Preparation and development of equine hyperimmune globulin F(ab')2 against severe acute respiratory syndrome coronavirus1
Introduction
Severe acute respiratory syndrome (SARS) emerged in Southeast Asia in late 2002 and subsequently spread internationally. The causative agent was quickly identified as a previously unknown member of the Coronaviridae family[1–3]. According to the World Health Organization, up to 2004 Apr 21, SARS coronavirus infected more than 8000 people in various countries worldwide and caused approximately 800 deaths[4]. Although SARS infection of human beings has been contained through infection-control measures, resurgence is still a threat because the causative agent remaining in animal reservoirs is not fully understood, and sporadic cases continue to be reported in Singapore[5,6], Taiwan[7] and mainland China[8,9].
There are no specific vaccines and effective drugs currently available for SARS-CoV[1,2,10,11]. Until an effective vaccine is developed, the best hope for the treatment of infection and the prevention and control of future outbreaks is the development of passive immunotherapy with SARS-CoV-specific antibodies[11]. Immunoglobulin is an effective method used in protection against animal coronavirus: transmissible gastroenteritis virus (TGEV)[12,13], mouse hepatitis virus[14], and bovine coronavirus[15]. There is clinical evidence that serum from recovered patients is effective in infected individuals[16,17]. These observations suggest that hyperimmune serum could be developed for the passive treatment of SARS. The use of equine antisera for emergent prevention and treatment of infectious diseases has been proven to be an effective and safe strategy, such as in rabies virus[18,19]. Therefore, immunoprophylaxis and treatment of SARS coronavirus infection with equine hyperimmune globulin might be a viable strategy for controlling SARS.
Materials and methods
Virus strains Severe acute respiratory syndrome corona-virus Z2-Y3 (AY394989) and F69 (AY313906), isolated from the samples of 2 different Cantonese onset SARS patients in 2003, were sequenced and compared, showing certain differences (Table 1). Viral titres of SARS-CoV Z2-Y3 and F69 strains were determined to be 106.5 50% tissue-culture-infective doses (TCID50)/mL and 106.7 TCID50/mL with the Reed-Muench method, respectively[20–22].
Full table
Antigen preparation F69 strain was used as antigen for immunization. African green monkey kidney (Vero-E6) cells, infected with SARS CoV F69 strain, were cultivated in serum-free minimum essential medium (MEM) (GIBCO) and observed periodically for cytopathic effect (CPE). When 75%–100% cytopathy was reached, infected Vero-E6 cells were frozen and thawed 3 times, which was subsequently centrifuged at 8000×g for 30 min, and then the cell debris was decanted. The supernatant was collected and stored at –70 ºC until used. The viral supernatant was then centrifuged at 30 000×g for 3 h. The precipitate was diluted with phosphate buffered saline (PBS), which was used as antigen for immunization.
Animal immunization Six 4–9 year-old healthy horses were provided by the Quartermaster University of PLA. Immunization of horses was performed according to the State Food and Drug Administration (SFDA) standard operating procedures. On d 0 and d 10, all horses were injected with 1.0 mL antigen intramuscularly (SARS-CoV F69) with complete Freund’s adjuvant (FCA, Sigma). On d 21 and d 28, horses were injected with the same antigen 2.5 mL im, with incomplete Freund’s adjuvant (FIA, Sigma). Eight batches of sera were collected from trachelo veins on d 0, d 10, d 21, d 28, d 35, d 42, d 49, and d 55 after the first immunization, which were stored at -20 °C for the measurement of antibody titers.
Enzyme-linked immunosorbent assay (ELISA) Severe acute respiratory syndrome coronavirus specific IgG was measured using an indirect enzyme-linked immunosorbent assay (ELISA) and whole purified SARS-CoV F69 as antigen. In brief, polystyrene micro well plates were coated with antigen (100 µL/well containing 1.0 µg/mL virus protein) in carbonate-bicarbonate buffer (pH 9.6). The wells were washed 3 times with PBS and then blocked with 15% bovine serum in PBS containing 0.05% Tween-20 (PBST) at 37 °C for 1 h. After 3 washes with PBST, serially twofold diluted serum samples (from 1:100 to a final 1:51200) were added to the plates and incubated at 37°C for 1 h. Horseradish peroxidase (HRP)-conjugated goat anti-horse IgG (Sigma, USA) diluted 3000-fold in PBS was added, followed by 3 washes. Following incubation at 37 °C for 1 h, the plates were washed as above and the substrate tetramethylbenzidine (TMB) solution (Sigma) was added to the wells. After incubation at 37 °C for 15 min, the reaction was stopped by adding 2.0 mmol/L sulfuric acid, and the absorbance value at 450 nm (A450) was measured with a microplate reader (Model 550, BioRad). IgG antibody titer was defined as the highest dilution of serum when the A450 ratio (A450 of negative serum) was greater than 2.0.
Microneutralization assay The neutralization assay was performed according to modified operating procedures of the Manual for the virological investigation of polio formulated by WHO/EPI/GEN/97.01. (http://www.who.ch/programmes/gpv/gEnglish/avail/gpvcatalog/catlog.htm). Each serum sample was serially diluted in twofold 1:10 dilution in MEM maintenance medium to a final dilution of 1:20 480 and incubated with an equal volume of 100 TCID50/25 µL of purified F69 or Z2-Y3 strain for 3 h at 36 °C. The virus-antibody mix was then inoculated onto Vero-E6 cell (3×105 cells/mL) monolayers in 96-well plates at 37 °C for approximately 6 d. Wells for normal cell control and virus control were added to 100 µL maintenance medium and unneutralized active virus dilution, respectively. The plates were incubated until CPE developed in all the virus controls but the cell control remained normal. Neutralizing antibody titer was the highest dilution of serum, which protected 50% of the cultures against 100TCID50 of the challenge virus, when the virus control (no serum) showed complete CPE.
Purification of immunoglobulin Horse antiserum was thawed in 37 °C water bath, added to 45% saturated ammonium sulphate solution, then mixed gently at 22 °C for 30 min, centrifuged at 10 000×g, and the precipitation generated was collected and stored at 4 °C overnight. The ammonium sulphate precipitation was diluted using an equal volume of 0.9% NaCl solution and dialyzed to remove the salt. Then pH was adjusted to 3.5 with 0.36 mol/L HCl. Then horse serum was added to 2% pepsin (Sigma) solution and digested at 37 °C for 8 h, 24 h, 36 h, 48 h, 60 h, and 72 h, respectively. The reaction was stopped by adjusting the pH to 8.0 using 1.0 mol/L NaOH. Then the digested material was ultrafiltrated. Anion-exchange separations of ultrafiltrated material were further performed using diethylaminoethyl(DEAE) Sepharose Fast Flow (Pharmacia) Column, pre-equilibrated with 200 mL of buffer A (50 mmo/L Tris-HCl, pH 8.0). The ultrafiltrated material was pumped down the column, while the A280 nm of the eluted material was monitored, followed by pumping fresh buffer A until the A280 nm returned to the baseline. All the unbounded material, corresponding to the F(ab')2 fragments was collected and stored at 4 °C. Bound contaminants can then be eluted to regenerate the column using a gradient of buffer B (containing 50 mmo/L Tris-HCl, pH 8.0, 1 mmol/L NaCl, pH 8.0). The product obtained using anion-exchange chromatography was ultrafiltrated, concentrated and added to 0.3 mol/L aminoacetic acid to obtain stock solution. According to the demanded standards of biological product, the product characteristics (eg, pH, the protein concentration and bacterial endotoxin content) were detected using serial procedures.
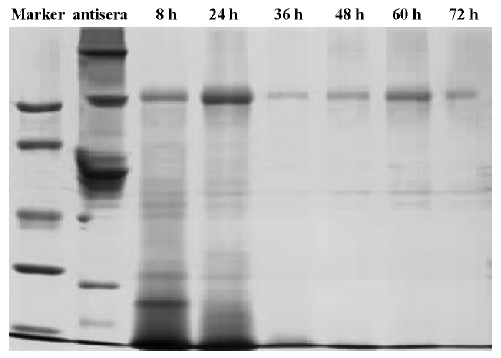
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) Non-reducing SDS-PAGE gels, using the buffer system described by Laemmli (1970)[23], were used to monitor the digestion process and to check for traces of undigested IgG and other unwanted materials.
Results
Identification of SARS coronavirus The virus (SARS-CoV F69) was electron microscopically visualized, and the characteristic coronavirus particle form was observed (Figure 1).
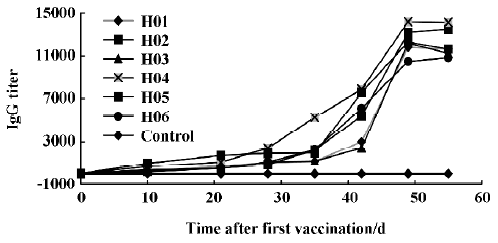
Level of the specific IgG antibody Six health horses were immunized with purified SARS-CoV F69 strain. The titers of total anti-SARS-CoV IgG was measured using an indirect ELISA. The dynamic changes of specific anti-SARS-CoV IgG antibody titers are shown in Figure 2. On d 10, all sera distinctly showed positive reactions, with the range of specific IgG antibody titers from 1:160 to 1:980. Titers of specific IgG antibodies increased rapidly from week 4 and peaked at week 7 after the first immunization; the maximum value was 1:14210.
Titers of neutralizing antibodies The antiserum was measured using a micro-neutralization test. The kinetics of formation of neutralizing antibodies following immunization for horses were observed (Figure 3). The neutralizing antibodies were partially detectable on d 10 (from 1:10 to 1:60). After the third immunization, the neutralizing antibody titers of all the immunized horses increased rapidly on d 28. On d 48 after the first immunization, the neutralizing antibody titers of 4 of 6 equines reached the highest level. The other 2 continued increasing and reached the highest titer at 1:14240.
Cross neutralization response Z2-Y3 strain was used in micro-neutralization test in vitro to measure the hyperimmune sera from the SARS-CoV F69 strain. The results indicate that the horse antiserum induced by the inactivated SARS-CoV F69 strain is capable of neutralizing the SARS-CoV Z2-Y3 strain completely.
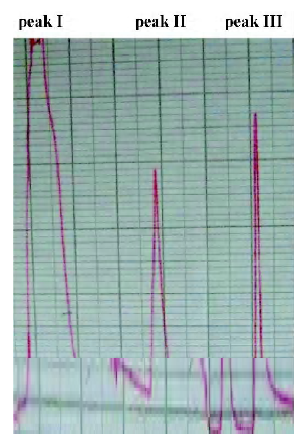
F(ab')2 preparation Digestion with pepsin at different time points was assessed using SDS-PAGE (Figure 5). The results indicate that IgG could be digested completely at pH 3.5 within 48 h and unwanted protein bands (eg albumin and transferrin) could be eliminated as well. The reaction was stopped by adjusting the pH to 8.0 using 1.0 mol/L NaOH. The anion-exchange chromatography with a salt gradient was performed to further remove high molecular weight aggregates and pepsin. Digested antisera in buffer A are separated into 3 peaks (Figure 6). Material from peak I was then concentrated. Finally, approximately15 g F(ab')2 fragments were obtained from 1 litre antiserum with the purity above 90%. The titer of neutralizing antibodies after purification was detected as 1:5120.
The product obtained above was dissolved in a suitable volume of 0.9% NaCl to adjust the protein concentration to 10 g/L. Then pH determined at 20 °C was 7.0. The bacterial endotoxin content was also detected at no more than 200 EU/mL. The terminal product according with the standard of SFDA [(2003) No.267], was stored at 4 °C.
Discussion
Severe acute respiratory syndrome has resulted in important challenges for the medical community. There are no available specific vaccines and effective drugs for use against SARS-CoV[11]. Control depends on prompt detection and isolation of cases, good infection control in hospitals, and the tracing and quarantine of contacts[24]. The widespread clinical successful application of immunoglobulins derived from heterogenous animals against rabies has a long history[25]. The passive administration of neutralizing antibodies could be an effective strategy for emergency prophylaxis and the treatment of SARS[26].
The results of our research indicate that healthy horses immunized with the SARS-CoV F69 strain can be induced to generate effective, specific and neutralizing antibodies. Analysis indicates that sequence difference existed among SARS-CoVs[27]. The sequence of the SARS-CoV F69 strain is different from that of the SARS-CoV Z2-Y3 strain (Table 1). Immunoglobulin prepared from SARS-CoV F69 strain isolated in April, 2003 could neutralize another SARS-CoV Z2-Y3 strain, which was isolated from SARS patient in February, 2003. This showed that SARS-CoV F69 and Z2-Y3 strain owned identical or similar neutralizing epitopes.
Heterogenous antisera used for treatment possibly result in anaphylactoid severe acute side-effects[28]. To avoid the side-effects caused by horse antiserum, IgG against SARS-CoV was digested with pepsin and purified with anion-exchange separations to exclude the immunogenicity of Fc fragments and to retain the special activity of binding the antigen of F(ab')2 fraction. The titers of neutralizing F(ab')2 against SARS-CoV was detected at higher level (1:5120). And approximately 15 g F(ab')2 fragments were obtained from 1 litre antiserum, with the purity above 90%.
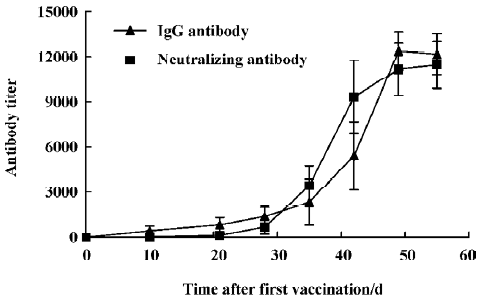
Until we have an efficacious vaccine and have implemented effective epidemiologic infection control measures, and given the presence of effective anti-SARS-CoV agents, SARS is likely to remain a major health threat to the world. In this article, we provide an alternative pathway of prevention and treatment of SARS, with the purpose of combating any resurgence of SARS. The profile of the antibody titer was observed, while an effective, specific and neutralizing hyperimmunoglobulin was prepared. The results indicate that the kinetics of the induced specific IgG and neutralizing antibodies are similar (Figure 4). This data paves the way for the development of an inactivated SARS vaccine.
References
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med 2003;348:1967-76.
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348:1953-66.
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, et al. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 2003;300:1394-9.
- World Health Organization [homepage on the Internet]. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Geneva: The Organization. [cited 2005 Jul 9] Available from: http://www.who.int/csr/sars/country/table2004_04_21/en/
- Lim PL, Asok K, Gopalakrishna G, Chan KP, Wong CW, et al. Laboratory-acquired severe acute respiratory syndrome. N Engl J Med 2004;250:1740-5.
- World Health Organization [homepage on the Internet]. Severe acute respiratory syndrome (SARS) in Singapore. Geneva: The Organization. [cited 2005 April 10] Available from: http://www.who.int/csr/don/2003_09_10/en/
- World Health Organization [homepage on the Internet]. Severe Acute Respiratory Syndrome (SARS) in Taiwan, China. Geneva: The Organization. [cited 2005 May 17] Available from:http://www.who.int/csr/don/2003_12_17/en/
- World Health Organization [homepage on the Internet]. SARS: one suspected case reported in China. Geneva: The Organization. [cited 2005 April 22] Available from: http://www.who.int/csr/don/2004_04_22/en/
- World Health Organization [homepage on the Internet]. China’s latest SARS outbreak has been contained, but biosafety concerns remain-Update 7. Geneva: The Organization. [cited 2005 July 18] Available from: www.who.int/csr/don/2004_05_18a/en/
- Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, et al. Newly discovered coronavirus as the primary cause of Severe Acute Respiratory Syndrom. Lancet 2003;362:263-70.
- Holmes KV. SARS-Associated Coronavirus. N Engl J Med 2003;348:1948-51.
- Saif LJ, Bohl EH. Passive immunity to transmissible gastroenteritis virus: intramammary viral inoculation of sows. Ann N Y Acad Sci 1983;409:708-23.
- Sestak K, Lanza I, Park SK, Weilnau PA, Saif LJ. Contribution of passive immunity to porcine respiratory coronavirus to protection against transmissible gastroenteritis virus challenge exposure in suckling pigs. Am J Vet Res 1996;57:664-71.
- Homberger FR, Barthold SW. Passively acquired challenge immunity to enterotropic coronavirus in mice. Arch Virol 1992;126:35-43.
- Arthington JD, Jaynes CA, Tyler HD, Kapil S, Quigley JD 3rd. The use of bovine serum protein as an oral support therapy following coronavirus challenge in calves1. J Dairy Sci 2002;85:1249-54.
- Li G, Chen X, Xu A. Profile of specific antibodies to the SARS-associated coronavirus. N Engl J Med 2003;349:508-9.
- Wong VW, Dai D, Wu AK, Sung JJ. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J 2003;9:199-201.
- Wilde H, Chomchey P, Punyaratabandhu P, Phanupak P, Chutivongse S. Purified equine rabies immune globulin: a safe and affordable alternative to human rabies immune globulin. Bull World Health Organ 1989;67:731-6.
- Wilde H, Chutivongse S. Equine rabies immune globulin: a product with an undeserved poor reputation. Am J Trop Med Hyg 1990;42:175-8.
- Yan XG, Wan ZY, Zhang X, Chen QX. Isolation and identification of SARS coronavirus in Guangdong province. Chin J Exp Clin Virol 2003;17:213-6.
- Lu JH, Yan XG. Establishment of SARS virus vaccine line (F69) Guangdong Med J 2003;24 SARS Suppl II:225-7.
- Lu JH. Establishment of SARS virus vaccine line (Y3) Guangdong Med J 2003;24 SARS Suppl II:234-6.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680-5.
- Sampathkumar P, Temesgen Z, Smith TF, Thompson RL. SARS: epidemiology, clinical presentation, management, and infection control measures. Mayo Clin Proc 2003;78:882-90.
- Quiambao BP, Lang J, Vital S, Montalban CG, Le Mener V, Wood SC, et al. Immunogenicity and effectiveness of post-exposure rabies prophylaxis with a new chromatographically purified Vero-cell rabies vaccine (CPRV): a two-stage randomised clinical trial in the Philippines. Acta Trop 2000;75:39-52.
- Sui J, Li W, Murakami A, Tamin A, Matthews LJ, Wong SK, et al. Potent neutralization of severe acute respiratory syndrome (SARS) coronavirus by a human mAb to S1 protein that blocks receptor association. Proc Natl Acad Sci USA 2004;101:2536-41.
- Chinese SARS Molecular Epidemiology Consortium. Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 2004;303:1666-9.
- Jahrling PB, Geisbert TW, Geisbert JB, Swearengen JR, Bray M, Jaax NK, et al. Evaluation of immune globulin and recombinant interferon-alpha2b for treatment of experimental Ebola virus infections. J Infect Dis 1999;179 Suppl 1:224-34.