Identification of some benproperine metabolites in humans and investigation of their antitussive effect1
Introduction
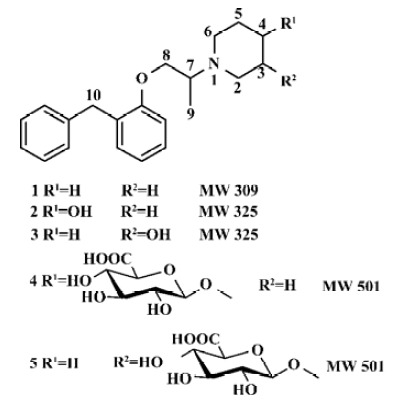
Benproperine, 1-[1-methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-piperidine (BPP, 1, Figure 1) is widely used as a cough suppressant for non-productive coughs. It has a peripheral and central action and can be given to humans po in forms of embonate or phosphate[1]. The antitussive activity is comparable to that of codeine, but is devoid of the undesirable codeine’s side effects[2].
In general, drugs are metabolized to more polar, hydrophilic entities, which can be excreted from the body more easily. At the same time, drugs can be inactivated, be activated or become a toxicant. There is a growing interest in identifying metabolites and in establishing their pharma-cokinetic, pharmacological, and toxicological properties. Activated metabolites are sometimes drug candidates for the treatment of a variety of diseases.
In our previous research, 5 mono-hydroxylated metabolites of benproperine and their conjugates were detected in human urine and 2 of them that were hydroxylated in phenyl rings have been identified. Mass spectra indicated that the other 2 mono-hydroxylates were probably hydroxylated in the piperidyl ring[3]. To identify metabolites, liquid chromatography-tandem mass spectrometry is an efficient approach, which is used widely[4–6]. A comparison of the high performance liquid chromatography (HPLC) retention times, as well as MS/MS spectra of putative metabolite and authentic standard, might be sufficient to make a more definitive identification[4].
The purposes of the present study was to synthesize 4 putative benproperine metabolites: 1-[1-methyl-2-[2-(phenyl-methyl)phenoxy]ethyl]-4-piperidinol (2), 1-[1-methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-3-piperidinol (3), as well as their glucuronides 1-O-[1-[1-methyl-2- [2-(phenylmethyl)phenoxy]ethyl]-piperidin-4-yl]-β-D-glucopyranosiduronic acid (4) and 1-O-[1- [1-methyl-2-[2-(phenylmethyl)phenoxy]-ethyl]-piperidin-3-yl]-β-D-glucopyranosiduronic acid (5) (Figure 1), to identify the chemical structures of the metabolites and to evaluate the antitussive effects of 2 and 3 phos-phates.
Materials and methods
Instruments and chemicals Melting points were determined in open capillary tubes and the thermometer was uncorrected. MS/MS spectra were recorded on a Finnigan LCQ ion trap mass spectrometer via an electrospray ionization (ESI) source in positive ion detection mode. 1H and 13C NMR spectra were recorded on a Bruker ARX-600 instrument with tetramethylsilane as an internal standard. Ultraviolet (UV) spectra were recorded on a Shimadzu UV-2201 instrument.
3-Piperidinol hydrochloride and 4-piperidinol were obtained from the Aldrich Chemical Co. We prepared 1-[2-(phenylmethyl)phenoxy]-2-propanol p-toluensulfonate (6) ourselves, according to published procedures[7,8], from o-benzylphenol, which was kindly supplied by Aosen Pharmaceutical Co. Methyl (2,3,4-tri-O-acetyl-1-O-trichloroaceti-midoyl)-α-D-glucopyranuronate (7) was prepared by us, according to Soliman et al[9] from D(+)-glucuronolactone, which was obtained from Wako Chemical Co. The structures of 6 and 7 were identified using 1H and 13C NMR. Column chromatography was carried out on silica gel BW-820MH, which was obtained from Fuji Silysia Chemical Co. Benproperine phosphate capsule was obtained from Shenyang Pharmaceutical Co. Benproperine phosphate was kindly supplied by Aosen Pharmaceutical Co and was recrystallized by us; the purity was 98.5% by HPLC. Citric acid was obtained from Shenyang Dongxing Reagent Factory. Test compounds and citric acid used to investigate antitussive were all dissolved in physiological saline. Methanol was of HPLC grade, and the other chemicals used were of analytical grade. Distilled water, prepared from demineralized water, was used in LC/MS/MS.
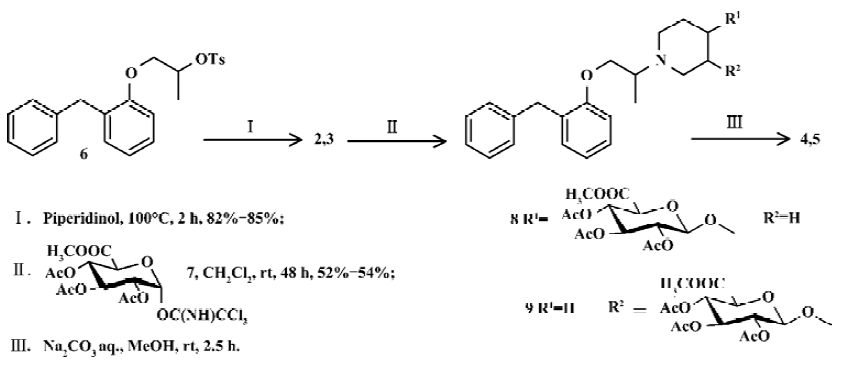
The putative metabolites were synthesized with the procedure shown in Figure 2.
1-[1-Methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-4-piperidinol (2) A total of 3.64 g (36.0 mmol) of 4-piperidinol and 3.6 g (9.0 mmol) of 1-[2-(phenylmethyl)phenoxy]-2-propanol p-toluensulfonate (6)[7,8] were fused on an oil bath at 100°C for 3 h, and stirred. After cooling to an ambient temperature, 15 mL of water was introduced. The resultant was extracted with dichloromethane (20 mL×3). The combined dichloromethane extracts were washed with water and brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, leaving a brown oil. The residue was purified using silica gel column chroma-tography, eluting with CHCl3/MeOH to leave the titled compound 2 (2.49 g, 85.3%) as a pale-yellow oil. The oil 2 (2.49 g, 0.77 mmol) was dissolved in an aqueous solution of 0.3 mol/L H3PO4 (25 mL) and the water was removed under reduced pressure. The residue was recrystallized from ethanol, leaving phosphate of 2 (2.60 g, 80.2%) as a colorless needle.
1-[1-Methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-3-piperidinol (3) 3-Piperidinol was treated as in the procedure described for 4-piperidinol, leaving 3 as a pale yellow oil (82.1%) and its phosphate of 3 as a white powder.
1-O-[1-[1-methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-piperidin-4-yl]-β-D-glucopyrano-siduronic acid (4) Phosphate of 2 (85 mg, 0.20 mmol) was suspended and stirred in CH2Cl2 (4.0 mL) at -15°C under N2 atmosphere. BF3 Et2O (10 μL) was added to in one portion. After 10 min of stirring, methyl (2,3,4-tri-O-acetyl-1-O-trichloroacetimidoyl)-α-D-glucopyranuronate (7) [9] (95 mg, 0.20 mmol) was added. Stirring was continued for 48 h at an ambient temperature. EtOAc (50 mL) was added, and the mixture was washed with saturated Na2CO3 aqueous solution (10 mL×2) and brine (10 mL), and dried over Na2SO4. The solution was evaporated and purified on silica gel column chromatography, eluted with n-C6H14-EtOAc-MeOH in gradient. 1-O-[1-[1-Methyl- 2-[2-(phenylmethyl)phenoxy]ethyl]-piperidin-4-yl]-2,3,4-tri-O-acetyl-β-D-glu-copyranosiduronic acid methyl ester (8, 65 mg, 50.4%) was obtained as a colorless syrup. Compound 8 was dissolved in MeOH (2 mL), and Na2CO3 aqueous solution (0.1 mol/L, 1 mL) was added at an ambient temperature and stirred. After 2.5 h of stirring, the mixture was desalted and concentrated. The titled compound 4 was obtained as a colorless syrup.
1-O-[1-[1-methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-piperidin-3-yl]-β-D-glucopyranosiduronic acid (3-OH-BPP glucuronide, 5) Phosphate of 3 was treated as in the procedure described above for phosphate of 2 to give the intermediate 9. Then, the titled compound 5 was obtained as a pale-yellow syrup after hydrolysis.
Urine sample Seven healthy male volunteers, whose mean (SD) age and weight were 22.7 (0.6) years and 62.2 (5.6) kg, gave written informed consent to take part in the study and local ethics approval was obtained. Each of the volunteers swallowed 3 benproperine phosphate capsules (60 mg BPP) with 200 mL of water. Urine samples were collected at 0 h−24 h. Blank urine was collected from the same volunteer directly before being given the capsules. The urine samples were stored at -20°C until analysis.
A 1.0 mL urine sample was filtered through 0.45 μm of precut membrane. The filter was applied to a 3.0-mL Bond-Elute C18 cartridge (0.5 g silica gel) preconditioned with 2 mL aliquots of methanol and water. After loading the sample, the column was washed with 1 mL of water. Metabolites were eluted with 1 mL of methanol. The eluate was evaporated to dryness at 40°C under a gentle stream of N2. The residue was dissolved in 100 μL of the mobile phase. A 20-μL aliquot of the resulting solution was injected onto the LC/MS/MS system for analysis.
A 1.0 mL portion of blank urine sample was treated as in the above procedure, in which the solutions of putative metabolites (2, 3, 4, or 5) in mobile phase were used instead of mobile phase to obtain the spiked urine sample for LC/MS/MS analysis.
LC/MS/MS analysis and identification of metabolites A Shimadzu LC-10AD pump was used in the LC/MS/MS system. Chromatography was performed on a Diamonsil C18 column (150×4.6 mm inner diameter, 5 μm, Dikma), which was coupled with a Security Guard C18 guard column (4×3.0 mm inner diameter, Phenomenex). The components were eluted with an isocratic mobile phase of methanol-water-formic acid (50:50:1, v:v:v), and the flow rate was 0.5 mL/min. The column temperature was maintained at 25 °C. A Finnigan LCQ ion trap mass spectrometer interfaced with liquid chromatography via an electrospray ionization (ESI) source was used for mass analysis in positive ion detection mode. The capillary voltage was fixed at 16 V, and its temperature was maintained at 200 °C. The spray voltage was set at 4.25 kV. The HPLC fluid was nebulized using N2 as both the sheath gas at a flow rate of 0.75 L/min, and the auxiliary gas at a flow rate of 0.15 L/min. The MS/MS spectra were produced by collision-induced dissociation (CID) of the selected precursor ions with He present in the mass analyzer, and the relative collision energy was set at 30%–40%. Data were collected and analyzed using the Navigator software (version 1.2).
Animals Dunkin Hartley guinea pigs of both sexes, weighing 200–300 g (Experimental Animal Center of Shen-yang Pharmaceutical University, Shenyang, China) were used. All animal studies were in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (China, 1988) and Implementing Regulations of the Administration on Medical Experiments on Animals (China, 1989). The guinea pigs were maintained in standard animal rooms, with food and water freely available, and on a natural light-dark cycle. They were allowed to adapt to the conditions for at least 1 week before being used in experi-ments.
Investigation of antitussive effect The antitussive effect of BPP, 2 and 3 phosphates was evaluated in conscious guinea-pigs against citric acid-induced coughs[10,11]. Guinea pigs were placed individually in a transparent plexiglass cylinder chamber (10 cm×10 cm×21 cm) and exposed to a nebulized solution of 7.5% citric acid for 3 min. An ultrasonic nebulizer (402 AI, Shanghai Yuyue Medical Facilities) was used to produce an aerosol with particles with an aerodynamic mass median diameter of 1 mm; the volume of solution aerosolized was approximately 0.6 mL/min. Animals were selected for the study according to the number of coughs observed 24 h before the test, and animals with more than 20 or fewer than 6 coughs during the 3 min test were not used. The compounds and the vehicle (physiological) saline were given ip (2 mL/kg) for 1.5 h (for BPP×H3PO4) or for 40 min (for 2×H3PO4 and 3×H3PO4) before the test. The number of coughs during the 3 min test and during the 5 min immediately after the test was determined. The animals with different doses (3 mg/kg, 9 mg/kg, 27 mg/kg for 2×H3PO4 and 3×H3PO4, 27 mg/kg for BPP×H3PO4) of drugs and the vehicle were grouped according to a random table. Animals were used only once because of tachyphylaxis of the cough response. Coughing sounds were recorded and amplified using a microphone and loudspeaker. During the experiment, the animals were continuously watched by a trained observer who was unaware of the treatment. Sneezes and coughs were differentiated by visual observation of the animals.
Statistical analysis Pharmacological results are represented as mean±SEM. Data were analyzed using one-way analysis of variance (ANOVA). Whenever ANOVA was significant, further comparisons between vehicle- and drug-treatment groups were performed using Dunnett’s t-test. The above analysis was performed using the software SPSS V11.0 for Windows.
Results
Chemical synthesis Compound 6, which was synthesized from 2-benzylphenol[8], was fused with 4-piperidinol to give 2 in 85% of yield. 3-Piperidinol was treated according to the procedure for 4-piperidinol to give 3 in 82% of yield. Glucuronides were synthesized from mono-hydroxylates (2 and 3) treated with trichloroacetimidate donor (7) followed by basic hydrolysis (Figure 2). The structures of products were identified using ESI-MS, 1H NMR, and 13C NMR (Table 1).
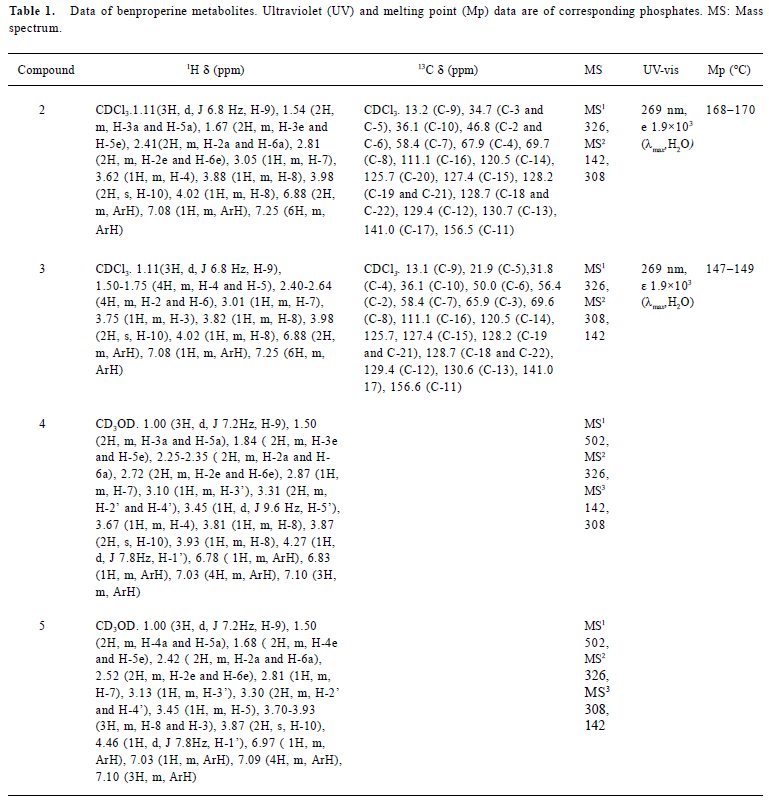
Full table
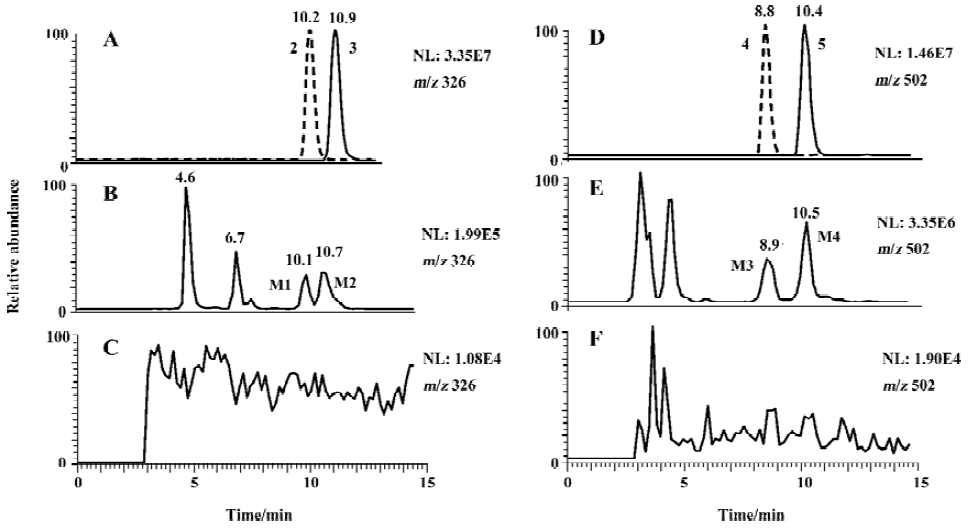
Metabolites of benproperine Compared with corresponding blank samples, the urine after being given of BPP showed 5 peaks corresponding to hydroxylated metabolites and 5 peaks corresponding to their glucuronides in LC/MS/MS analysis (Figure 3).
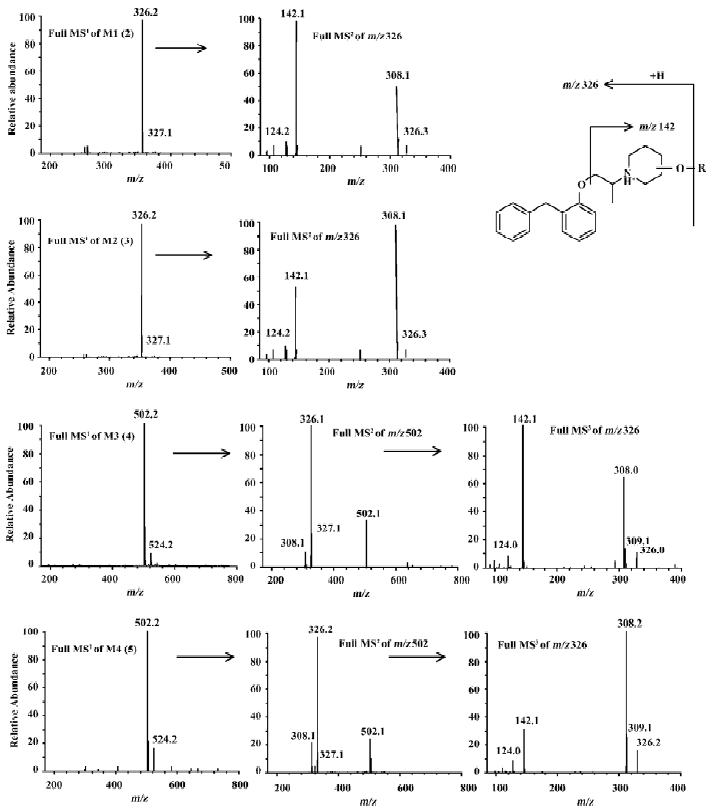
Metabolites M1 and M2 M1 and M2 gave the same pseudo-molecular ions [M+H]+ at m/z 326, similar MS/MS spectra (Figure 4), but different retention times (Figure 3), indicating that they were isomers. Their pseudo-molecular ions were 16 u higher than that of the parent drug, indicating the addition of a hydroxyl group to the parent drug.
The MS2 spectra of M1 and M2 displayed the same fragment ions at m/z 308 and m/z 142 (Figure 4). The presence of the prominent ion at m/z 308 was 18 u lower than precursor ions, indicating the loss of a water molecule from pseudo-molecular ions and further proving the presence of hydroxyl group. The presence of the ion at m/z 142 indicates that the hydroxyl group was in the piperidylpropanyl moiety. However, the MS/MS data did not provide useful information for assigning the exact site of hydroxylation. Therefore, M1 and M2 were definitely confirmed by comparing their retention time and mass spectra with synthesized 2 and 3 (Figures 3 and 4). M1 possessed the same retention time and mass spectra with 2, and was confirmed as 1-[1-methyl-2-[2-(phenylmethyl)-phenoxy]ethyl]-4-piperidinol and, in the same way, M2 was identified as 1-[1-methyl-2-[2- (phenylmethyl)phenoxy]-ethyl]-3-piperidinol.
Metabolites M3 and M4 M3 and M4 gave the same pseudo-molecular ions [M + H]+ at m/z 502, which were 176 u higher than monohydroxylate metabolites, indicating conjugation of a glucuronic acid. The MS2 spectra of M3 and M4 displayed same fragment ions at m/z 326, which possessed the same mass to charge ratio as the pseudo-molecular ions of M1 and M2. The MS3 spectra of M3 and M4 displayed the fragment ions at m/z 308 and m/z 142, which were consistent with MS2 spectra of M1 and M2, respectively (Figure 4). It indicated that M3 and M4 were the glucuronides of M1 and M2, respectively. The further confirmation was obtained by comparing their retention times and MS/MS spectra with the synthesized glucuronides 4 and 5 (Figure 3 and Figure 4). M3 was identified as 1-O-[1-[1-methyl-2-[2-(phenylmethyl)phenoxy]-ethyl]-piperidin-4-yl]-β-D-glucopyranosiduronic acid. In the same way, M4 was identified as 1-O-[1-[1-methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-piperidin-3-yl]-β-D-glucopyranosiduronic acid.
The peaks at 4.6 min and 6.7 min (Figure 3) corresponded to the two identified monohydroxylate metabolites[3]. Two peaks gave the same pseudo-molecular ions [M + H]+ at m/z 326. The MS/MS spectra showed the fragment ion at m/z 163 and 126 for the peak at 4.6 min and m/z 126 for the peak at 6.7 min, respectively.
Pharmacology Exposure to a nebulized solution of 7.5% citric acid aerosol caused coughing in control animals within 88.1±5.9 s (n=10), and both test compounds prolonged the latency of cough without dose-dependency as shown in Table 2. Immediately after exposure to citric acid, there is a hypersensitive period and the animals tend to cough continuously. For the control group, the number of coughs during the 3 min test was 8.2±1.2; all three compounds significantly decreased the number of coughs during the 3 min test, but 2 and 3 failed to decrease the number of coughs during the 5 min immediately after the test (Table 2).
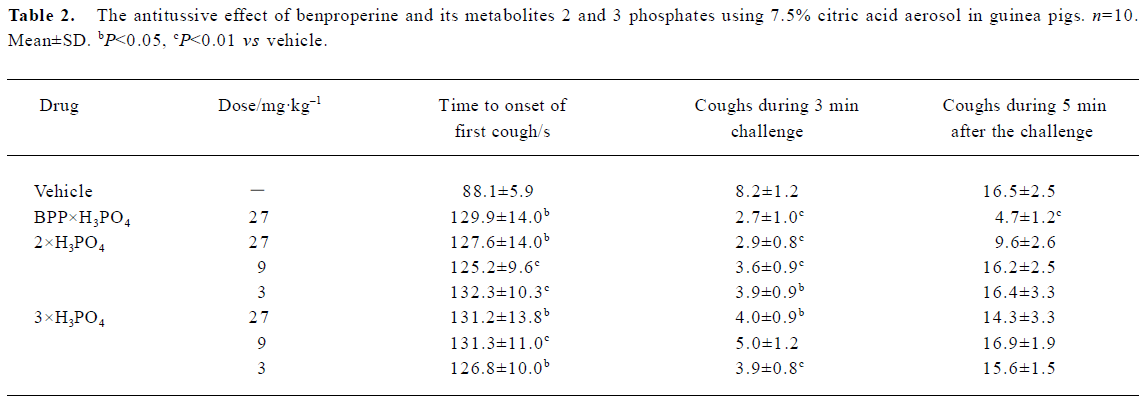
Full table
Discussion
In synthesis of putative metabolites 4 and 5, the form of basic acceptor, reaction promoter, and the order of addition are all important variables in glucuronidation. The strong electron-withdrawing character of the 6β-methoxycarbonyl group in any activated glucuronate makes such species notoriously poor donors[12], so that glucuronidation is difficult. The phosphate of 2 was treated with a trichloroacetimidate donor in the presence of BF3×Et2O to give protected glucuronide 4 in 52% of yield. However, in free form of 2, no desired product was obtained. The salt form of the basic acceptor was better than its free form for glucuronidation, with Lewis acid used as the promoter in the present study. When trimethysilyl trifluoromethane sulfonate (TMSOTf)[9] was used as a promoter instead of BF3×Et2O, more of the by-product, 2 acetylate, was obtained and the yield of the desired product was increased. An inverse addition, which means trichloroacetimidate 7 was added into the mixed solution of 2 phosphate and BF3×Et2O, improved the yield to 52% from 21%. This result is consistent with the prior report for glucuronidation of other compounds[13].
In positive-ion mode, compounds 2 and 3 formed pseudo-molecular ions [M+H] + at m/z 326. They are two isomers; each of them contains a hydroxyl group in the piperidyl ring and an ether bond. The same product ions were observed in the full scan of MS/MS spectra at m/z 308 and 142, with different relative abundance for 2 compounds. The fragment ion at m/z 142 was formed by cleavage of the ether bond (Figure 4). The fragment ion at m/z 308 was formed by dehydration, which was easier going in 3 than in 2 because p- π conjugated system was formed after dehydration in the former. Therefore, m/z 308 was the base peak for 3 and m/z 142 was the base peak for 2.
Hydroxylation and glucuronidation are 2 general pathways of drug metabolism, by which drug can be metabolized to more polar, hydrophilic entities, which can be excreted from the body more easily. The presence of M1 (2), M2 (3) and their glucuronides M3 (4) and M4 (5) is consistent with the general rule of metabolism. In human urine, some dihydroxylate metabolites besides monohydroxylate were detected; no dealkylation metabolite was detected. In the present study, 4 monohydroxylate metabolites and 2 glucuronide metabolites were identified. The chromatogram (Figure 3) indicates that the hydroxylated metabolites of benproperine were recovered in urine mainly as glucuronides, and in very low concentrations as free forms. M1 and M2 were also found in human plasma.
The free forms of compound 2 (M1) and 3 (M2) are oils, which are difficult to be weighed, and the water-solubility of the free forms are poor. They are not suitable for use in the study of antitussive activity. Benproperine is generally used in clinical treatments as dihydrogenphosphate. Therefore, we converted the free form of compounds 2 and 3 to their phosphates for pharmacological study.
The experimental model to induce coughing using citric acid is the model most frequently adopted and extensively studied in animals and in humans[14]. Citric acid can induce coughing in guinea pigs by increasing the volume of bronchial secretion[10], by acting on capsaicin sensitive sensory neurons[15,16] or by disturbing the pH of the airway surface liquid[17]. However, it induces the airway hyper-reactivity in guinea pigs[18]. Although phosphates of 2 and 3 produced an increase in the latency of the first cough and decreased the number of coughs during the 3 min test using citric acid, they did not decrease the number of coughs during the 5 min immediately after the test. The results showed that phosphates 2 and 3 did not inhibit the coughing induced by citric acid in guinea pigs.
In conclusion, 8 novel compounds, 2−5, 8, 9, and phosphates of 2 and 3 were synthesized successfully for the first time. 1-[1-Methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-4-piperidinol (2), 1-[1-methyl-2-[2-(phenylmethyl)phenoxy]ethyl]-3-piperidinol (3), 1-O-[1-[1-methyl-2-[2-(phenyl methyl)-phenoxy]ethyl]-piperidin-4-yl]-β-D-glucopyrano-siduronic acid (4) and 1-O-[1-[1-methyl- 2-[2-(phenylmethyl)phenoxy]ethyl]-piperidin-3-yl]-β-D-glucopyranosiduronic acid (5) were identified to be metabolites of benproperine in human urine. Compounds 2 and 3 are inactive metabolites of benproperine.
Acknowledgments
The authors thank Dr Osamu HARA from Meijo University for his helpful discussions. We also thank Ms Wen LI, Mr Yi SHA and Ms Ai-hua SONG in Shenyang Pharmaceutical University for obtaining part of the NMR data and UV data.
References
- Sweetman SC, editor. Martindale, the Extra Pharmacopoeia 34th Edition. London: Pharmaceutical Press; 2005. p1115.
- Kamei J, Ogawa M, Kasuya Y. Monoamines and the mechanisms of action of antitussive drugs in rats. Arch Int Pharmacodyn Ther 1987;290:117-27.
- Du ZM, Huang HH, Chen XY, Zhong DF. Study on hydroxylated metabolites of benproperine in human urine. Acta Pharm Sin 2000;35:916-20.
- Clarke NJ, Rindgen D, Korfmacher WA, Cox KA. Systematic LC/MS metabolite identification in drug discovery. Anal Chem 2001;73:430A-439A.
- Desai RB, Schwartz MS, Matuszewski BK. The identification of three human metabolites of a peptide-doxorubicin conjugate using HPLC-MS-MS in positive and negative ionization modes. J Chromatogr Sci 2004;42:317-22.
- Tevell A, Bondesson U, Torneke K, Hedeland M. Identification of some new clemastine metabolites in dog, horse, and human urine with liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2004;18:2267-72.
- Fei X, Lin H. Preparation of benproperine. Hei Long Jiang Yi Yao 1999;112:194-5.
- Li Y, Chen S, Zhong D, Gan C, Min L, Sun Y, et al. Synthesis of R(+)- and S(–)-benproperine phosphate and their antitussive activity. Chin J Med Chem 2004;14:19-22.
- Soliman SE, Bassily RW, El-Sokkary RI, Nashed MA. Acetylated methyl glucopyranuronate trichloroacetimidate as a glycosyl donor for efficient synthesis of disaccharides. Carbohydr Res 2003;338:2337-40.
- Karlsson JA. lanner AS, Persson CG. Airway opioid receptors mediate inhibition of cough and reflex bronchoconstriction in guinea pig. J Pharmacol Exp Ther 1990;252:863-8.
- Chen S, Min L, Li Y, Li W, Zhong D, Kong W. Anti-tussive activity of benproperine enantiomers on citric-acid-induced cough in conscious guinea-pigs. J Pharm Pharmacol 2004;56:277-80.
- Stachulski AV. Glucuronidation of alcohols using the bromosugar–iodonium reagent method. Tetrahedron Lett 2001;42:661-13.
- Ferguson JR, Harding JR, Lumbard KW, Scheinmann F, Stachulski AV. Glucuronide and sulfate conjugates of ICI 182,780, a pure anti-estrogenic steroid. Order of addition, catalysis and substitution effects in glucuronidation. Tetrahedron Lett 2000;41:389-91.
- BRAGA PC. BOSSI R, PIATTI G, DAL SASSO M. ANTITUSSIVE EFFECT OF OXATOMIDE ON CITRIC ACID-INDUCED COUGH IN CONSCIOUS GUINEA PIG. ARZNEIM FORSCH 1993;43:550-3.
- Forsberg K, Karlsson JA, Theodorsson E, Lundberg JM, Persson CG. Cough and bronchoconstriction mediated by capsaicin-sensitive sensory neurons in the guinea-pig. Pulm Pharmacol 1988;1:33-9.
- Ricciardolo FL. Mechanisms of citric acid-induced bronchoconstriction. Am J Med 2001;111:18S-24S.
- Wong CH, Matai R, Morice AH. Cough induced by low pH. Respir Med 1999;93:58-61.
- Hay DW, Giardina GA, Griswold DE, Underwood DC, Kotzer CJ, Bush B, et al. Nonpeptide tachykinin receptor antagonists. III. SB 235375, a low central nervous system-penetrant, potent and selective neurokinin-3 receptor antagonist, inhibits citric acid-induced cough and airways hyper-reactivity in guinea pigs. J Pharmacol Exp Ther 2002;300:314-23.