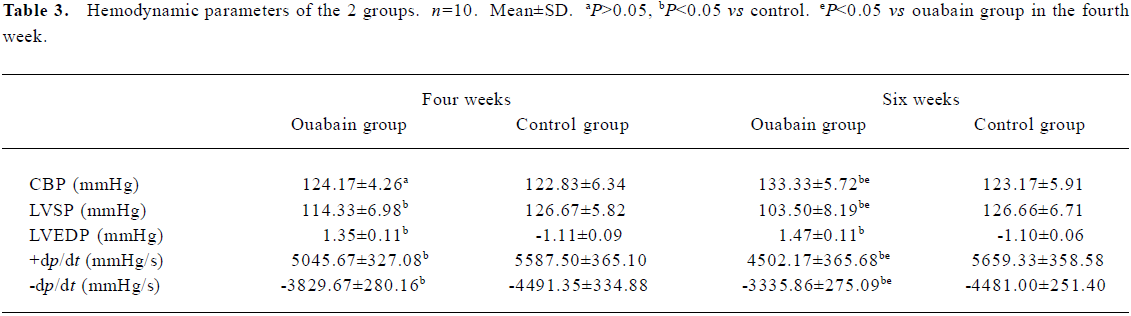
Full table
Changes in myocardial ultrastructure Cardiac muscle fibers in the control group were abundant, with regular arrays of myofibrils closely arranged within the sarcomere. The mitochondria were intact and had no swelling or disruption. There were little collagen fibers between the myocardial cells in the control group. Cardiac muscle fibers, mitochondria, and collagen fibers in the ouabain group were significantly different from those in the control group. The differences included reduction of the sarcoplasm content, disorganization of myofilaments and Z line, mitochondrial swelling, disruption and vacuolation, and hyperplastic collagen fibers. The differences were obvious in the rats treated with ouabain for 4 weeks (Figure 3).

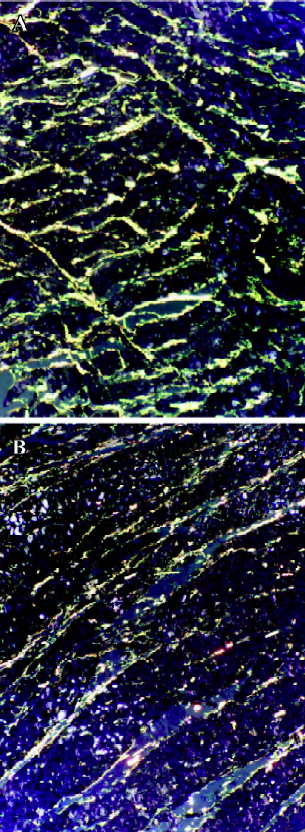
Morphometric histology After treatment with ouabain for 4 weeks, Picrosirius red observation under polarized light showed that CFLV significantly increased in the rats of the ouabain group, which indicated that the collagen metabolism in left ventricle was affected by ouabain treatment [at 4 weeks 5.97±0.39 (ouabain group) vs 4.92±0.50 (control group); at 6 weeks 6.38±0.53 vs 5.05±0.62, P<0.05] (Figure 4).
Expression of MHC-α and MHC-β mRNA in the left ventricles After treatment with ouabain for 4 weeks, real-time quantitative RT-PCR showed a 0.74× fold decrease (0.65–0.83, CV 10.81%) of MHC-α mRNA expression in the ouabain-treated rats compared with the vehicle rats. In contrast, MHC-β mRNA expression increased 2.26× fold (1.93–2.58, CV 14.60%). Six weeks after treatment with ouabain, MHC-α mRNA expression decreased 0.55×fold (0.43–0.67, CV 21.82%), and MHC-β mRNA increased 3.28×fold (2.83–3.73, CV 13.41%) compared with the control group (Figure 5).

Discussion
It has been demonstrated that ouabain administered ip is readily absorbed, and that plasma ouabain levels are not significantly different between intraperitoneal and intravenous groups at 10 min after administration[16]. The doses of ouabain infused in our study were determined based on previously published pharmacokinetic data for ouabain in rats. The doses administered were estimated to increase average plasma levels 0.5–1.0 nmol/L above the physical level[17] (the physical concentration of plasma ouabain is approximately 0.3–0.9 nmol/L[18]), so the plasma ouabain concentration in the animals is similar to the levels present in many pathological states such as hypertension and congestive heart failure (the pathological concentration of plasma ouabain is approximately 0.9–1.8 nmol/L[18]). Moreover, based on the animals’ weight gain, behavioral changes, and cardiac rhythms, the effects of ouabain did not appear to be associated with substantial toxicity.
In this study, we investigated ouabain’s effects on cardiac remodeling in rats. The primary findings are as follows: left ventricular hypertrophy, myocardial ultrastructure deterioration, and extracellular matrix remodeling were induced by ouabain treatment; meanwhile, cardiac systolic and diastolic performance both worsened. Moreover, the cardiac MHC-β mRNA, a marker of hypertrophy or failure, was upregulated by ouabain treatment, whereas MHC-α mRNA was downregulated. The effects of ouabain were found before the increase of blood pressure, indicating that ouabain might damage the structure and function of the rat heart and be involved in cardiac remodeling independent of blood pressure.
There are other studies providing evidence that ouabain may cause pathological cardiac hypertrophy independent of blood pressure. In a study in rats, a chronic infusion of very low doses of ouabain to double the plasma concentration of ouabain triggered a signal transduction pathway that produced cardiac hypertrophy[5]. Another study showed that partial inhibition of Na+/K+ ATPase by ouabain causes hypertrophic growth and transcriptional regulation of several growth-related marker genes in cultured neonatal rat cardiac myocytes[19].
However, how does ouabain exert such effects without a change in blood pressure? Recent studies have provided novel insights into ouabain’s role. When ouabain binds to Na+/K+ ATPase, it converts the enzyme to a signal transducer and initiates multiple gene regulatory pathways[19]; the cellular effects of ouabain on the heart may include actions independent of Na+/K+ ATPase inhibition[20]. We suppose that the mechanisms underlying these actions of ouabain are as follows: first, ouabain interaction with Na+/K+ ATPase initiates multiple growth-related gene regulatory pathways, triggers growth and the proliferation of cardiac myocytes and fibroblasts. It is reported[19] that ouabain’s effects includes the activation of the Src kinase and tyrosine phosphorylation of the epidermal growth factor receptors and other proteins, followed by the activation of Ras, the Ras/Raf/MEK//MAPK cascade, and increased production of reactive oxygen species. Thus, cardiac hypertrophy and extracellular matrix remodeling are induced by ouabain treatment. Second, ouabain is a steroid hormone in nature; it has a complicated interaction with other neurohormonal systems, such as the renin-angiotensin and the endothelin system[21]. Saunders and Scheiner-Bobis suggest that ouabain stimulates endothelin release and expression in human endothelial cells[22]. Our research group previously found that plasma angiotensin (Ang) II and endothelin were increased by ouabain treatment in rats[23,24]. It is well known that Ang II and endothelin have toxic effects on cardiac myocytes and play an important role in the development of pathological cardiac remodeling. Ouabain’s effects on the renin-angiotensin and the endothelin system might be involved in the damage of cardiac structure and function.
Echocardiography is one of the most widely used, noninvasive techniques to provide quantitative measurements of ventricular structure and function in humans and experimental animals. In rats, the heart is generally 1 g in weight with a 2 mm left ventricle wall thickness and a rapid heart rate of 300–400 bpm. Conventional transthoracic echocardiography transducers often do not provide enough resolution. However, high-frequency transducers (7.5–15 MHz) are now available to monitor morphometric and functional changes in small animals in vivo[25]. Hemodynamics monitoring is one of the reliable methods to evaluate left ventricle performance by invasive catheterization. We chose the 2 kinds of techniques to evaluate ouabain’s effects on the function of the rat heart.
Our results suggested that systolic and diastolic performance both worsened with ouabain treatment. Hypertrophic left ventricle and hyperplastic collagen fibers might be the reasons of diastolic dysfunction. The reasons for systolic dysfunction are multiple. At first our study showed that the mitochondria were damaged by ouabain treatment, and deteriorated energy metabolism could be predicted. Ouabain might affect systolic performance through disturbing the energy metabolism of cardiac myocytes. Second, the hypertrophy-associated switch of adult and fetal isoforms of myosin heavy chain expression might contribute to the systolic dysfunction in the ouabain-treated rats. In normal young adult rats, 80%–90% of the myosin heavy chain expressed is the α isoform[26]. The MHC-α is associated with higher ATPase activity, and so hearts rich in MHC-α have a high intrinsic contractility. In contrast, MHC-β is associated with low ATPase activity and a low intrinsic contractility. As showed in this study, decreased MHC-α mRNA expression and increased MHC-β mRNA expression were induced by ouabain treatment, so the animal’s cardiac systolic performance was affected. Finally, as an inhibitor of Na+/K+ ATPase, a short-term administration of ouabain can exert a positive inotropic effect on cardio-myocytes by inhibiting the plasma membrane Na+/K+ ATPase, increasing the intracellular Ca-concentration and decreasing the Ca-extrusion by the sodium/calcium exchanger (NCX). However, there are data suggesting that long-term administration of ouabain has no effect on myocardial contractility in rats. According to Muller-Ehmsen et al[27], chronic ouabain treatment increases the protein expression of the NCX, and the positive inotropic effect can no longer be observed after chronic treatment for 2 d. Further more, NCX overexpression impairs ouabain-dependent cell shortening in adult rat cardiomyo-cytes[28]. In this study, chronic ouabain treatment for 4 or 6 weeks might induce NCX overexpression, and myocardial contractility might be impaired subsequently; this may be one of the ways through which ouabain affects systolic performance.
In conclusion, our study provides evidence that ouabain might damage the structure and function of the rat heart independent of blood pressure. Ouabain does not only trigger mechanisms initiating primary hypertension, but also plays an important role in the development of cardiac remodeling. Clinical research during the past several decades has shown the importance of cardiac remodeling as a basic mechanism in the progression of heart failure. In the future, these novel insights into the role of ouabain in cardiac remodeling might allow the development of novel therapeutic strategies to treat cardiac remodeling and failure.
Acknowledgement
We would like to thank all the other members of our ouabain research group for their suggestions and technical assistance.
References
- Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem 2002;269:2440-8.
- Manunta P, Messaggio E, Ballabeni C, Sciarrone MT, Lanzanic C, Ferrandi M, et al.
- Plasma ouabain-like factor during acute and chronic changes in sodium balance in essential hypertension. Hypertension 2001;38:198-203.
- Manunta P, Stella P, Rivera R, Ciurlino D, Cusi D, Ferrandi M, et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension 1999;34:450-6.
- Pierdomenico SD, Bucci A, Manunta P, Rivera R, Ferrandi M, Hamlyn JM, et al. Endogenous ouabain and hemodynamic and left ventricular geometric patterns in essential hypertension. Am J Hypertens 2001;14:44-50.
- Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem 2004; 279: 33 306–14.
- Manunta P, Iacoviello M, Forleo C, Messaggio E, Hamlyn JM, Lucarelli K, et al. High circulating levels of endogenous ouabain in the offspring of hypertensive and normotensive individuals. J Hypertens 2005;23:1677-81.
- Pitzalis MV, Hamlyn JM, Messaggio E, Iacoviello M, Forleo C, Romito R, et al. Independent and incremental prognostic value of endogenous ouabain in idiopathic dilated cardiomyopathy. Eur J Heart Fail 2006;8:179-86.
- Ren YP, Huang RW, Lv ZR. Ouabain at pathological concentrations might induce damage in human vascular endothelial cells. Acta Pharmacol Sin 2006;27:165-72.
- Tipton CM, Sebastian LA, Overton JM, Woodman CR, Williams SB. Chronic exercise and its hemodynamic influences on resting blood of hypertension rats. J Appl Physiol 1991;71:2206-11.
- Shimizu G, Hirota Y, Kita Y, Kawamura K, Saito T, Gaasch WH. Left ventricular midwall mechanics in systemic arterial hyper-tension. Myocardial function is depressed in pressure-overload hypertrophy. Circulation 1991;83:1676-84.
- Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantification of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on quantification of two-dimensional echocardio-grams. J Am Soc Echocardiogr 1989;2:358-67.
- Hou XW, Son J, Wang Y, Ru YX, Lian Q, Majiti W, et al. Granulocyte Colony-Stimulating Factor Reduces Cardiomyocyte Apoptosis and Improves Cardiac Function in Adriamycin-Induced Cardiomyopathy in Rats. Cardiovasc Drugs Ther 2006;20:85-91.
- Zhang H, Sun L, Wang W, Ma X. Quantitative analysis of fibrosis formation on the microcapsule surface with the use of picro-sirius red staining, polarized light microscopy, and digital image analysis. J Biomed Maser Res A 2006;76:120-5.
- Tao X, Liu GL. Protection of organic trauma in sinoaortic-denervated rats treated with fosinopril. Acta Pharm Sin 2003;38:743-7.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2—ΔΔCT method. Methods 2001;25:402-8.
- Wang H, Yuan WQ, Lu ZR. Differential regulation of the sodium pump α-subunit isoform gene by ouabain and digoxin in tissues of rats. Hypertens Res 2000;23:S55-60.
- Tian G, Dang CX, Lu ZR. The changes and significance of the Na+, K+ -ATPase α-subunit in ouabain-hypertensive rats. Hypertens Res 2001;24:729-34.
- Hamlyn JM, Manunta P. Ouabain, digitalis-like factors and hypertension. J Hypertens 1992;10:S99-111.
- Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie ZJ. Multiple signal transduction pathways link Na/K-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 1998; 273: 15 249–56.
- Nishio M, Rush SW, Wasserstrom JA. Positive inotropic effects of ouabain in isolated cat ventricular myocytes in sodium-free conditions. Am J Physiol Heart Circ Physiol 2002;283:H2045-53.
- Xavier FE, Yogi A, Callera GE, Tostes RC, Alvarez Y, Salaices M, et al. Contribution of the endothelin and renin-angiotensin systems to the vascular changes in rats chronically treated with ouabain. Br J Pharmacol 2004;143:794-802.
- Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur J Biochem 2004;271:1054-62.
- Guo N, Jiang X, Lv ZR, Ai WT. The expression of angiotensin II and its subtype 1 and 2 recepors in the myocardium of ouabain-induced hypertensive rats. J Xi’an Jiaotong Univ 2005;26:526-9. (Med Sci).
- Jiang X, Guo N, Lv ZR, Ai WT. Effects of ouabain on endothelin system in rat heart. J Xi’an Jiaotong Univ 2005;26:519-22. (Med Sci).
- Kokubo M, Uemura A, Matsubara T, Murohara T. Noninvasive evaluation of the time course of change in cardiac function in spontaneously hypertensive rats by echocardiography. Hypertens Res 2005;28:601-9.
- Nakanishi K, Nakata Y, Kanazawa F, Imamura SI, Matsuoka R, Osada H, et al. Changes in myosin heavy chain and its localization in rat heart in association with hypobaric hypoxia-induced pulmonary hypertension. J Pathol 2002;197:380-7.
- Muller-Ehmsen J, Nickel J, Zobel C, Hirsch I, Bolck B, Brixius K, et al. Longer term effects of ouabain on the contractility of rat isolated cardiomyocytes and on the expression of Ca and Na regulating proteins. Basic Res Cardiol 2003;98:90-6.
- Bolck B, Munch G, Mackenstein P, Hellmich M, Hirsch I, Reuter H, et al. Na+/Ca2+ exchanger overexpression impairs frequency- and ouabain-dependent cell shortening in adult rat cardiomyocytes. Am J Physiol Heart Circ Physiol 2004;287:H1435-45.

