Curcumin regulated shift from Th1 to Th2 in trinitrobenzene sulphonic acid-induced chronic colitis
Introduction
Curcumin (Cur), the prominent yellow pigment in turmeric, is a widely used spice and food coloring agent with anti-inflammatory and anti-cancer properties[1]. Previoulsy published studies have reported that cur inhibits IL-12 production from macrophages, and results in a reduced ability to induce IFN-γ and an increased ability to induce IL-4, which suggests that cur may inhibit Th1 and enhance Th2 cytokines synthesis and may be therapeutic for Th1-mediated immune disease[2,3]. However, a published study of atopic asthmatics indicates that cur inhibited the expression of IL-4 and IL-5, both of which are Th2 cytokines. From this study, it appears that cur has the ability to also inhibit Th2[4].
Inflammatory bowel disease (IBD) comprises the 2 conditions, Crohn’s disease (CD) and ulcerative colitis (UC) which are characterized by chronic relapsing and remitting an inflammatory condition in the colon[5]. Recently, various animal models for IBD have been developed, and it has been revealed that the dysfunction of T cells plays an important role in the pathogenesis of this disease[6]. Trinitrobenzene sulphonic acid (TNBS)-induced colitis model mimics human CD, which has a high level of T help 1 (Th1) cytokines (IL-12, IFN-γ, TNF-α, IL-1), but low levels of Th2 cytokines (IL-4, IL-5, IL-10)[7]. From these cytokines, it has been reported that IL-12 plays a pivotal role in the pathogenesis of TNBS-induced colitis[8].
Frontline drugs, such as dexamethasone (Dex, one of the most commonly used drugs in the control of acute inflammation), used in the treatment of IBD, have varying efficacy from patient to patient. They are expensive and inevitably without side effects. New agents, including traditional herb medicines such as Cur (used in most chronic inflammation), have been tried in the treatment of IBD. Applying cur on colitis models has been reported[9–11], but different conclusions have been reached on the aspect of the expression of cytokines. Sugimoto et al[10] reported that IL-4 could not be detected in all the experimental animals in their study, but Ukil et al[11] reported that IL-4 increased in the Cur-treated groups. Therefore, we were prompted to investigate the expression of Th1 and Th2 cytokines in the Cur-treated colitis model. We also wanted to see if Cur and Dex, two agents used in different phases of inflammation, have additive and/or synergistic effects on rats that receive the 2 agents simultaneously.
Materials and methods
Animals and experimental protocols Specific pathogen-free Sprague-Dawley rats (200−220 g) were obtained from the Laboratory Animal Central of Wuhan University (Wuhan, China) and kept in a room with a constant temperature of 23±2 oC and humidity around 50%–70% with a 12-h light-dark cycle. The rats were allowed to adapt to our laboratory environment for 1 week before the onset of the experiment, during which they had free access to standard rodent chow and tap water. Seventy five rats were distributed to 5 groups randomly: control (Con) group (receiving ethanol only without therapy), TNBS group (receiving TNBS without therapy), Cur group (30 mg·kg-1·d-1 Cur, ip), Dex group (2 mg·kg-1·d-1 Dex, ip), Cur+Dex group (30 mg·kg-1·d-1 Cur, ip+2 mg·kg-1·d-1 Dex, ip). Cur (=95.0%) and Dex were purchased from Sigma (St Lonis, MO, USA). The rats in the Con and TNBS groups received the same volume of 5% ethanol (vehicle of Cur) everyday until the end of the experiment. All the rats were checked daily for behavior and body weight.
Induction of colitis Colitis was induced by TNBS using a modification of the method described by Morris et al[12]. Briefly, all the rats were made to fast on d 1; on d 2, colitis was induced. Each rat was anesthetized with ether, and 100 mg/kg of TNBS (Sigma, St Lonis, MO, USA; 10% TNBS in 50% ethanol; total volume 1.0 mL) was then instilled via a rubber catheter inserted 8 cm into the colon via the anus. The rubber catheter was modified with numerous holes in the last 4 cm of its length. The instillation procedure required only a few seconds to be completed, and the rats were maintained in a vertical position for 3 min to prevent solution leakage. The Con group rats received 50% ethanol of the same volume using the same technique. On d 15, all the rats were killed. The experiments were approved by the institution’s animal care and use committee.
Macroscopic and histological assessment of the severity of colitis After rapid removal of the colon, the specimen was flushed with ice cold PBS, cut open, and photographed with a Pentax camera (Pentax Co, Tokyo, Japan) before being snap-frozen in liquid nitrogen and stored at -80 oC. Scores were assessed using the following damage scoring system[12]: (0) no damage; (1) localized hyperaemia, but no ulcers; (2) linear ulcers with no significant inflammation; (3) linear ulcers with inflammation at 1 site; (4) 2 or more sites of ulceration and/or inflammation; and (5) 2 or more major sites of inflammation and ulceration or 1 major site of inflammation and ulceration extending more than 1 cm along the length of the colon.
For histological analyses, tissues were fixed in 10% paraformaldehyde in phosphate-buffered saline formalin and paraffin-embedded tissue sections were stained with hematoxylin and eosin (H-E) using the standard techniques.
Assessment of myeloperoxidase activity Myelopero-xidase (MPO) activity was assessed as a marker of neutrophil infiltration. The tissue was thawed, weighed and homogenized; the homogenate was then centrifuged and the pellet was again homogenized in PBS, containing 0.5% hexadecyl-trimethylammonium bromide (HETAB) and 10 mmol/L ethylenediamine tetraacetic acid (EDTA). The homogenate was subjected to 3 cycles of freezing/thawing and brief sonication. A sample of homogenate was added to the reaction volume. The mixture was incubated at 37 oC for 5 min and the reaction was started by the addition of H2O2. The complete reaction mixture was incubated for exactly 3 min at 37 oC and terminated by the sequential addition of catalase and sodium acetate. The changes in absorbance at 655 nm were measured with a spectrophotometer. The results were quantified as U/mg protein.
Real-time quantitative reverse-transcription polymerase chain reaction Total RNA were isolated using TRIzol Reagent (Invitrogen). Two micrograms of total RNA were reverse-transcribed into complementary deoxyribonucleic acids (cDNA) by Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. Real-time PCR was performed using the SYBR green PCR kit (Qiagen), according to the manufacturer’s instructions. Annealing temperature was optimized to create a 1-peak melting curve and the productions of PCR were checked by agarose gel electrophoresis for a single band of the expected size. The abundance of each mRNA was detected normalized to that of GAPDH mRNA. The sequences of all the primers used in this study are shown in Table 1.
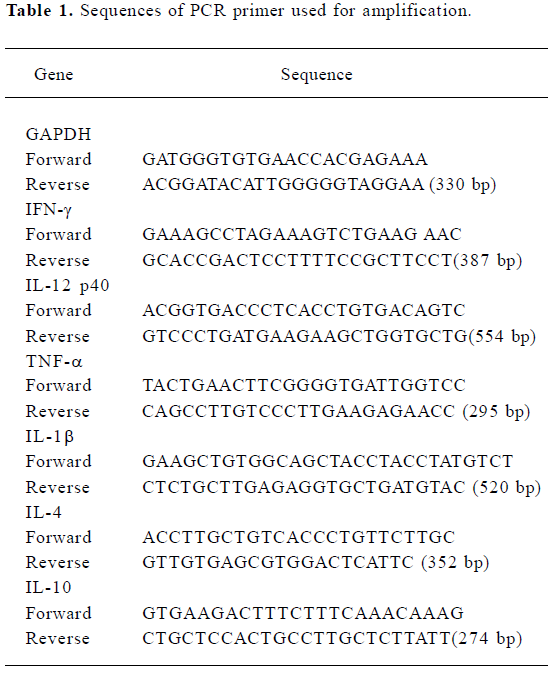
Full table
Splenocyte isolation and flow cytometry The isolation of splenocytes and flow cytometry (FCM) were performed using the methods described by Maisel et al[13]. Briefly, the spleens were excised aseptically 15 min after rats were killed and placed in RPMI-1640. Single cell suspensions were then prepared. The tissue was passed through a 70-µm-pore mesh and washed with RPMI-1640. Samples were spun and supernatants were aspirated. Erythrocytes were lysed by ammonium chloride (BD Bioscience, San Diego, CA, USA). Cells were resuspended in supplemented RPMI-1640 and counted on a hemocytometer in trypan blue to ensure viability. After being stimulated with PMA, ionomycin and monensin (GolgiStop), the cells were stained with Cy-Chrome-labeled anti-rat CD4 antibody (BD PharMingen). The fixation and membrane permeabilization were performed with FACSTM Perm 2 (BD) according to the manufacturer’s instructions. Intracellular cytokines were stained with FITC-labeled IFN-γ or PE-labeled IL-4 monoclonal antibodies (BD Pharmingen). The IFN-γ- and IL-4-producing CD4+ T cells were analyzed with FACSTM Calibur (BD, PharMingen).
Enzyme-linked immunosorbent analysis (ELISA) Before the rats were killed, 1.5 mL of blood was collected from each rat and centrifuged immediately to remove the blood cells. Concentrations of IFN-γ and IL-4 in the animal sera were estimated by commercially available ELISA kits (R&D), according to the manufacturer’s instruction. All samples were assayed in duplicate. The proportions of IFN-γ/IL-4 were then calculated.
Statistics Data are expressed as mean±standard error of the mean (SEM). The statistical significance was evaluated by one-way ANOVA followed by least significant difference (LSD) or Dunnett’s test. A P-value less than 0.05 was considered statistically significant. In the experiment involving histology, the figures shown are representative of at least 3 experiments performed on different days.
Results
Cur therapy improves body weight recovery The Con group showed transient and slight body weight loss and recovered quickly (Figure 1A). Administration of TNBS caused a dramatic decrease of body weight in all the groups. At the end of the experiment, no difference was observed between the Con and Cur groups in body weight, but there was a significant difference between the 2 groups and the other 3 groups. No difference was observed between the Dex and Cur+Dex groups, but they differed from the TNBS group.
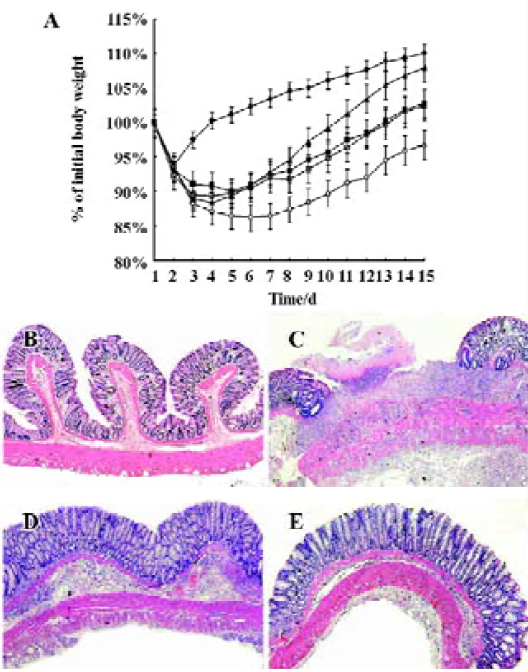
With the exception of faint infiltration of inflammatory cells, no pathological changes were observed in the Con group (Figure 1B). Necrosis of epithelium, distortion of crypts, destruction of glands and infiltration of inflammatory cells were observed in the TNBS group (Figure 1C). Treatment with Cur was found to decrease the necrosis of epithelium, and the infiltration of inflammatory cells and granulation could be seen (Figure 1D). Dex was found to dramatically decrease the necrosis of epithelium cells and the infiltration of inflammatory cells. However, it had little effect on the formation of granulation; edema existed in most cases (Figure 1E). The effects of Cur+Dex on histological images were similar to that of the Dex group.
Cur therapy decreases macroscopic scores and MPO activity The different treatment effects on macroscopic scores in TNBS-induced colitis were evaluated (Table 2). The macroscopic score of the Cur group was significantly lower than that of the TNBS group (P<0.01). The Dex group also had decreased macroscopic scores. The combined therapeutic effects of Cur+Dex on macroscopic scores were similar to that of Dex alone. MPO activity significantly (P<0.01) increased in the TNBS group. Both Cur and/or Dex was found to significantly reduce its activity.
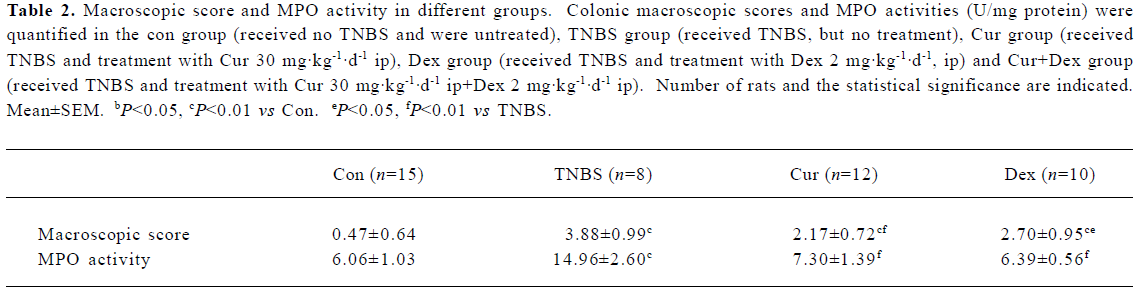
Full table
Cur therapy decreased Th1 and increased Th2 cytokines in colon mucosa Relative mRNA abundance of Th1 cytokines in the TNBS group were elevated (10–50 fold) over the Con group (Table 3), however, that of Th2 cytokines decreased. The Cur-treated group suppressed the expression of Th1 cytokines efficiently, which was significantly high in the Con group. Th2 cytokines all increased after the treatment with Cur. Treatment with Dex was also found to decrease the expression of Th1 cytokines. However, it seemed that Dex could not increase Th2 cytokines. The effects of Cur+Dex on the expression of cytokines were similar to that of Dex.
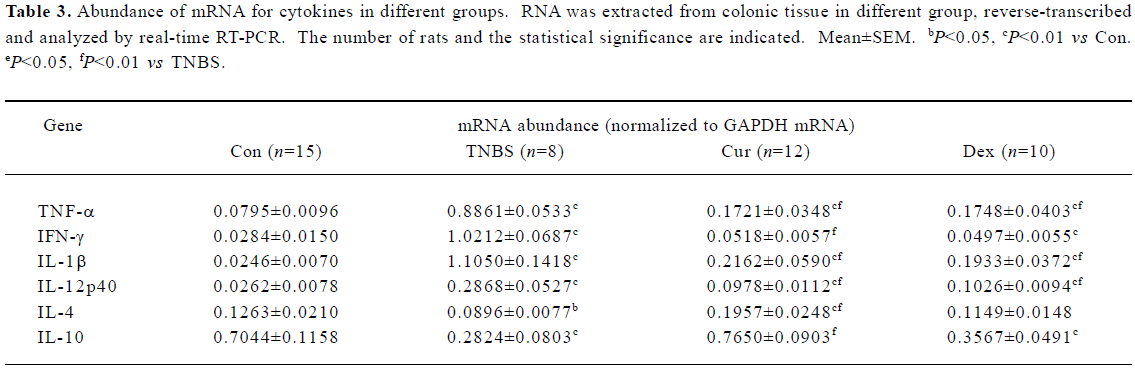
Full table
Cur therapy decreased IFN-γ and increased IL-4 in splenocytes The frequencies of IFN-γ-producing CD4+ T cells increased, IL-4-producing CD4+ T cells decreased and the proportion increased dramatically after colitis induced by TNBS (Table 4). Cur decreased the frequencies of IFN-γ-producing CD4+ T cells and the proportion and increased IL-4-producing CD4+ T cells. Frequencies of IFN-γ-producing CD4+ T cells decreased in the Dex-treated group, frequencies of IL-4-producing CD4+ T cells increased slightly but show no difference from the TNBS group. The proportion decreased in the Dex-treated group, but was higher than that of the Con group. The effect of Cur+Dex was similar to that of Dex.
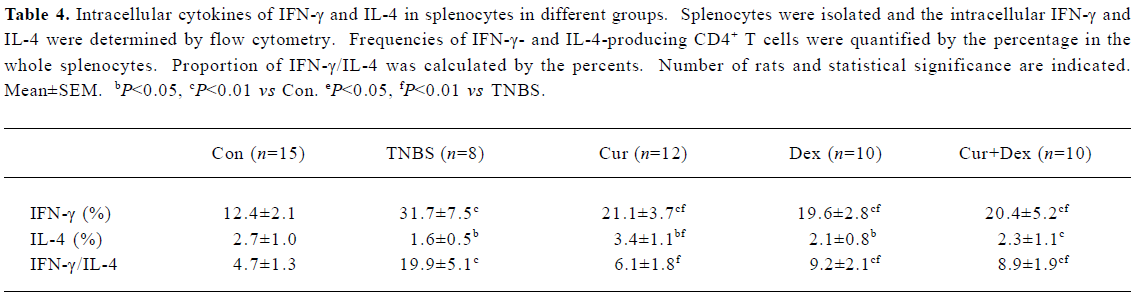
Full table
Cur therapy decreased IFN-γ and increased IL-4 in circulation Compared to the Con group, the concentrations of IFN-γ increased and IL-4 decreased, thus, the proportion of IFN-γ/IL-4 increased significantly in the TNBS group (Table 5). Treatment with Cur could dramaticly decrease the concentration of IFN-γ, increase that of IL-4 and decrease the proportion significantly (P<0.01). Dex and Cur+Dex could decrease IFN-γ but did not show the same effects on IL-4 and the proportion.
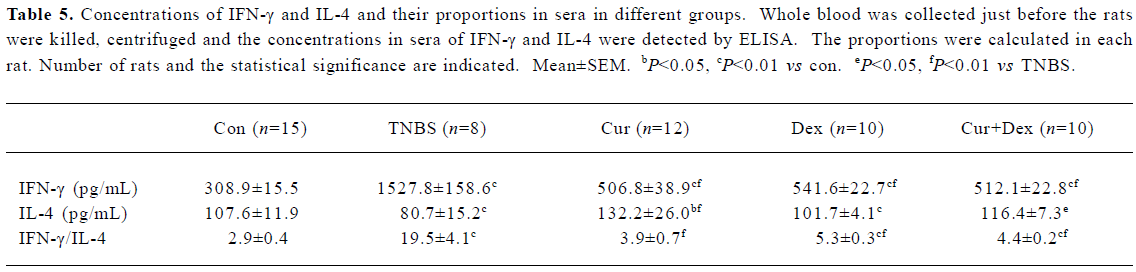
Full table
Discussion
The results of the present study indicate that Cur could (1) reduce body weight loss in colitis induced by TNBS; (2) decrease macroscopic scores and MPO activity; (3) decrease the mRNA of Th1 cytokines; and (4) increase the mRNA of Th2 cytokines and decrease the proportion of IFN-γ/IL-4. Dex exerted similar effects on the first 3 findings, but not on the last one.
To control the precise dosage and to compare it with Dex, which usually administered by injection, Cur and Dex were given to animals separately or combined intra-peritoneally. Both Cur and Dex increased body weight, but it was much slower than reported in the findings of Sugimoto et al[10]. The reason for this might be because (1) mice and rats may have different sensitivity and endurance to TNBS; and (2) the different route of medication, especially if it is intraperitoneal as it was in our experiment, was traumatic to the animal. However, the concurrent conclusion is that treatment with Cur is capable of increasing the body weight in TNBS-induced colitis.
Administration of TNBS resulted in hyperaemia, edema, ulceration (some break through the whole wall) and adhesion. All of these changes were expressed as the elevation of the macroscopic score. MPO is an enzyme found in neutrophils, and its activity in the colon is linearly related to neutrophil infiltration. After colitis was induced by TNBS, the macroscopic score and MPO activity increased. Treatment with cur was found to decrease the macroscopic score, MPO activity and ameliorate histological images. Dex and Cur+Dex showed similar effects.
TNBS-induced colitis is an experimental model of Th1-like gut inflammation[7]. IL-12, the inducer of Th1 cytokines and an inhibitor of Th2 cytokines, plays an important role in TNBS-induced colitis[8,14,15]. Inhibiting IL-12 has been shown to prevent development and block the progression of Th1-mediated disease in experimental models of autoimmunity[16,17]. Our results, which are consistent with previously published reports[2,3,10,17], indicate that Cur could inhibit the production of IL-12 mRNA in colon mucosa, sequentially IFN-γ, IL-1β, TNF-α decreased and IL-4 increased in TNBS-induced colitis. Dex was found to inhibit IL-12 and also other Th1 cytokines. The effect of Cur+Dex was similar to that of Dex.
IL-4 is the inducer and the production of Th2 cells. After colitis was induced by TNBS, it decreased. Treatment with Cur could increase its level, which is consistent with the report of Ukil et al[11], but different from the findings in the study by Sugimoto et al[10]. Different durations of treatment with Cur may have resulted in the different findings in our study and that of Sugimoto et al. In their study, the animals were treated for only 1 week, but in our experiment all the animals received Cur for 2 weeks. However, the differences between the study of Sugimoto et al and that of Ukil et al should be explored in future. Kobayashi et al[4] reported on the contrary that Cur inhibited IL-4 and improved IL-2 (Th1 cytokine) in atopic asthmatics. The reason for this may be because their experiment focused on allergic diseases in which Th2 cytokines increased and Th1 cytokines decreased, whereas ours focused on Th1-mediated inflammatory diseases. These different results indicate that Cur might exert its pharmacological effects by different mechanisms. IL-10 also increased after the treatment with Cur. Dex induced the expression of IL-4, slightly to the level of the con group, even though it did not show differences from that of TNBS group. These results are consistent with the report of DeKruyff et al[18]. Combined Cur with Dex did not show additive or synergistic effect on the expression of Th2 cytokines.
The proportion of IFN-γ/IL-4 is reported as an important factor in regulating the shift of Th1/Th2[19]. The relative abundance of mRNA in colon mucosa is a local index of Th1/Th2. To determine the whole body balance of Th1/Th2, the intracellular concentrations of IFN-γ and IL-4 in splenocytes and in circulation were detected. The results in splenocytes and in circulation were similar. Increased IFN-γ and the proportion and decreased IL-4 were observed in the TNBS group. Treatment with Cur was found to significantly decrease IFN-γ and increase IL-4, thus the proportion decreased markedly to the level of the Con group. Treatment with Dex decreased IFN-γ significantly and slightly increased IL-4; the proportion also decreased, although it was higher than that of the Con group. Contrary to our results, Xie et al[20] reported that Dex decreased IL-4 and increased IFN-γ, thus the proportion of IFN-γ/IL-4 increased. The possible reason for this difference may be because that experiment, similar to that of Kobayashi et al[4], focused on the allergic asthmatics model, different dosages were administered (0.5 mg·kg-1·d-1 vs 2 mg·kg-1·d-1), and the course of treatment differed (6 d vs 14 d). Cur+Dex showed similar effects as Dex alone. All of these results were similar to the results of the colon mucosa.
Our unpublished data indicates that treatment with Cur can significantly increase the concentration of prostaglandin E2 (PGE2) in colon mucosa, but Dex decreases it. PGE2, participated in epithelium repairing, has been implicated in the enhancement of Th2 responses by inhibiting the production of Th1 cytokines[21]. Thus, changes of cytokines in this experiment suggest that Cur regulates the shift from Th1 to Th2, possibly by inhibiting Th1 cytokines directly or indirectly by increasing PGE2 or by the two pathways simultaneously.
To investigate if Cur, the traditional Chinese medicine, shows additive and/or synergistic effects with Dex, the most commonly-used Western medicine, some rats were given the 2 agents simultaneously. Our results indicate that they do not show any additive and/or synergistic effects. The reason may be because of the long length of time (2 weeks) and high-dosage (2 mg·kg-1·d-1) delivery of Dex which resulted in the over-suppression of the immune system. Another reason may be that Cur and Dex acted on some common signal pathways. In addition to its anti-inflammation effects, Cur has been reported to have chemo-preventive characteristics in animal models of colon cancer[22,23]. One important clinical issue in the management of patients with long term IBD is a high risk for development of dysplasia and neoplasia. This manifests our belief that in acute phases of IBD, Dex is powerful, but in chronic phases, Cur may be more useful, not only in the control of inflammation and minimal side effects, but also in chemo-preventive activity.
In conclusion, we provide evidence that both Cur and Dex can attenuate TNBS-induced colitis. We also provide evidence that Cur can regulate the shift from Th1 to Th2 in TNBS-induced colitis. Even though it has previously been reported to suppress Th1 and improve Th2 cytokines[24], we found that Dex can also inhibit Th1 cytokines, but its effect on improving Th2 cytokines is not as powerful as Cur. Combined Cur and Dex did not show additive and/or synergistic effects. Thus, Cur may be a new agent in the clinical in the treatment of IBD.
References
- Ammon HPT, Wahl MA. Pharmacology of curcuma longa. Planta Med 1991;57:1-7.
- Kang BY, Song YJ, Kim KM, Choe YK, Hwang SY, Kim TS. Curcumin inhibits Th1 cytokine profile in CD4+ T cells by suppressing interleukin-12 production in macrophages. Br J Pharmacol 1999;128:380-4.
- Kang BY, Chung SW, Chung W, Im S, Hwang SY, Kim TS. Inhibition of interleukin-12 production in lipopolysaccharide-activated macrophages by curcumin. Eur J Pharmacol 1999;19:191-5.
- Kobayashi T, Hashimoto S, Horie T. Curcumin inhibition of dermatophagoides farinea-induced interleukin-5 (IL-5) and granulocyte macrophage-colony stimulating factor (GM-CSF) production by lymphocytes from bronchial asthmatics. Biochem Pharmacol 1997;54:819-24.
- Schmidt C, Stallmach A. Etiology and pathogenesis of inflammatory bowel disease. Minerva Gastroenterol Dietol 2005;51:127-45.
- Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology 1995;109:1344-67.
- Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, et al. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol 1996;157:2174-85.
- Tozawa K, Hanai H, Sugimoto K, Baba S, Sugimura H, Aoshi T, et al. Evidence for the critical role of interleukin-12 but not interferon-gamma in the pathogenesis of experimental colitis in mice. J Gastroenterol Hepatol 2003;18:578-87.
- Salh B, Assi K, Templeman V, Parhar K, Owen D, Gomez-Munoz A, et al. Curcumin attenuates DNB-induced murine colitis. Am J Physiol Gastrointest Liver Physiol 2003;285:G235-43.
- Sugimoto K, Hanai H, Tozawa K, Aoshi T, Uchijima M, Nagata T, et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 2002;123:1912-22.
- Ukil A, Maity S, Karmakar S, Datta N, Vedasiromoni JR, Das PK. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol 2003;139:209-18.
- Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989;96:795-803.
- Maisel A, Cesario D, Baird S, Rehman J, Haghighi P, Carter S. Experimental autoimmune myocarditis produced by adoptive transfer of splenocytes after myocardial infarction. Circ Res 1998;82:458-63.
- Egi H, Hayamizu K, Yoshimitsu M, Shimamoto F, Oishi K, Ohmori I, et al. Regulation of T helper type-1 immunity in hapten-induced colitis by host pretreatment with granulocyte colony-stimulating factor. Cytokine 2003;23:23-30.
- Colon AL, Menchen LA, Hurtado O, De Cristobal J, Lizasoain I, Leza JC, et al. Implication of TNF-alpha convertase (TACE/ADAM17) in inducible nitric oxide synthase expression and inflammation in an experimental model of colitis. Cytokine 2001;16:220-6.
- Caspi RR. IL-12 in autoimmunity. Clin Immunol Immunopathol 1998;88:4-13.
- Chandramohan N, John JB. Curcumin inhibits experimental allergic encephalomyelitis by blocking IL-12 signaling through janus kinase-STAT pathway in T lymphocytes. J Immunol 2002;169:6506-13.
- DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol 1998;160:2231-7.
- Cao W, Chen Y, Alkan S, Subramaniam A, Long F, Liu H, et al. Human T helper (Th) cell lineage commitment is not directly linked to the secretion of IFN-gamma or IL-4: characterization of Th cells isolated by FACS based on IFN-gamma and IL-4 secretion. Eur J Immunol 2005;35:2709-17.
- Xie QM, Chen JQ, Shen WH, Bian RL. Correlative changes of interferon-gamma and interleukin-4 between cortical layer and pulmonary airway of sensitized rats. Acta Pharmacol Sin 2002;23:248-52.
- Sarah GH, Josue P, Laura K, Denise R, Richard PP. Prostaglandins as modulators of immunity. Trends Immunol 2002;23:144-50.
- Pereira MA, Grubbs CJ, Barnes LH, Li H, Olson GR, Eto I, et al. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 1996;17:1305-11.
- Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, et al. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene 1999;18:6013-20.
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci 2004;1024:138-46.
