Induction of murine interleukin-1 beta expression by water-soluble components from Hericium erinaceum1
Introduction
Numerous studies have demonstrated that certain components present in dietary mushrooms have been responsible for the modulation of cellular and physiological changes in the host. It is for this reason that mushrooms are often used as cancer therapeutic agents[1–4]. Hericium erinaceum, a well-known traditional edible mushroom, contains valuable constituents including polysaccharides, lectins, proteins, lipids, hericenone, erinacol, erinacine, and terpenoids[5–7]. Recently these components, including water-soluble polysaccharides of H erinaceum, were isolated from its fruiting bodies and induced intriguing biological activities such as cytotoxicity, synthesis of a nerve growth factor, and antimicrobial function[6,8–11]. However, not much attention has been given to the elucidation of other immunological activities and therefore, limited data and references are available.
Interleukin (IL)-1 is a pluripotent and proinflammatory cytokine that orchestrates inflammatory and host-defense responses. Biologically active IL-1β is a 17.5-kDa protein resulting from cleavage of an inactive 31–34 kDa pro-IL-1β[12,13]. IL-1β augments T-cell responses to mitogens[14,15], indirectly activates B cells[16,17], increases expression of vascular adhesion molecules[18], and induces other proinflammatory cytokines and chemokines[19,20]. IL-1 is produced mainly by monocytes and macrophages when stimulated with various antigenic stimulants, including viruses or bacterial components such as lipopolysaccharide (LPS)[21–23]. Numerous studies have demonstrated that nuclear factor-kappa B (NF-κB), activator protein 1 (AP-1), nuclear factor interleukin-6 (NF-IL6), and cAMP response element (CRE)/activating transcription factor (ATF) regulate IL-1 transcription in macrophages upon stimulations[24–27].
Since IL-1 is a proinflammatory cytokine, agents that induce the activity of IL-1 have recently gained particular therapeutic and clinical interest[28–31]. In the present study, in order to characterize the immunological properties of H erinaceum, we prepared water extract from H erinaceum (WEHE) and investigated the inductive effect of WEHE on IL-1β expression in vitro.
Materials and methods
Reagents and chemicals LPS (from Salmonella typhosa) was obtained from Sigma-Aldrich (St Louis, Missouri, USA) and β-D-(1,3)-(1,6)-glucan from VPGmbH (Hergestellt, Germany). All reagents for RT-PCR were purchased from Promega (Madison, Wisconsin, USA), with the exception of recombinant Taq DNA polymerase (rTaq) and dNTP, which were purchased from Takara Bio Inc. (Otsu, Shiga, Japan). Rabbit anti-mouse IL-1β antibody and anti-actin antibody were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA) and Sigma-Aldrich, respectively. The mouse macrophage-like cell line RAW 264.7 (TIB-71) was purchased from the American Type Culture Collection (Manassas, Virginia, USA).
WEHE extraction, isolation and chemical analysis Dried mushrooms, H erinaceum, were obtained from the Korean Mushroom Corporation (Pochon, Korea). One hundred grams of the mushroom was washed several times with distilled water, soaked in 1.5 L of pyrogen-free water for 2 h, and then boiled for 2 h. Solid particles and aggregates were removed by centrifugation at 3000×g for 30 min and the supernatants were lyophilized. Finally, 26.35 g of the lyophilized water extract was obtained and used in this experiment. The general chemical composition of WEHE was analyzed in triplicate according to the methods of the Association of Official Analytical Chemists[32]. Analyses were conducted for moisture (AOAC method 930.15), crude protein (AOAC method 984.13), acid detergent fiber (AOAC method 962.09), ash (AOAC method 942.05) and ether extract (AOAC method 920.39). For the high-performance thin-layer chromatography (HPTLC) analysis, Interlucan 500 [β-D-(1,3)-(1,6)-glucan from Pharmode] and WEHE were dissolved in HPLC-grade methanol and applied to the pre-washed silica gel 60 F254 HPTLC plates (size 10×10 cm; thickness of the silica gel 0.2 mm; Merck, Darmstadt, Germany) with an automated applicator (Linomat IV, CAMAG, Merck KGaA, Germany). The samples were then separated (migration distance 75 mm) using HPLC-grade chloroform/methanol/water/formic acid (48:48:2:2). The migrated components were visualized at 254 nm using Reprostar 3 with a digital camera (CAMAG, Germany).
Culture of RAW 264.7 cells RAW 264.7 cells were cultured with Dulbecco’s modified Eagle’s medium (DMEM, Cellgro Mediatech, Herndon, Virginia, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, Utah, USA), 1×105 unit/L penicillin, and 100 mg/L streptomycin at 37 oC in a 5% CO2 humidified incubator.
Animals Specific pathogen-free 6-week-old C3H/HeJ mice were purchased from Charles River Japan Inc (Hino Breeding Center, Yokohama, Japan). On arrival, the randomized mice were transferred to cages (5 mice per cage) containing a saw dust bedding and quarantined for 1 week. The mice were given food (Purina Certified Lab Chow) and water ad libitum. Their thymocytes were isolated and used for IL-1 bioassay when their body weight reached 17 to 20 g. The temperature of the animal care facility was kept at 21–24 oC and 40%–60% relative humidity with a 12 h light/dark cycle. All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health.
IL-1 bioassay An IL-1 bioassay to determine functional IL-1 levels in media was performed as previously described[33]. Briefly, RAW 264.7 cells were plated at 5×105 cells/mL in 24-well culture plates and stimulated with 0, 0.1, 1, or 10 mg/L WEHE for 48 h. Indomethacin was added to prevent prostaglandin synthesis in the IL-1 assay. The culture media were harvested and assayed for the activity of IL-1 to induce proliferation of thymocytes isolated from the 6-week-old C3H/HeJ mice. The thymocytes (1.5×106) were cultured for 68 h with 10-fold dilutions of the IL-1-containing supernatants in the presence of phytohemagglutinin and then incubated with [3H]-thymidine for 4 h. The cultures were harvested onto glass filter paper and the [3H]-thymidine uptake was measured by scintillation counting.
RT-PCR RAW 264.7 cells (5×105 cells/mL, 10 mL) were treated with 0, 1, 5, or 10 mg/L of WEHE or 500 µg/L of LPS as a positive control for 3 h. The total RNA was isolated from each group of the cells using TRIzol reagent (InVitrogen, Carlsbad, California, USA) and equal amounts of RNA were reverse transcribed into cDNA with random hexamers (Promega Corporation, Madison, Wisconsin, USA). For PCR, the amplifications were performed in a total volume of 30 µL containing 0.5 units of rTaq and 10 pmol of primers specific to murine IL-1β (5'- AAGCTCTCACCTCAATGGA-3' and 5'-TGCTTGAGAGGTGCTGATGT-3') and β-actin (5'-GTG GGGCGCCCCAGGCACCA-3' and 5'-CTCCTTAATGTCA CGCACGATTTC-3'). The amplifications were performed for 25 cycles for β-actin and for 30 cycles for IL-1β. Equal volumes of RT-PCR products were separated on an agarose gel (1%) and visualized by ethidium bromide staining with a gel documentation system (Gel Doc 2000, Life Science Research, Hercules, CA, USA). Relative expression of the IL-1β to the β-actin control was quantitated using a densitometer with Multi gauge software (Fujiphoto Film Co Ltd, Tokyo, Japan).
Transient transfection of RAW 264.7 cells and CAT assay RAW 264.7 cells were transiently transfected with an IL-1β reporter gene construct pIL-1(870 bp)-CAT, which expresses CAT reporter genes solely regulated by the activity of the IL-1β promoter[33], using the DEAE-Dextran method as previously described[34]. The cells were then adjusted to 5×106 cells per 10 mL of the media, placed onto 100 mm plates, and incubated in a 5% CO2 humidified incubator at 37 oC for 24 h. The transfectants were treated with 2, 5 or 10 mg/L of WEHE. Eighteen hours later, the cells were washed with ice-cold PBS, resuspended in 0.25 mmol/L Tris (pH 7.8) and subjected to 3 cycles of freezing and thawing. The lysates were centrifuged (12 000×g for 10 min at 4 oC) and the supernatant was assayed for CAT activity by the TLC method[35]. The amount of radioactivity was determined by an image analyzer (Phosphor Imager, Molecular Dynamics, Sunnyvale, CA, USA) for quantitative analysis.
Western blotting RAW 264.7 cells (5×105 cells/mL, 10 mL) were placed onto a 100 mm tissue culture dish in DMEM supplemented with 10% FBS and antibiotics (1×105 unit/L penicillin and 100 mg/L streptomycin). Cells were treated with 0, 1, 5 or 10 mg/L of WEHE for 24 h. At the end of incubation, cells were washed once with PBS and lysed with RIPA buffer (Upstate Biotechnology, Lake Placid, NY, USA) as recommended by the manufacturer. Twenty micrograms of the whole-cell lysate were separated by 10% SDS-polyacrylamide gel electrophoresis and electro-transferred to a PVDF membrane (Millipore, Bedford, MA, USA). The membrane was incubated with a blocking buffer (5% BSA/1X TBS/0.1% Tween-20) at room temperature for 1 h and then was kept on ice overnight with the same buffer containing rabbit polyclonal antibodies against IL-1β or actin. After washing 3 times with TBS-T (1×TBS/0.1% Tween-20), the membrane was incubated with HRP-conjugated anti-rabbit IgG in the blocking buffer at room temperature for 1 h. Then, after washing 3 times with TBS-T, the immunoreactive bands were detected with enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ, USA).
Electrophoretic mobility shift assay (EMSA) The cells (5×106 cells/mL) were treated with 1, 5, or 10 mg/L of WEHE or 500 µg/L of LPS as a positive control for 90 min. Nuclear extracts were prepared as previously described[34]. Briefly, cells were lysed with hypotonic buffer (10 mmol/L Hepes, 1.5 mmol/L MgCl2, pH 7.5) and the nuclei were pelleted by centrifugation at 3000×g for 5 min. Nuclear lysis was performed using a hypertonic buffer (30 mmol/L Hepes, 1.5 mmol/L MgCl2, 450 mmol/L KCl, 0.3 mmol/L EDTA, 10% glycerol, 1 mmol/L dithiothreitol (DTT), 1 mmol/L phenylmethyl-sulfonyl fluoride (PMSF), and 1 mg/L each of aprotinin and leupeptin). Following lysis, the samples were centrifuged at 14 500×g for 20 min, and the supernatant was retained for use in the DNA binding assay. Double-stranded deoxyoligo nucleotides containing each consensus recognition site (in italics) of the NF-κB, AP-1, NF-IL6, and CRE/ATF are as follow: NF-κB; 5'-GATCTCAGAGGGGACTTTCCGAG AGA-3', AP-1; 5'-GATCTGCATGAGTCAGACACA-3', NF-IL6; 5'-GATCTACATGTTGTGCAACTTGCCTA-3', and CRE/ATF; 5'-CTCGAGAGAGATTGCCTGACGTCAGAGAGCTA GAGATCT-3'. The oligonucleotides were synthesized and end-labeled with [γ-32P]-dATP. Nuclear extracts (5 µg) were incubated with 1 μg poly (dI-dC) and the 32P-labeled DNA probe in the binding buffer (100 mmol/L KCl, 30 mmol/L Hepes, 1.5 mmol/L MgCl2, 0.3 mmol/L EDTA, 10% glycerol, 1 mmol/L DTT, 1 mmol/L PMSF, and 1 mg/L of each aprotinin and leupeptin) for 20 min at room temperature. Protein/DNA binding complexes were separated from free probe using a 4.8% polyacrylamide gel in 0.5×TBE (44.5 mmol/L Tris, 44.5 mmol/L boric acid, and 1 mmol/L EDTA). Following electrophoresis, the gel was dried and subjected to autoradiography.
Statistical analysis The mean±SD was determined for each treatment group in a given experiment. The treatment groups were compared to appropriate controls to find significant differences using a Dunnett’s two-tailed t-test.
Results
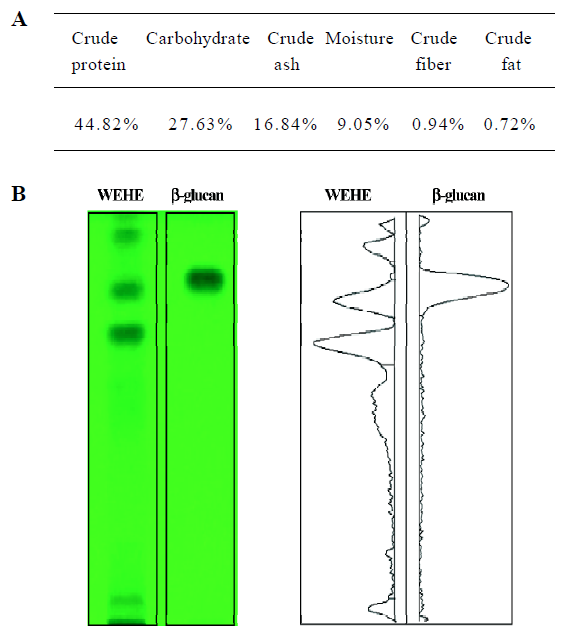
Basic chemical composition of WEHE The general chemical composition of WEHE showed a relatively higher concentration of crude protein (44.82%) and carbohydrate (27.63%) than other components (Figure 1A). Further analysis of carbohydrate using HPTLC showed that β-glucan was one of the major components of WEHE (Figure 1B).
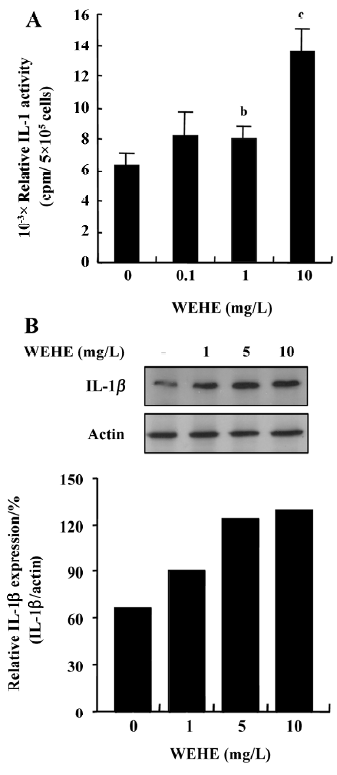
WEHE treatment augments IL-1 production in RAW 264.7 cells The effect of WEHE on IL-1 induction was examined in RAW 264.7 cells using bioassay. A significant (P<0.05) increase in the activity of IL-1 was observed when the cells were stimulated with 1 or 10 mg/L of WEHE (Figure 2A). The IL-1β protein expression was examined using Western blotting since the bioassay may have been interferred with other similar or closely related molecules although we added appropriate inhibitors. As shown in Figure 2B, the level of IL-1β in the macrophage cells was increased in proportion to the concentration of WEHE. Meanwhile, no change in the actin expression was observed, indicating specific induction of IL-1 in macrophages by WEHE. Cell viabilities in any of the WEHE treatment groups were not affected, in which live cells always exceeded 90% as determined by trypan blue staining (data not shown).
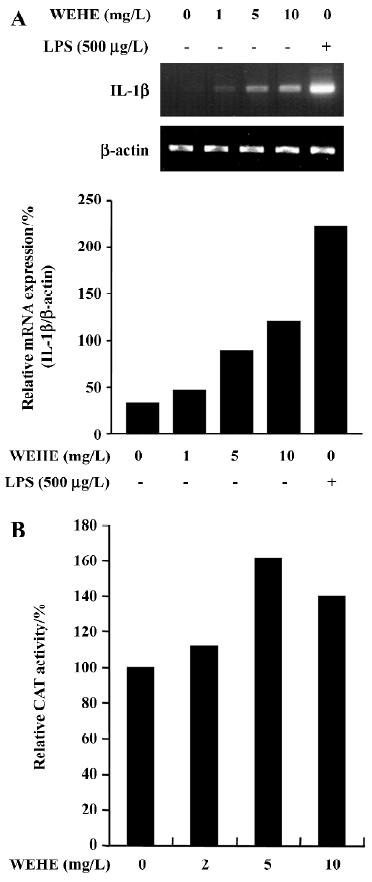
WEHE-mediated augmentation of IL-1β production was due to up-regulation of its transcription Next we examined the steady-state levels of mRNA encoding IL-1β in RAW 264.7 cells treated with WEHE. As shown in Figure 3A, the cells maintained in normal conditions expressed undetectable levels of IL-1β mRNA, whereas the level of expression dramatically increased when the cells were stimulated with 500 µg/L of LPS, which served as a positive control. It is evident that WEHE induced a dose-dependent increase of IL-1β mRNA. Since the enhancement of IL-1β transcripts could be due to either increased stability of IL-1β mRNA or up-regulation of IL-1β transcription, we performed an in vitro transfection assay with a reporter gene, pIL-1(870bp)-CAT, where the expression of CAT is regulated by IL-1β promoter. As shown in Figure 3B, the activity of IL-1β promoter increased in cells treated with WEHE compared to the negative control.
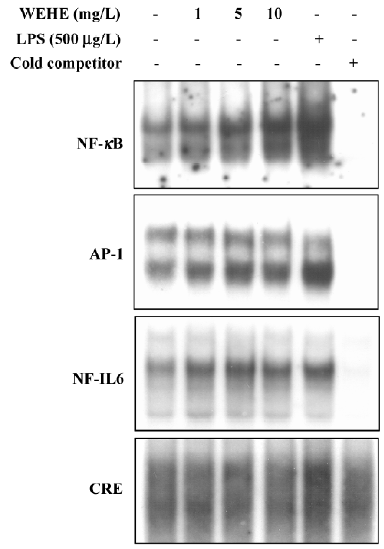
Positive regulation of transcription factors, NF-κB, AP-1, and NF-IL6 by WEHE We have further investigated the mechanism of WEHE for the transcriptional activation of the IL-1β gene. DNA-binding activity of transcription factors, NF-κB, NF-IL6, AP-1, and CRE/ATF, all of which have been known to exist on the promoter sequence of IL-1β and contribute to its transcriptional regulation, are examined using EMSA. As shown in Figure 4, the DNA-binding ability of NF-κB increased in the cells upon exposure to WEHE in a dose-dependent manner. It was further evident that the DNA-binding activities of NF-IL6 and AP-1 also increased, although the effects were less dramatic than NF-κB. However, no change was observed in the DNA binding activity of CRE/ATF to its cognate DNA recognition sequences (Figure 4).
Discussion
There is increasing interest in the use of mushrooms and mushroom extracts, not only as dietary supplements, but also as therapeutic supplements based on theories and findings, with a paucity of data, which indicate that they modulate immune function. In the present study, WEHE induced the secretion of IL-1β and its mRNA expression in murine macrophages. Although some other possibilities cannot be excluded, our study indicates that WEHE induction of IL-1β expression in murine macrophage might be primarily due to the up-regulation of IL-1β mRNA as determined by the in vitro CAT reporter gene assay. This is further supported by the fact that WEHE induced activation of responsible transcription factors such as NF-κB, AP-1 and NF-IL6, leading to an increase in IL-1β transcription.
IL-1 represents a potent inflammatory cytokine with numerous biological activities that regulate host defense and immune responses[12,18]. In addition to the role of IL-1 as an important immunoregulator, it also appears to be involved in anticancer activity. IL-1 has a suppressive effect on the proliferation of human prostate cancer[36], ovarian cancer[37], and breast cancer[38]. Conversely, a strong carcinogen, 2-acetylaminofluorene inhibits IL-1β expression[25]. Together with these aforementioned reports, our findings on the inductive effect of WEHE on the expression of IL-1 could give an important insight into the explanation of a possible mechanism by which polysaccharides isolated from H erinaceum produce antitumor activities[11].
We further demonstrated that WEHE stimulating IL-1β expression in murine macrophages was due to the up-regulation of transcription factors, especially NF-κB, where the changes of NF-κB were superior to other transcription factors. It is well established that the activation of NF-κB is strictly regulated by the binding of its inhibitor, IκBα[39]. Phosphorylation and subsequent proteasomal degradation of IκBα disrupts the IκBα-NF-κB complex, thus allowing for the translocation of activated NF-κB into the nucleus[39–42]. In the nucleus, NF-κB binds onto its consensus sequence on the promoter regions of various genes, including IL-1β, TNF-α, IL-6, and iNOS[40,43]. Therefore, it is very likely that the expression of not only IL-1β, but also other proinflammatory mediators, could be affected by WEHE through a similar mechanism to those observed in this study.
In conclusion, the present study suggests that WEHE has a stimulatory effect on IL-1 production in macrophages via activation of transcription factors such as NF-κB. This study could be valuable in that WEHE might have therapeutic potential, although further examination is necessary in terms of efficacy in animal studies and safety, including adverse effects.
References
- Zaidman BZ, Yassin M, Mahajna J, Wasser SP. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl Microbiol Biotechnol 2005;67:453-68.
- Carrizo ME, Capaldi S, Perduca M, Irazoqui FJ, Nores GA, Monaco HL. The antineoplastic lectin of the common edible mushroom (Agaricus bisporus) has two binding sites, each specific for a different configuration at a single epimeric hydroxyl. J Biol Chem 2005;280:10614-23.
- deVere White RW, Hackman RM, Soares SE, Beckett LA, Sun B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology 2002;60:640-4.
- Chang R. Functional properties of edible mushrooms. Nutr Rev 1996;54:S91-3.
- Ko HG, Park SH, Kim SH, Park HG, Park WM. Detection and recovery of hydrolytic enzymes from spent compost of four mushroom species. Folia Microbiol (Praha) 2005;50:103-6.
- Wang HX, Ng TB. A new laccase from dried fruiting bodies of the monkey head mushroom Hericium erinaceum. Biochem Biophys Res Commun 2004;322:17-21.
- Kawagishi H, Mori H, Uno A, Kimura A, Chiba S. A sialic acid-binding lectin from the mushroom Hericium erinaceum. FEBS Lett 1994;340:56-8.
- Nakatsugawa M, Takahashi H, Takezawa C, Nakajima K, Harada K, Sugawara Y, et al. Hericium erinaceum (yamabushitake) extract-induced acute respiratory distress syndrome monitored by serum surfactant proteins. Intern Med 2003;42:1219-22.
- Lee EW, Shizuki K, Hosokawa S, Suzuki M, Suganuma H, Inakuma T, et al. Two novel diterpenoids, erinacines H and I from the mycelia of Hericium erinaceum. Biosci Biotechnol Biochem 2000;64:2402-5.
- Kenmoku H, Shimai T, Toyomasu T, Kato N, Sassa T. Erinacine Q, a new erinacine from Hericium erinaceum, and its biosynthetic route to erinacine C in the basidiomycete. Biosci Biotechnol Biochem 2002;66:571-5.
- Mizuno T, Wasa T, Ito H, Suzuki C, Ukai N. Antitumor-active polysaccharides isolated from the fruiting body of Hericium erinaceum, an edible and medicinal mushroom called yamabushitake or houtou. Biosci Biotechnol Biochem 1992;56:347-8.
- Rossi B. IL-1 transduction signals. Eur Cytokine Netw 1993;4:181-7.
- Dayer JM. Evidence for the biological modulation of IL-1 activity: the role of IL-1Ra. Clin Exp Rheumatol 2002;20:S14-20.
- Khoruts A, Osness RE, Jenkins MK. IL-1 acts on antigen-presenting cells to enhance the in vivo proliferation of antigen-stimulated naive CD4 T cells via a CD28-dependent mechanism that does not involve increased expression of CD28 ligands. Eur J Immunol 2004;34:1085-90.
- Wesa A, Galy A. Increased production of pro-inflammatory cytokines and enhanced T cell responses after activation of human dendritic cells with IL-1 and CD40 ligand. BMC Immunol 2002;3:14.
- Nakae S, Asano M, Horai R, Sakaguchi N, Iwakura Y. IL-1 enhances T cell-dependent antibody production through induction of CD40 ligand and OX40 on T cells. J Immunol 2001;167:90-7.
- Chelvarajan RL, Gilbert NL, Bondada S. Neonatal murine B lymphocytes respond to polysaccharide antigens in the presence of IL-1 and IL-6. J Immunol 1998;161:3315-24.
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol 2002;20:S1-13.
- Calkins CM, Bensard DD, Shames BD, Pulido EJ, Abraham E, Fernandez N, et al. IL-1 regulates in vivo C-X-C chemokine induction and neutrophil sequestration following endotoxemia. J Endotoxin Res 2002;8:59-67.
- Yamada T, Fujieda S, Yanagi S, Yamamura H, Inatome R, Yamamoto H, et al. IL-1 induced chemokine production through the association of Syk with TNF receptor-associated factor-6 in nasal fibroblast lines. J Immunol 2001;167:283-8.
- Furutani Y. Molecular studies on interleukin-1 alpha. Eur Cytokine Netw 1994;5:533-8.
- Uhing RJ, Adams DO. Molecular events in the activation of murine macrophages. Agents Actions 1989;26:9-14.
- Gao B, Tsan MF. Induction of cytokines by heat shock proteins and endotoxin in murine macrophages. Biochem Biophys Res Commun 2004;317:1149-54.
- Schilling D, Beissert T, Fenton MJ, Nixdorff K. Negative regulation of IL-1beta production at the level of transcription in macrophages stimulated with LPS. Cytokine 2001;16:51-61.
- Kang JS, Jeon YJ, Suh J, Park SK, Yang KH, Kim HM. 2-Acetylaminofluorene inhibits interleukin-1beta production in LPS-stimulated macrophages by blocking NF-kappaB/Rel activation. Cancer Lett 2004;203:91-8.
- Goto M, Katayama KI, Shirakawa F, Tanaka I. Involvement of NF-kappaB p50/p65 heterodimer in activation of the human pro-interleukin-1beta gene at two subregions of the upstream enhancer element. Cytokine 1999;11:16-28.
- Muller JM, Ziegler-Heitbrock HW, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology 1993;187:233-56.
- Yang H, Tuzun E, Alagappan D, Yu X, Scott BG, Ischenko A, et al. IL-1 Receptor antagonist-mediated therapeutic effect in murine myasthenia gravis is associated with suppressed serum proinflammatory cytokines, C3, and anti-acetylcholine receptor IgG1. J Immunol 2005;175:2018-25.
- Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med 2005;201:1355-9.
- Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 2005;201:1479-86.
- Braddock M, Quinn A. Targeting IL-1 in inflammatory disease: new opportunities for therapeutic intervention. Nat Rev Drug Discov 2004;3:330-9.
- Association of Official Analytical Chemists. 15th ed. Washington, DC: Official Methods of Analysis; 1990.
- Jeon YJ, Han SH, Lee YW, Lee M, Yang KH, Kim HM. Dexamethasone inhibits IL-1 beta gene expression in LPS-stimulated RAW 264.7 cells by blocking NF-kappa B/Rel and AP-1 activation. Immunopharmacology 2000;48:173-83.
- Han SH, Yea SS, Jeon YJ, Yang KH, Kaminski NE. Transforming growth factor-beta 1 (TGF-beta1) promotes IL-2 mRNA expression through the up-regulation of NF-kappaB, AP-1 and NF-AT in EL4 cells. J Pharmacol Exp Ther 1998;287:1105-12.
- Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol 1982;2:1044-51.
- Hsieh TC, Chiao JW. Growth modulation of human prostatic cancer cells by interleukin-1 and interleukin-1 receptor antagonist. Cancer Lett 1995;95:119-23.
- Kilian PL, Kaffka KL, Biondi DA, Lipman JM, Benjamin WR, Feldman D, et al. Antiproliferative effect of interleukin-1 on human ovarian carcinoma cell line (NIH: OVCAR-3). Cancer Res 1991;51:1823-8.
- Paciotti GF, Tamarkin L. Interleukin-1 directly regulates hormone-dependent human breast cancer cell proliferation in vitro. Mol Endocrinol 1988;2:459-64.
- Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol 2000;12:85-98.
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 1994;12:141-79.
- Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol 1994;10:405-55.
- May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today 1998;19:80-8.
- Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol 2001;166:3873-81.
