High-throughput fluorescence polarization method for identifying ligands of LOX-11
Introduction
Recent advances in automation and in the identification of new molecular targets for therapeutic intervention, and the increasing number of compounds available for screening are the driving forces behind the development of new drug discovery systems. High-throughput screening (HTS) has become increasingly important in the process of drug development[1]. Thereinto, measurement techniques are particularly important with respect to the quality of HTS. During the past 7 years, fluorescence polarization (FP) assays have gained popularity in HTS because the method is homo-geneous, highly sensitive, and easily miniaturizable, has a rapid readout, and is neither radioactive nor labor intensive[2].
FP is a versatile technique for measuring equilibrium binding, nucleic acid hybridization, and enzymatic activity. FP assays are homogenous in that they do not require a separation step and do not require attachment to an immobilized phase. Polarization values can be measured repeatedly and after the addition of reagents, because measuring the polarization is rapid and does not destroy the sample. Generally, this technique can be used to measure the polarization values of fluorophores from low picomolar to micromolar levels. Polarization does not change with fluorescence intensity; therefore a 1 pmol/L and a 1 nmol/L solution of fluorescein should have the same polarization values[3].
For HTS assays, FP can be used to directly measure the binding and dissociation between two molecules if the binding partners are small and fluorescently tagged. The binding can be simply monitored by reading the polarization value of the small fluorescent molecule before and after it binds to a large molecule. Developing competitive binding assays that can be used to measure analyte concentrations directly in solution is straightforward[4,5].
In the present study, we developed a novel high-throughput assay based on competitive FP to screen the ligands of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), a membrane receptor that is expressed on the surface of endothelial cells, and has roles in endothelial injury and atherosclerosis[6,7]. The basic principle of this assay is that cloned, expressed, and purified LOX-1 is labeled by fluorescein isothiocyanate (FITC), and allowed to bind its natural ligand, oxidized low-density lipoprotein (oxLDL), in a competitive displacement assay in a homogeneous solution to find compounds that are active with respect to LOX-1.
Materials and methods
Reagent preparation Human low-density lipoprotein (LDL; density 1.019–1.063 g/mL) was isolated by sequential ultracentrifugation at 4 °C from normol lipidemic fasting volunteers and oxidized to oxLDL by CuSO4[8,9]. oxLDL was labeled with 1,1'-dioctadecyl-3,3,3',3'-tetramethylindo-carbocyanine perchlorate (DiI; Molecular Probe, Sigma, St Louis, USA) according to the method of Zouhair and Yurachek[10].
Construction, expression and purification of recombinant hLOX-1 The human LOX-1 gene was obtained by RT-PCR from THP-1 cells stimulated with 50 µmol/L histamine. The purified hLOX-1 gene was cloned into a pMD 18-T (TaKaRa, Dalian, China) vector, amplified in Escherichia coli strain DH5α, and the positive plasmid-harboring hLOX-1 cDNA was digested with EcoRI and NotI. hLOX-1 cDNA was subcloned into pPIC9K (Invitrogen, Carlsbad, CA, USA), and digested with the same restriction enzymes, resulting in the recombinant plasmid pPIC9K-His-hLOX-1.
The plasmid pPIC9K-His-hLOX-1 was linearized with the restriction enzyme SacI and transformed into Pichia pastoris GS115 (Invitrogen) with PEG1000 as recommended in the instructions of the Pichia expression kit (Version F; Invitrogen). The yeast strain was cultured on YEPD medium (1% yeast extract, 2% peptone, 2% glucose). MD medium (1.34% yeast nitrogen base, 0.04% biotin, 2% glucose and 1.5% agar) was used for the selection and maintenance of His+ (His 4 genotype) transformants. Transformants were incubated for 18 h in buffered minimal glycerol medium, then the cultures were centrifuged and the cell pellet was resuspended in buffered minimal methanol medium. Cells were cultured in a baffled shake flask for 5 d at 30 ºC. Methanol was added to maintain a concentration of 1% (v/v) every day. Recombinant hLOX-1 was purified using HiTrap ChelatingHP (Amersham Biotech, Piscataway, USA) according to the manufacturer’s instructions[11,12].
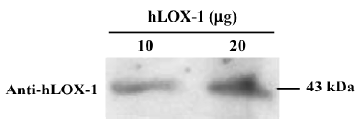
Validation of hLOX-1 Western blot analysis was used to validate the recombinant hLOX-1 receptor. The purified hLOX-1 receptor (10 and 20 µg) was separated on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to polyvinylidene difluoride membrane (PVDF). After incubation in blocking solution (5% nonfat milk; Sigma), the membrane was incubated with a 1:150 dilution of the anti-hLOX-1 monoclonal primary antibody (JTX92; a gift from Prof T SAWAMURA, Osaka, Japan)[13] overnight at 4 °C. The membrane was washed and then incubated with a 1:2000 dilution of the second antibody (Amersham) for 1 h, and fragments binding to the membrane were detected with the Amersham enhanced chemiluminescence (ECL) kit.
Three concentrations of purified hLOX-1 (0.4, 0.2, and 0.1 mg/mL) plus bovine serum albumin (BSA; 1 mg/mL) were used for printing on poly-L-lysine-coated slides by robotic printers equipped with split pins (diameter 300 µm). The slides were incubated for 5 min at 4 °C. Ten microliters of DiI-oxLDL (5 ng/mL) was added to each slide, then slides were covered with a slip, incubated in a humidified chamber at room temperature for approximately 30 min, and then rinsed with a gentle stream of phosphate-buffered saline (pH 7.4) for 3 min. Slides were air-dried and then used in the microarray scanner (ScanArray Express; PerkinElmer, Boston, USA).
Fluorescent labeling Purified hLOX-1 receptor was labeled with FITC after it was dialyzed overnight against 0.01 mol/L carbonate buffer (pH 9.5). A stock solution of FITC (Sigma) was prepared by dissolving 1 mg FITC in 1 mL of dimethyl sulfoxide (Me2SO). The FITC solution was added to the hLOX-1 solution drop by drop, and the final ratio of FITC to hLOX-1 is 50 µg to 1 mg. After this mixture was incubated for 16 h at 4 °C in the dark with a magnetic stirrer, FITC-hLOX-1 was collected by using a Sephadex G25 column. Absorbance at 495 and 280 nm was measured in order to calculate the labeling efficiency as follows: F/P=(2.87×A495)/(A280–0.35×A495). Only FITC-hLOX-1 in which the value of F/P was between 2 and 4 was used for the assay.
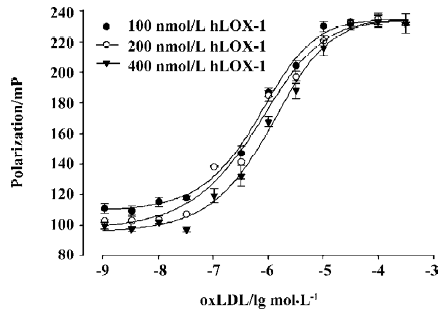
Optimization of the FP assay Optimization of the FP assay was achieved by titrating to determine the optimal oxLDL and FITC-hLOX-1 concentrations[14]. Three FITC-hLOX-1 concentrations (100, 200, and 400 nmol/L) were selected to betitrated by oxLDL at concentrations ranging from 0.05 nmol/L to 100 µmol/L.
FP measurements were carried out using a SpectraMaxM5 (Molecular Devices, California USA) and black Costar 96-well plates (Corning Incorporated, Corning, NY, USA). Polarization was measured by using an excitation filter of 485 nm and an emission filter of 520 nm, each well was flashed 50 times, and average values were obtained. Binding experiments were performed in 96-well microplates of 150 µL total volume per well, and both FITC-hLOX-1 and oxLDL were diluted with 20 nmol/L phosphate-buffered saline (pH 7.4), which contained 0.24 nmol/L ethylenediamine tetraacetic acid (EDTA).
Me2SO tolerance and stability Me2SO was necessary to maintain the solubility of the chemical library components. To assess the effect of Me2SO on polarization in all experiments, 0%, 1%, 3%, or 5% final Me2SO concentrations were used in the displacement assay buffer. To determine the optimal time of incubation and the stability of the signal, FITC-hLOX-1 with oxLDL was incubated for 15 min, 30 min, 1 h, or 2 h before the polarization value was measured.
FP competition assay Assay was carried out according to optimal condition, and the maximal bound oxLDL concentrations and optimal concentrations of FITC-hLOX-1 were determined. To assess the competition assay, the displacement reaction was investigated by adding unlabeled hLOX-1, after the binding of FITC-hLOX-1 and oxLDL reached a maximum. The titration concentration of unlabeled hLOX-1 spanned from 5 nmol/L to 1 mmol/L.
High-throughput screening The optimized FP assay was used to screen 12 700 single chemicals. In homogenous solution, a polarization value was obtained when only fully displacement FITC-hLOX-1 was present. When oxLDL was added and binding reached a maximum, the sample (final concentration 2.5 mg/mL) was added to competitively displace oxLDL and bind hLOX-1. Three polarization values were used to determine the binding affinity of the sample with hLOX-1, and unlabeled hLOX-1 was used as a positive control. The reaction system and assay conditions were all as described earlier for the optimized assay.
Data analysis All polarization values are expressed in millipolarization units (mP). The polarization values of the FP assay were calculated by using Analyst software based on the equation[15]: mP =1000×[(I|| -G*I⊥)/(I|| +G*I⊥)], where I|| is the fluorescence intensity measured when the excitation and emission polarizers are parallel, I⊥ is the fluorescence intensity measured when the excitation and emission polarizers are perpendicular, and G is the grating factor that corrects for instrument bias. The G factor was calculated for each experiment by using the basal polarization value determined with the FITC-only wells.
To assess the robustness of the HTS of FP, the Z' value was calculated by using the equation[15]: Z'=1–[(3*σ free+3*σbound)/(mPbound–mPfree)], where σ is the standard deviation in the signal, the subscript “bound” corresponds to the mean signal obtained in the absence of a displacing substance, and the subscript “free” corresponds to a completely displaced tracer. Data from 96 positive and negative controls were used to calculate the Z' factor. Data are presented as mean±SD.
Results
Validation of hLOX-1 To validate the purity of the hLOX-1, the purified hLOX-1 protein was blotted with anti-hLOX-1 monoclonal antibody. Purified hLOX-1 and anti-hLOX-1 monoclonal antibody had a high binding at 43 kDa (Figure 1). Incubation of purified hLOX-1 (at 3 concentrations: 0.4, 0.2, and 0.1 mg/mL) and BSA (1 mg/mL) with DiI-oxLDL showed that binding of hLOX-1-oxLDL occurred in a concentration-dependent manner, and that BSA did not bind with DiI-oxLDL (Figure 2). This demonstrated the binding activity of hLOX-1 with oxLDL.

FP assay optimization The oxLDL and fluorescently labeled-hLOX-1 titration curves are shown in Figure 3. Labeled-hLOX-1 concentrations of 100, 200, and 400 nmol/L were tested and used to determine the optimal concentration of oxLDL. With increasing oxLDL concentration, the polarization value increased and reached a plateau at an oxLDL concentration of 30 µmol/L. Once this plateau reached, the polarization value barely changed with further increases in the concentration of oxLDL. A system with a total volume of 150 µL containing 200 nmol/L FITC-hLOX-1 and 50 µmol/L oxLDL was selected to use in the actual assay.
FP Me2SO tolerance and assay stability For the HTS chemical library, all compounds needed to be dissolved in Me2SO. To obtain a tolerance value for Me2SO in the FP assay, 0%, 1%, 3%, and 5% Me2SO was used to assess the effect on the polarization value. As shown in Figure 4, the more Me2SO was added to each well, the lower the polarization value became. However, for up to 5% Me2SO, the Z' factor was not significantly different for the different concentrations of Me2SO.

Different durations for the receptor-ligand reaction were tested to determine the stability of the signal. FITC-LOX-1 at a concentration of 200 nmol/L was titrated with oxLDL, and the reaction was incubated for varying periods of time before measurement. There was little difference in the polarization values after 15 min, 30 min, 1 h, and 2 h incubation. An incubation time of 20 min was chosen for the actual FP HTS assay (Figure 5).

Robustness of the FP assay for HTS The ultimate aim of the HTS assay is to identify active compounds, so the validation and design of assays for robust HTS is critically important. The Z' factor is widely used to measure the quality of an assay. The average polarization values were approximately 106.0±7.6 and 232±6.8 mP in negative controls (free) and positive controls (maximal binding), respectively (Figure 6). The Z' factor for this assay was 0.66, which indicates that it should be a robust HTS assay.

FP competition assay Given fixed concentrations of FITC-hLOX-1 and oxLDL, when the binding of receptor and ligand reached an equilibrium, unlabeled hLOX-1 was titrated to assess the competition effect (Figure 7). At a concentration of 100 nmol/L of unlabeled hLOX-1, the polarization value began to reduce and the FITC-labeled hLOX-1-oxLDL began to dissociate and be displaced by unlabeled hLOX-1. At a titrating concentration of 300 µmol/L unlabeled hLOX-1, the polarization value was unchanged and FITC-hLOX-1 was almost displaced by unlabeled hLOX-1.

High-throughput screening results A total of 12 700 single chemicals were screened using the optimized FP-based HTS assay system. After the primary screening, 28 chemicals were retested in duplicate. The distribution of the duplicate test results was very consistent. During retesting with 5 multiple diluted concentrations, 3 chemicals were confirmed to have activity with respect to LOX-1, and their IC50 values are shown in Table 1.

Full table
Discussion
Membrane receptors are large receptors that are expressed on the cell surface, one of the most important classes of therapeutic targets for drug discovery. In the present study, we selected hLOX-1 as a target, and developed a competitive FP-based HTS assay. LOX-1 receptor is critically related to the progression of atherosclerosis and endothelial cell injury, so the identification of antagonists of LOX-1 will have potential significance for diseases induced by endothelial activation, dysfunction, and injury[16].
FP provides information on the rotational mobility of a fluorescent molecule. The rotational speed of a molecule is dependent on the size of the molecule, and the temperature and viscosity of the solution. Therefore, if viscosity and temperature are held constant, polarization is directly proportional to molecular volume[17]. The basic theory of FP is that mobilized small molecules have low polarization values and that large molecules have high polarization values. In a homogeneous solution, the labeled compound is small and rotates rapidly (low polarization); when the labeled compound binds to the larger molecule, its rotation slows down considerably (polarization changes from low to high)[18,19]. Because the mobility of a fluorescent molecule will change if it binds to another molecule, FP can be used to study and quantify biomolecular interactions. Therefore FP is a powerful technique for the study of biomolecular interactions in solution. It has been used to investigate DNA-protein interactions, protein-protein interactions, and small molecule-protein interactions[20,21].
For FP assay, the change in polarization value comes from the change of molecular volume, so fluorophores generally were labelled to a small volume molecule, and were obtained high polarization values when small labeled-tracers with low polarization values bind to large molecules in solution. In an FP-based receptor-ligand reactive system, the fluorophore is always labeled onto ligands, because the ligands are almost smaller than the correlated receptors[17]. In our FP-based competition assay, the molecular weight of hLOX-1 was 43 kDa, even though it is a membrane receptor, and that of its ligand, oxLDL, was 3000 kDa. According to the theory of FP-based assay, FITC was used to label hLOX-1. The compounds of chemical libraries are almost all small molecules, and far smaller than oxLDL. If the natural ligands competitively displace oxLDL from hLOX-1, the polarization value will be greatly decreased relative to that of hLOX-1-oxLDL. Therefore it was feasible to use the designed FP assay for screening hLOX-1 ligands.
In FP-based assays, the concentration of the fluorophore in the assay mixture is a major factor determining interference of the compounds. A high labeled-tracer concentration in the screening process would result in reduced sensitivity for weak inhibitors, because it is difficult for the weak inhibitors to compete with the ligand[22]. However, a low labeled-tracer concentration would produce low fluorescence counts, decreasing the signal-to-noise ratio. In the present study, 3 labeled-tracer concentrations were selected to determine the optimal concentration, and a concentration of 200 nmol/L for FITC-hLOX-1 was selected for use in the actual assay as the final concentration. At a concentration of 30 µmol/L oxLDL, the binding plateaued, and the fixed 200 nmol/L concentration of FITC-hLOX-1 was titrated by oxLDL. In actual FP-based HTS, it is possible that free tracer binding to compounds could conceal some displacement, so an excess oxLDL concentration (50 µmol/L) was used to prevent free FITC-hLOX-1 occurring in the reaction solution and reducing the background signal.
For HTS, identification of an active compound is known as a “hit”, and the sensitivity of an assay is the critical determinant of the hit ratio. The ability of a particular HTS assay to identify hits depends largely on the suitability or quality of the assay used in the screening. The Z' factor, a characteristic parameter for the quality of the assay itself, was used to evaluate the quality of the competitive FP-based HTS assay developed in the present study. The closer the Z' factor is to 1.0, the more robust the assay for HTS is, and if Z' is =0.5, it indicates that the HTS assay is robust[15]. The Z' value for our assay was 0.66, indicating that it is robust and well suited for HTS.
In summary, an FP assay for the binding of oxLDL to FITC-hLOX-1 was optimized and miniaturized for HTS. The assay performance was excellent, with a Z' value of 0.66. The distributions of the duplicates of the retest were very consistent, indicating the reliability and robustness of the assay. The competitive FP assays described here for the detection of ligands of LOX-1 are sensitive, robust, and suitable for adaptation to the HTS format.
References
- Hertzberg RP, Pope AJ. High-throughput screening: new technology for the 21st century. Curr Opin Chem Biol 2000;4:445-51.
- Silverman L, Campbell R, Broach JR. New drug assay technologies for high throughput screening. Curr Opin Chem Biol 1998;2:397-403.
- Banks P, Harvey M. Consideration for using fluorescence polarization in the screening of G protein-coupled receptor. J Biomol Screen 2002;7:111-7.
- Rodiger M, Haupts U, Moore KJ. Single-molecule detection technologies in miniaturized high throughput screening: binding assays for G protein-coupled receptors using fluorescence intensity distribution analysis and fluorescence anisotropy. J Biomol Screen 2001;6:29-37.
- Banks P, Gosselin M, Prystay L. Impact of a red-shifted dye label for high throughput fluorescence polarization assays of G protein-coupled receptors. J Biomol Screen 2000;5:329-34.
- Sawamura T, Kume N, Aoyama T, Moriwakl H, Hoshikawa H, Alba Y, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 1997;386:73-7.
- Masaki T. Endothelial dysfunction and LOX-1: forty years from muscle to endothelium. Circ Res 2003;92:819-20.
- Cominacini L, Garbin U, Davoli A, Micciolo R, Bosello O, Gaviraghi G, et al. A simple test for predisposition to LDL oxidation based on the fluorescence development during copper-catalyzed oxidative modification. J Lipid Res 1991;32:349-58.
- Bradford MM. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 1976;72:248-54.
- Zouhair FS, Yurachek EC. Rapid fluorometric assay of LDL receptor activity by Dil-labeled LDL. J Lipid Res 1993;34:325-30.
- Takefumi N, Yasuda T, Hoshikawa H, Shimizu M, Kakinuma T, Chen MY, et al. LOX1-1 expressed in culture rat chondrocytes mediates oxidized LDL-induced cell death: possible role of dephosphorylation of Akt. Biochem Biophys Res Commun 2002;299:91-7.
- Huang ZT, Zhang TT, Yang JL, Zhu P, Du GH, Chen KD. Cloning and expression of human lectin-like oxidized low density lipoprotein receptor-1 in Pichia pastoris. Biotech Lett 2005;27:49-52.
- Li DY, Liu L, Chen HJ, Sawamura T, Ranganathan S, Mehta JL. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation 2003;107:612-7.
- Jameson DM, Seifried SE. Quantification of protein-protein interaction using fluorescence polarization. Methods 1999;19:222-33.
- Zhang JH, Chung T, Oldenburg K. A simple statistical parameter for use in evaluation of high throughput screening assays. J Biomol Screen 1999;4:67-73.
- Thum T, Borlak J. Mechanistic role of cytochrome P450 monooxygenases in oxidized low-density lipoprotein-induced vascular injury: therapy through LOX-1 receptor antagonism? Circ Res 2004;94:e1-e13.
- Jameson DM, Croney JC. Fluorescence polarization: past, present and future. Comb Chem High Throughput Screen 2003;6:167-76.
- Kakehi K, Oda Y, Kinoshita M. Fluorescence polarization: analysis of carbohydrate–protein interactions. Anal Biochem 2001;297:111-6.
- Banks P, Gosselin M, Prystay L. Fluorescence polarization assays for high throughput screening of G protein-coupled receptors. J Biomol Screen 2000;5:159-67.
- Heyduk T, Ma Y, Tang H, Ebright RH. Fluorescence anisotropy: rapid, quantitative assay for protein-DNA and protein-protein interaction. Methods Enzymol 1996;274:492-503.
- Fisher BM, Ha JH, Raines RT. Coulombic forces in protein-RNA interactions: binding and cleavage by ribonuclease A and variants at Lys7, Arg10 and Lys66. Biochemistry 1998;37:12121-32.
- Qian J, Voorbach MJ, Huth JR, Coen ML, Zhang H, Ng SC, et al. Discovery of novel inhibitors of Bcl-xL using multiple high-throughput screening platforms. Anal Biochem 2004;328:131-8.
