Antagonist peptides of human interferon-α2b isolated from phage display library inhibit interferon induced antiviral activity
Introduction
Human type I interferons (IFN), including IFN-α, -β, -τ and –ω, are cytokines best known for their antiviral activities[1]. IFN binding to its receptor triggers a cascade of events, activating a number of proteins that inhibit viral replication and cell growth and control apoptosis[2]. IFN-α and -β bind to a common cell-surface receptor comprised of IFNAR1 and IFNAR2. IFNAR2 is the major ligand binding component of the receptor complex, exhibiting nanomolar affinity to both IFN-α and -β subtypes[2]. The IFN-α/β signal is transduced by the induction of specific intracellular responses mediated by tyrosine phosphorylation of janus kinases (JAK). The activation of these kinases induces phosphorylation of the cytoplasmic tail of the receptor itself, which lacks intrinsic kinase activity. The subsequent phosphorylation of signal transducer and activator of transcription (STAT) factors and activation of the transcriptional activator IFN-α-stimulated gene factor3 (ISGF3), resulting in the induction of an antiviral state[1].
Suppression of any one of these steps would inhibit the IFN-induced antiviral activity. So far, several interferon antagonists have been identified including the Newcastle disease virus V protein[3], which blocks the antiviral functions of IFN-α by targeting STAT1 for degradation. Another example is the sarcolectin[4], an interferon antagonist extracted from hamster sarcomas and normal muscles, which has no direct action on IFN molecules and does not compete with it for specific cell membrane receptors, and could act by triggering or enhancing the synthesis of an IFN regulatory protein, which restores virus sensitivity in the cells. The vasoactive intestinal peptide[5] is another interferon antagonist, which inhibits Jak1-2 and STAT1 phosphorylation, resulting in the downregulation of IFN-induced gene expression. Not only do the antagonists block the IFN intracellular signaling but they also suppress IFN binding to its receptor, such as neutralizing antibodies. It has been reported that some patients treated with IFN have produced neutralizing antibodies inhibiting the antiviral activity of IFN[6]. Pervious studies have shown that IFN-α was able to induce the expression of MHC (major histocompatibility complex) class I antigens, and II antigens, both of which had been linked genetically and functionally to autoimmune diseases. There appears to be a correlation between these diseases and increased expression of IFN-α. Thus, IFN-α antagonists may be therapeutic candidates for autoimmune diseases[7,8].
The screening of phage-displayed libraries is a powerful technique for identifying peptides with desirable biological or physical properties, particularly when it is combined with iterative cycles of phage selection and amplification[9]. New agonists and antagonists of cell membrane receptors have been successfully identified using this process[10], and examples include the RGD(Arg-Gly-Asp)-containing peptides that bind to specific cell surface integrins and inhibit integrin-mediated cell adhesion[11,12]. Peptide display libraries have also been used to derive a peptide-(ATWLPPR), which specifically inhibited human endothelial cell proliferation in vitro and totally abolished VEGF(vascular endothelial growth factor) induced angiogenesis in vivo[13]. Thus, phage-displayed technology has been shown to be effective to the identification of novel peptides that may inhibit cell adhesion.
In this study, we attempted to identify IFN-α2b antagonist peptides that might block the binding of IFN-α2b to its receptor and be helpful in laying the foundation for the molecular mechanism of the interaction between IFN and its receptor.
Materials and methods
Cell lines WISH cells and VSV(vesicular stomatitis virus) viruses were kindly provided by Hualida Co (Tianjin, China). The WISH cells, naturally carrying the type I IFN receptors on the cell surface, were cultured in RPMI-1640 (Roswell Park Memorial Institute) medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 1×105 U/L of penicillin and 0.1 g/L of streptomycin.
Phage peptide library and bacterial strain The heptapeptide library [1.5×1016 plaque forming units (pfu)/L] and the host bacterial strain E coli ER2738 were purchased from New England Biolabs (Beverly, MA, USA).
Interferon, antibodies and other reagents Standard IFN-α 2b was kindly provided by Hualida and had a specific activity of 1.0×1011 U/g protein. Anti-IFN-α2b antibodies were prepared as outlined by Tian et al[14]. Wild-type M13 phage and horseradish peroxidase (HRP)-conjugated anti-M13 phage antibody were purchased from Pharmacia Biotech (Uppsala, Sweden). Vector pET32a(+) was purchased from Novagen Co LTD (Darmstadt, Germany). Vector pEGFPCII containing cDNA coding green fluorescence protein (GFP) was a kind gift from Prof Xiao-dong ZHANG (College of Life Sciences, Nankai University, Tianjin, China). O-Phenylenediamine (OPD) and diaminobenzidine (DAB) were purchased from Sigma (St Louis, MO, USA). Other chemicals used in this study were of analytical grade and commercially available.
Phage selection with WISH cells and polyclonal anti-IFN-α2b antibodies Selection procedure was based on the ph.D-7 kit standard procedure (New England Biolabs, Beverly, MA) with some modifications. The exponentially growing WISH cells were fixed on 96-well culture plates (~2.0×105 cells/well; Nunc, Roskilde, Denmark) with glutaraldehyde (0.25% final concentration). Phages of approximately 2.0×1010 pfu were preincubated for 1 h with blocking buffer [5 g/L bovine serum albumin (BSA), 0.1 mol/L NaHCO3] and then were transferred to the 96-well culture plate and incubated for 2 h at room temperature. Unbound phage particles were removed by washing with 0.1%TBST (0.05 mol/L Tris-HCl, pH 7.5, 0.15 mol/L NaCl, 0.1% Tween 20). Cell-bound phages were eluted for 10 min with 0.001g/L IFN-α2b. The eluted phages were replicated by infecting E coli ER2738 cells. The amplified phage particles were purified using polyethyleneglycol (PEG), and then used for the subsequent round of selection with WISH cells.
For the selection with antibodies, all the steps were the same as described for the selection with WISH cells except for the fact that the 96-well plates were coated with polyclonal anti-IFN-α2b antibodies (0.01g/L in 0.1 mol/L NaHCO3, pH 8.6).
Phage ELISA WISH cells naturally carrying type I IFN receptor on the cell surfaces were used to select IFN receptor-binding peptides from a phage displayed library that was described as phage ELISA A method. The exponentially growing WISH cells were fixed on 96-well culture plates (~2.0×105 cells/well) with glutaraldehyde (0.25% final concentration). Approximately 2×1011 pfu amplified phages (or mixed with0.01g/L IFN-α2b) were added to each well, and then incubated with the cells for 2 h at room temperature. The wells were treated with five 3-min washes with 0.1% TBST and the amount of bound phages was detected with horseradish peroxidase (HRP)-conjugated anti-M13 phage antibody. The development was performed by the addition of OPD, and read at 490 nm in an ELISA Reader (Bio Rad). Original phage peptide library without selection was used as the negative control.
For detecting the binding reactivity of phage clones to antibodies with phage ELISA B method, all the steps were the same as the phage ELISA A method except that the 96-well plates were coated with polyclonal anti-IFN-α2b antibodies (0.01g/L in 0.1 mol/L NaHCO3, pH 8.6).
Immunohistochemistry Exponentially growing WISH cells (~2.0×105 cells/well) were fixed on 96-well culture plates using glutaraldehyde and incubated with detective phage clones (about 1.0×1010 pfu) for 2 h at room temperature. The cells were washed five times with PBS and incubated with HRP-conjugated anti-M13 phage antibody at 37 ºC for 1 h. After washing five times with PBS, the wells were developed with DAB until brown. The DAB excess was washed away with water and photographed with an inverted microscope and digital camera.
Peptides sequencing and synthesis The single-stranded DNA (ssDNA) was prepared from identified phage clones as described in the phD-7 kit guidelines and sequenced by the Shanghai Sangon Company. The primer used for sequencing was -96pIII: 5’-CCCTCATAGTTAGCGTAACG-3’. Corresponding amino acid sequences were deduced from DNA sequences, and a multiple sequence alignment was done using BLAST software package (obtained from http://ncbi.nlm.nih.gov./BLAST) to determine the groups of related peptides. Two peptides corresponding to positive clones N
Cloning, expression and purification of GFP-IFN-α2b fusion protein This procedure has been described in a previous studies[15]. Briefly, the cDNA coding human IFN-α2b was obtained from peripheral blood mononuclear cells (PBMC) by reverse transcription-PCR and was then cloned into expression vector pET32a(+) to construct pET32a(+)/IFN-α2b. The cDNA coding GFP was digested from pEGFPCII and was cloned into pET32a(+)/IFN-α2b. Thus, the prokaryotic expression vector pET32a (+)/GFP-IFN-α2b was constructed and then transformed to E coli BL21. The expressed fusion protein after induction with isopropyl-beta-D-thiogalactopyranoside (IPTG) was purified by nickel chelation chromatography.
Competition assay of synthesized peptides Exponentially growing WISH cells were fixed approximately 2.0×105 cells per well on 96-well culture plates using glutaraldehyde. After blocking with 0.06 mol/L phosphate buffer (pH 7.4, containing 1% BSA and 0.1% NaN3) for 1 h at room temperature, 1 µg GFP-IFN-α2b was added to each well and incubated at 37 °C for 2 h. After washing five times with 0.1% TBST, synthetic peptides or IFN-α2b were added and incubated at 37 °C for 1 h with gentle shaking. The wells were then washed with 0.1% TBST again and observed with an inverted fluorescence microscope. Irrelative peptide 50 µg, (Lysine) 7, was used as a negative control.
Inhibition assay of antiviral activity The antiviral activity of IFN was determined in vitro by protection of human amnion WISH cells against VSV-induced cytopathic effects as described by following the traditional method[16]. In brief, 2.0×104 cells were seeded into each well of 96 well plates and incubated with two-fold serial dilutions of synthetic peptides samples [or (Lysine) 7 as a negative control] and IFN-α2b giving 50% protection of WISH cells for 18 h at 37 °C. After incubation, the cells were challenged with VSV and the plates were incubated at 37 °C for 24 h. Virus-induced cytopathic effects were assayed by the MTT method[17].
Results
Specific enrichment of positive phages In order to enrich IFNAR-binding phages from the phage display library, four rounds of selection with WISH cells were performed. The enrichment was determined by the use of the output/input ratio of phages after each round of selection. The ratio increased approximately 78-fold (from 4.5×10-7 to 3.5×10-5) after the second round of selection. After the third and the fourth rounds of selection, the output/input ratio of phages increased approximately 710-fold and 3700-fold respectively. When the library was screened with polyclonal antibodies, the output/input ratio of phages increased about 5-fold and 70-fold after the sixth and seventh rounds of selection, which indicates an obvious enrichment for the specific binding of phages to IFNAR-expressing cells and polyclonal antibodies against IFN-α2b (Table 1).
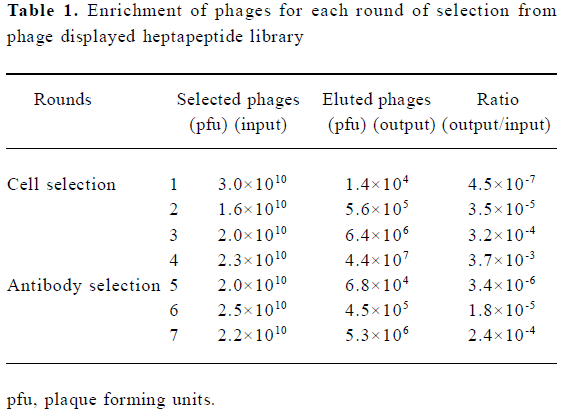
Full table
Identification of the positive phages Ninty six clones were picked out from the sample after the fourth round of selection with whole cells and the specificity was examined by phage ELISA A. Thirty-four of the 96 clones (35.4%) showed a binding ability to WISH cells, while the positive rate increased to 40/96 (41.6%) after the seventh round of selection with antibodies. In contrast, the original phage library cannot bind to WISH cells. Further testing demonstrated that 23 out of the 40 positive clones could be specifically competed by 1 µg IFN-α2b in the binding to WISH cells and anti-IFN antibodies using phage ELISA A and B method (Figure 1). These results indicated the enrichment for the specific binding of phages to IFNAR-carrying cells and polyclonal antibodies against IFN-α2b.
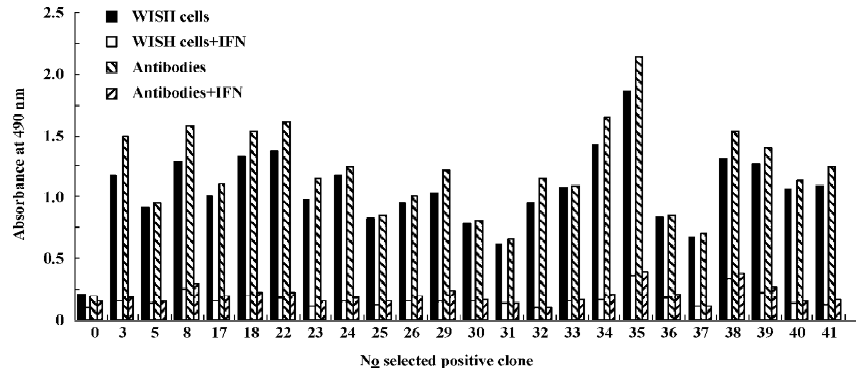
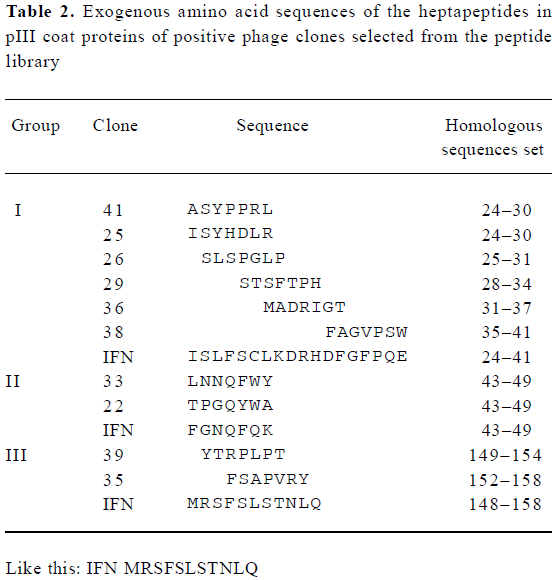
Analyses of exogenous sequences of positive phage clones The ssDNA was prepared from positive phage clones and sequenced, and the amino acid sequences of the mimetic peptides were then deduced from DNA sequences. Homologous analysis was performed to find an optimal alignment between the selected motifs and the primary sequence of IFN-α2b. The homologous analysis of amino acid sequences with IFN-α2b showed that 3 groups were obtained, which corresponds to three domains defined by residues (group I, 24–41; group II, 43–49; and group III, 148–158) of IFN-α2b (Table 2). It had been proposed that the AB loop (residues 26–35) and E helix (residues 144–153) are implicated in the interaction with receptors[18]. By comparing the homology with functional domain amino acid sequences of IFN-α2b and the absorbance at 490 nm, two clones N
Full table
Specificity of positive phage clones to IFNAR The interaction of the positive phages and WISH cells was detected by immunohistochemical staining. As a result, dark brown staining on the cell surfaces indicated that positive phage clones (N
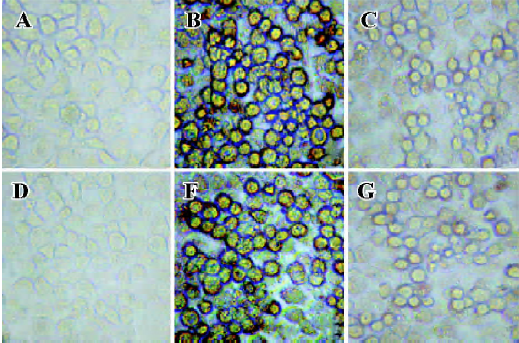
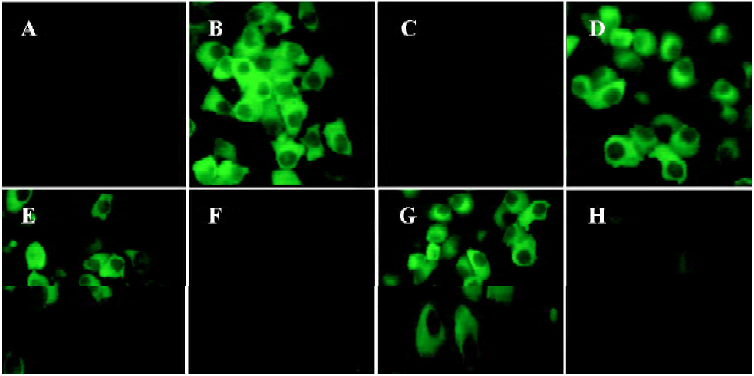
Inhibition reactivity of synthetic peptides to IFNAR The ability of the synthetic peptides, SP-7 and FY-7 to inhibit the binding of GFP-IFN-α2b to IFNAR was determined by competitive assay. The fluorescence reduction was almost not observed when 1 µg synthetic peptides (Figure 3E, 3G) added, while the fluorescence was obviously reduced or obsolescent in the conditions of 10 µg synthetic peptides (Figure 3F, 3H), equaling approximately 1 µg IFN-α2b (Figure 3C). This result revealed that the binding of IFN-α2b to WISH cells was inhibited by the two synthetic peptides.
Inhibition of IFN-induced antiviral activity by synthesis peptides It is well known that the binding of IFN to its receptor mediates the activation of different signal transduction pathways. Because peptides SP-7 and FY-7 have the ability to block the interaction between IFN and its receptor, we decided to test whether they might also suppress the antiviral activity mediated by IFN. As a result, when the added amount was from 6.25 µg to 100 µg, both mimetic peptides could inhibit the IFN-induced antiviral activity in a dose-dependent manner. The IC50 values of both peptides were approximately 25 µg (Figure 4).
Discussion
There is a growing interest in the development of active mimetic or synthetic peptides for IFN. For instance, a synthetic peptide (WLDPRH) was recognized by a neutralizing antibody, in the presence of which, the protective effects of suboptimal dose of IFN-α were increased[19]. Another example is the peptide mimetic of IFN-β isolated by phage-display screening also using a neutralizing monoclonal antibody (mAb), which elicits antiviral activity on cultured cells[20]. In contrast, there are no reports of the isolation of synthetic peptides for IFN that inhibit the antiviral activity as antagonists. We report here the identification of two IFN antagonist peptides by screening a phage-displayed peptide library.
To obtain peptides blocking the binding of IFN to its receptor, screening of a phage-display library with IFN receptor should be a straightforward strategy. However, there are some difficulties for membrane receptors to be purified and maintain the natural conformation. Previous studies have shown that screening with a soluble form of type I IFN receptor failed to identify any peptide ligands, while the use of a neutralizing anti-IFN-β mAb as a target resulted in the successful mimicry of IFN-β[20]. It is better to use whole native cells or target receptor gene transfected cells to select the ligands[13,21]. Whole cells usually make receptors maintain the native conformation with normal post-translational modification, so the ligands of the receptor can be selected even without information about the receptor[22].
In this study, we performed four rounds of biopanning against WISH cells carrying IFN receptors on the cell membranes. This resulted in the enrichment of IFN-receptor-specific binding clones from the phage library. The second step was then subjected for three rounds of selection with polyclonal anti-IFN antibodies, which resulted in the enrichment of phage clones binding both to IFN receptors and anti-IFN antibodies. Compared with screening only using monoclonal antibody, this strategy has the advantage of allowing us to obtain different epitopes of IFN simultaneously. The phage ELISA and immunohistochemistry staining showed that positive clones could bind specifically both to WISH cells and anti-IFN antibodies.
Ten out of the 23 positive clones were randomly picked out and sequenced. The results revealed that these sequences were divided into 3 groups, which correspond to three domains defined by residues of IFN-α2b (group I, 24–41; group II, 43–49; and group III, 148–158) (Table 2). It has been recently determined by X-ray crystallography that the IFN-α2b molecule is composed of five α-helices (A–E) linked by one long connection (AB loop) and three short segments (BC, CD and DE loops)[23]. Mutational studies have revealed the mutual binding sites on IFN-α2. On IFN-α2, IFNAR2 binds to the A helix (residues 12–15), the AB loop (residues 26–35), and the E helix (residues 144–153)[19,24], which induced antiviral activity. Therefore, it could be possible that peptides of group I are a structural mimic of discontinuous or conformational epitopes of the IFN AB loop, and those of group III might be E helix.
Clone N
Research has shown that the increased expression of IFN-α correlated with several autoimmune diseases such as insulin-dependent diabetes mellitus (IDDM) and systemic lupus erythematosis (SLE), but IFN antagonists could be candidates for treatment of IFN-induced diseases[25]. In this paper, we demonstrated that two IFN antagonist peptides, SP-7 and FY-7, which can compete with IFN for binding to its receptors, inhibit IFN-induced antiviral activity. We hope they might also partly reduce IFN-induced autoimmune diseases or side effects.
References
- Tyring SK. Interferons: biochemistry and mechanisms of action. Am J Obstet Gynecol 1995;172:1350-3.
- Jordan HC, Sabine RQ, Rina L, Gideon S, Jacob A. The human type I interferon receptor: NMR structure reveals the molecular basis of ligand binding. Structure 2003;11:791-802.
- Huang ZH, Sateesh K, Aruna P, Siba KS. Newcastle disease virus V protein is associated with viral pathogenesis and functions as an alpha interferon antagonist. J Virol 2003;77:8676-85.
- Pan HJ, Francoise CF, Brigitte RG, Madeleine S, Charles C. Sarcolectin: an interferon antagonist extracted from hamster sarcomas and normal muscles. J Biol Chem 1983;258:12361-7.
- Mario D. Inhibition of interferon (IFN) α-induced jak-STAT1 activation in microglia by vasoactive intestinal peptide. J Biol Chem 2003;278:27620-9.
- Antonelli G, Simeoni E, Bagnato F, Pozzilli C, Turriziani O, Tesoro R, et al. Further study on the specificity and incidence of neutralizing antibodies to interferon (IFN) in relapsing remitting multiple sclerosis patients treated with IFN beta-1a or IFN beta-1b. J Neurol Sci 1999;168:131-6.
- Maguire JE, Gresser I, Williams AH, Kielpinski GL, Colvin RB. Modulation of expression of MHC antigens in the kidneys of mice by murine interferon-alpha/beta. Transplantation 1990;49:130-4.
- Baldini L, Cortelezzi A, Polli N, Neri A, Nobili L, Maiolo AT, et al. Human recombinant interferon alpha-2C enhances the expression of class II HLA antigens on hairy cells. Blood 1986;67:458-64.
- Felici F, Luzzago A, Monaci P, Nicosia A, Sollazzo M, Traboni C. Peptide and protein display on the surface of filamentous bacteriophage. Biotechnol Annu Rev 1995;1:149-83.
- Cortese R, Monaci P, Luzzago A, Santini C, Bartoli F, Cortese I, et al. Selection of biologically active peptides by phage display of random peptide libraries. Curr Opin Biotechnol 1996;7:616-21.
- O’Neil KT, Hoess RH, Jackson SA, Ramachandran NS, Mousa SA, DeGrado WF. Identification of novel peptide antagonists for GPIIb/IIIa from a conformationally constrained phage peptide library. Proteins 1992;14:509-15.
- Koivunen E, Gay DA, Ruoslahti E. Selection of peptides binding to the alpha 5 beta 1 integrin from phage display library. J Biol Chem 1993;268:20205-10.
- Binetruy-Tournaire R, Demangel C, Malavaud B, Vassy R, Rouyre S, Kraemer M, et al. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J 2000;19:1525-33.
- Tian W, Li ZH, Cao XL, Yang WB, Bai G. Epitope mapping of interferon-α2b using phage random peptide library. Chin J Microbiol Immunol 2005;25:578-82. Chinese..
- Li ZH, Tian W, Yang WB, Bai G. Expression and characterization of GFP-IFNα2b fusion protein. Chin J Microbiol Immunol 2005;25:582. Chinese..
- Jacob P, Gideon S. Biophysical analysis of the interaction of human ifnar2 expressed in E coli with IFNα2. J Mol Biol 1999;289:57-67.
- Kazuho A, Norio M. Measurement of cellular 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci Res 2000;38:325-9.
- Piehler J, Roisman LC, Schreiber G. New structure and functional aspects of the type I interferon-receptor interaction revealed by comprehensive mutational analysis of the binding interface. J Biol Chem 2000;275:40425-33.
- Hu R, Ma XJ, Wei KK, Wang H, Qian ZC, Hou YD. Influence of the synthetic peptide recognized by neutralizing antibody on IFN-induced biological response. Chin J Exp Clin Virol 1999;13:29-32.
- Sato A, Sone S. A peptide mimetic of human interferon (IFN)-beta. Biochem J 2003;371:603-8.
- Szardenings M, Tornroth S, Mutulis F, Ruta M, Kari K, Arja K, et al. Phage display selection on whole cells yields a peptide specific for melanocortin receptor 1. J Biol Chem 1997;272:27943-8.
- Cao J, Zhao P, Miao XH, Zhao LJ, Xue LJ, Qi ZT. Phage display selection on whole cells yields a small peptide specific for HCV receptor human CD81. Cell Res 2003;13:473-9.
- Ramaswamy R, Leigh JW, Alan H, Paul R, Paul PT, Tattanahalli LN, et al. Zinc mediated dimmer of human interferon-α2b revealed by X-ray crystallography. Structure 1996;4:1453-63.
- Roisman LC, Piehler J, Trosset JY, Scheraga HA, Schreiber G. Structure of the interferon-receptor complex determined by distance constraints from double-mutant cycles and flexible docking. Proc Natl Acad Sci USA 2001;98:13231-6.
- Anan C, Jadine L, Huang XJ, Verna G, Kim KJ, Leonard GP, et al. Characterization and humanization of a monoclonal antibody that neutralizes human leukocyte interferon: a candidate therapeutic for IDDM and SLE. Cytokine 2001;15:250-60.
