Histamine ameliorates spatial memory deficits induced by MK-801 infusion into ventral hippocampus as evaluated by radial maze task in rats1
Introduction
Recently, the differentiation of functions along the longitudinal axis of the hippocampus (dorsal–ventral in the rat and posterior–anterior in humans) has attracted interest[1,2]. Anatomically, the hippocampus includes CA1, CA2, CA3, and dentate gyrus subfields, with the pattern of efferent and afferent connectivity changing between the dorsal and ventral hippocampus[3]. Identification of distinct functional roles for the dorsal and ventral hippocampus may help to resolve differences among the various theoretical accounts of hippocampal involvement in learning behavior[2].
Considerable evidence shows that the dorsal hippocampus plays an important role in spatial memory[4–6]. Ablation of the dorsal hippocampus, in which between 20% and 37% of the total hippocampal volume is removed, results in profound impairment of spatial location of a submerged platform in the Morris water maze[5]. Bannerman et al also found that performance in the water maze and elevated T maze was impaired by selective dorsal but not ventral hippocampal lesions[4]. On the other hand, ventral hippocampal lesions are considered to affect anxiety but not spatial learning[7,8]. However, Ferbinteanu et al demonstrated that the ventral hippocampus might also be involved in acquisition of spatial memory in Long–Evans rats[9]. So far, little is known about the functions performed by the ventral hippocampus in spatial memory.
Both histamine and its precursor histidine facilitate memory retrieval deficits induced by aging, dorsal hippocampal lesions and scopolamine, as determined by passive and active avoidance tasks and an 8-arm radial maze test for rats[10–12]. α-Fluoromethylhistidine (FMH), a selective histidine decarboxylase (HDC) inhibitor, produces significant memory deficits in an active avoidance task and the 8-arm radial maze in rats[13,14]. Furthermore, N-methyl-D-aspartate (NMDA) receptors play an important role in learning and memory[15,16]. Bekkers[15] and Vorobjev et al[17] found that histamine could dramatically enhance NMDA receptor-mediated synaptic transmission and facilitate the induction of long-term potentiation in cultured hippocampal pyramidal cells. Our previous studies indicate that histamine in the dorsal hippocampus ameliorates spatial working memory and reference memory deficits induced by MK-801, and the action is mediated by postsynaptic histamine H1 receptors[18,19]. However, whether histamine in the ventral hippocampus is involved in spatial memory deficits induced by MK-801 remains unclear.
Therefore, the objectives of this investigation were to use the radial maze task with 4-arms baited to further clarify the effects of the histaminergic system in the ventral hippo-campus on the regulation of spatial memory in rats.
Materials and methods
Animals All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (220−270 g, grade II, certificate N
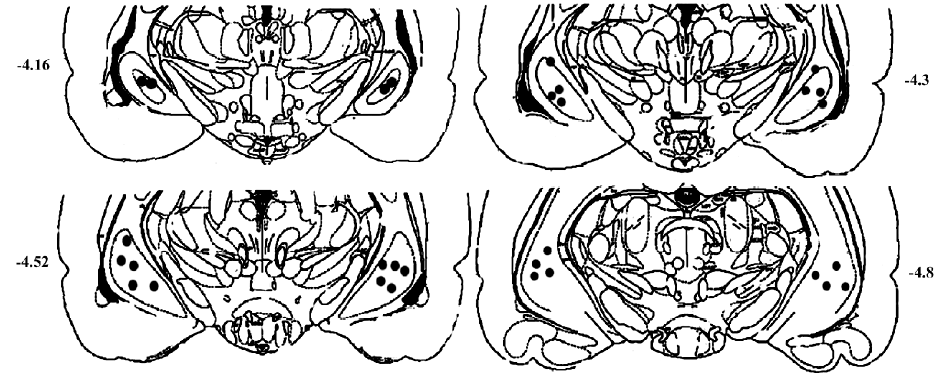
Surgical procedures The rats were anesthetized with sodium pentobarbital (35 mg/kg, ip), and fixed in a stereotaxic apparatus (Narishige, SR-5, Tokyo, Japan). Two guide cannulas made of stainless steel tubing (700 µm in outer diameter), were implanted bilaterally into the ventral hippocampus according to the following coordinates measured from the bregma: antero-posterior: -4.52 mm; medio-lateral: ±5.0 mm; dorso-ventral: 8.0 mm from the skull surface. The cannula was fixed to the skull with three screws and dental acrylic. A stylet was inserted into the cannula to keep it patent prior to injections. At least 10 d were allowed for recovery from the surgery. After the behavioral tests, Evans blue (1 µL) was injected bilaterally into the ventral hippocampus and the rats were killed by decapitation. The accuracy of the injection site was carefully determined (Figure 1). Based on histological examination, data from nine rats with incorrect injections were excluded from the results.
Intracerebral microinfusion Rats were manually restrained and the stylets were carefully removed from the guide tubes. Infusion cannulae, connected via flexible polyetheretherketone tubing to 5 µL Hamilton microsyringes mounted on a microinfusion pump (KN-201, Natsume, Tokyo, Japan), were then inserted into the guide tubes. Drugs in 1 µL vehicle (0.9% saline) or 1 μL vehicle per side were infused into the ventral hippocampus at a rate of 1 µL/min. To allow absorption of the infusion bolus, infusion cannulae were left in the brain for 60 s after infusion before being replaced by the stylets.
Radial-arm maze training The apparatus used is described in our previous reports[18,19]. The rats were familiarized with the radial maze once per day for 2 d prior to training. Food pellets (45 mg each, Bio-Serv, Frenchtown, NJ, USA) were scattered over the entire maze surface, and three or four rats were simultaneously placed in the maze and allowed to explore and take food freely for 10 min. After adaptation, all rats were trained with one trial per day. In each trial, only 4 arms (3, 5, 6, and 8) were baited, and the sequence was never changed throughout the experiment. The rat was placed on the center platform that was closed off by a door. After 15 s, the door was opened and the rat was allowed to make an arm choice to obtain food pellets until all 4 pellets had been eaten or 5 min had elapsed. The number of entries into never-baited arms was regarded as reference memory error (RME), whereas re-entry into arms where the pellet had already been eaten was considered as working memory error (WME). Rats continued training until reaching a criterion of less than 1 error per trial for 5 consecutive trials. Once a rat reached criterion, training for that rat was reduced to 2 trials per week until all rats reached criterion (range 35–50 d). After each trial, the maze was wiped clean using paper towels that were dampened with 10% ethanol and rotated 180º.
Drugs During the drug test, (+) MK-801 hydrogen maleate (Sigma, St Louis, MO, USA), histamine dihydrochloride (Sigma), cimetidine dimaleate (Sigma), or pyrilamine dihydrochloride (Sigma) were dissolved in saline and injected bilaterally into the ventral hippocampus. Histidine monohydrochloride (Sigma) was dissolved in saline and injected ip. Studies for drug effects were carried out once a week, on Tuesday or Friday.
Statistics All results are expressed as mean±SEM. Statistical significance was assessed by one-factor analyses of variance (ANOVA) or the Kruskal-Wallis non-parametric ANOVA test (when the data were not normally distributed or the variances of the groups differed significantly), followed by Dunnett’s test or the Mann-Whitney U test as a post hoc analysis. The software SPSS 11.5 (SPSS, Chicago, IL, USA) was used. P<0.05 was considered statistically significant.
Results
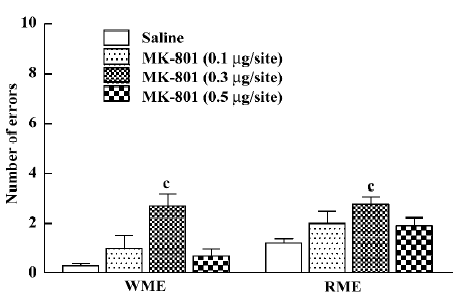
Effect of MK-801 on memory retrieval as evaluated by 4-arm baited radial maze performance Non-parametric ANOVA (Kruskal-Wallis) showed that bilateral ih injection of MK-801 caused a significant impairment of working memory and reference memory {WME: H[3]=13.873, P=0.003; RME: H[3]=25.161, P<0.01}. MK-801 at a dose of 0.1 μg/site slightly increased the memory error, but this was not significant (Mann-Whitney U test; WME: P=0.335; RME: P=0.145). At a dose of 0.3 µg/site it significantly increased the number of both WME and RME (Mann-Whitney U test; P<0.01; Figure 2). However, at a dose of 0.5 µg/site MK-801 did not significantly impair spatial working or reference memory (Mann–Whitney U test; WME: P=0.338; RME: P=0.061).
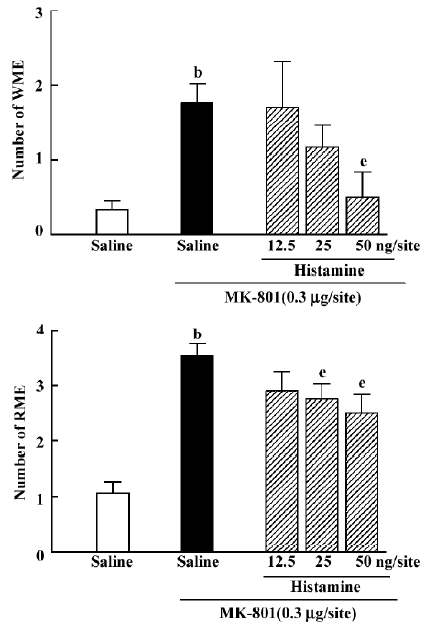
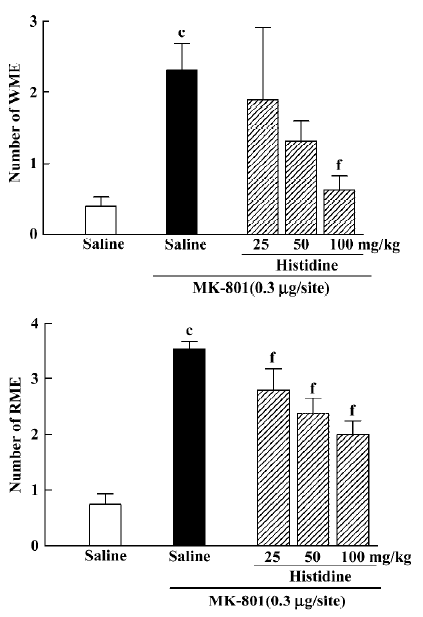
Effects of histamine and histidine on MK-801-induced memory deficits Intrahippocampal injection of histamine antagonized the effects of MK-801 {WME: H[3]=8.846, P=0.031; RME: H[3]=8.004, P=0.044}. At doses of 25 or 50 ng/site it significantly decreased the number of RME (Mann–Whitney U test; P<0.05; Figure 3), whereas histamine at a dose of 50 ng/site significantly decreased the number of WME(Mann-Whitney U test; P<0.05; Figure 3). Intraperitoneal injection of histidine produced an effect similar to histamine (WME: H[3]=11.314, P=0.010; RME: H[3]=20.415, P<0.01). At doses of 25, 50 or 100 mg/kg, histidine significantly inhibited MK-801-induced spatial reference memory deficits (Mann-Whitney U test; P<0.01; Figure 4). It significantly reversed the spatial working memory deficit induced by MK-801 only at the dose of 100 mg/kg (Mann-Whitney U test; P<0.01; Figure 4).
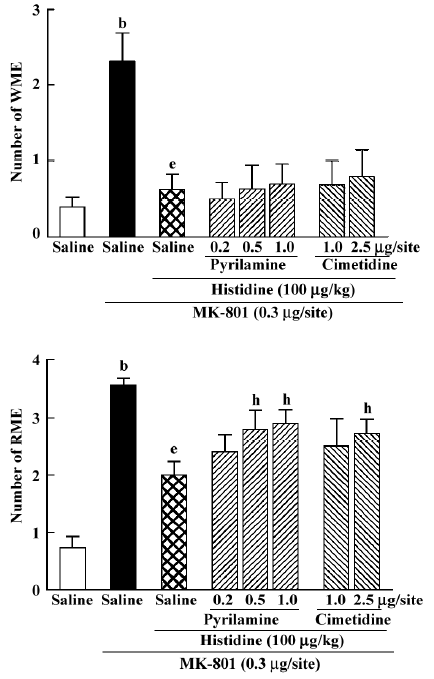
Effects of pyrilamine and cimetidine on memory amelioration of histidine on MK-801-induced memory deficits Intrahippocampal injection of pyrilamine, a representative central histamine H1 antagonist, significantly antagonized the ameliorating action of histidine on RME (H[3]=7.811, P=0.04) at doses of 0.5 and 1.0 µg/site (Mann-Whitney U test; P<0.05; Figure 5), but not on WME. In addition, cimetidine, a histamine H2 antagonist, at the high dose of 2.5 μg/site, also significantly antagonized the ameliorating action of histidine on RME (Mann-Whitney U test; P<0.05; Figure 5), but not on WME (Mann-Whitney U test; P=0.974).
Discussion
Previously, we observed that bilateral injection of MK-801, an NMDA receptor antagonist, into the dorsal hippocampus impaired both working and reference memory[18]. Our present results are consistent with previous studies as assessed by performance in the 8-arm (4-arm baited) radial maze. We found that blockade of NMDA receptors in the ventral hippocampus impaired the retrieval of spatial working and reference memory, which suggested that the ventral hippocampus might be involved in the spatial memory process in rats[9,20], although a chemical lesion study demonstrated that the ventral hippocampus was less involved in spatial memory modulation[5,7]. The different findings may arise from the different experimental procedures (eg using the Morris water maze, the T-maze, and the 8-arm maze). In addition, the lesion method is less specific than local delivery of com-pounds.
Intrahippocampal application of MK-801 (0.3 µg/site) did not lead to obvious changes in running time per choice (locomotor activity) or other behaviors, such as ataxia (data not shown), which usually occurs after systemic or intracerebroventricular (icv) treatment with MK-801[16]. However, we found that MK-801 at a dose of 0.5 µg/site did not impair spatial memory. A few rats exhibited epileptic symptoms or even died when given a dose of 0.5 µg/site, phenomena resembling those following systemic or icv treatment with MK-801[16]. Therefore, we used MK-801 (0.3 µg/site) to induce the spatial memory deficits in the following experiments.
Both histamine and histidine ameliorated the memory retrieval deficits induced by MK-801, including working memory and reference memory. Intraperitoneal injection of histidine significantly increased histamine levels in the cortex and hippocampus (data not shown). This was consistent with previous studies showing that ip or icv injection of histidine increased hippocampal histamine levels[11,12]. Our results were consistent with previous findings in which MK-801 was delivered to the dorsal hippocampus[18]. In addition, Chen et al reported that histidine at higher doses of 200, 500, and 1 000 mg/kg improved the impairment of radial maze performance induced by MK-801 (0.1 mg/kg)[14]. Our results indicate that histidine at lower doses (25, 50 and 100 mg/kg) can ameliorate the impairment induced by MK-801. The difference can be attributed to the different injection routes, different species, and different learning models. But these present results at least suggest that ventral hippocampal histamine may participate in the amelioration of both short-term and long-term spatial memory deficits.
In the present study, the amelioration of reference memory elicited by histidine was completely reversed by pyrilamine, a central histamine H1 antagonist. However, this result is different from those for the dorsal hippocampus in that pyrilamine infused into the ventral hippocampus produced no appreciable inhibition of the amelioration of working memory by histamine. This result suggests that the ameliorating effect of histidine on the working memory deficit induced by MK-801 is independent of histamine. Histamine can act directly on the NMDA receptor, as suggested by Bekkers[15] and Vorobjev et al[17]. Because the polyamine site of the NMDA receptor may be a binding site for histamine[21], the facilitating effect of histidine on MK-801-induced working memory deficit may be attributed to histamine acting directly on this site. So far, we have no explanation of why histamine H1 receptors in the dorsal and ventral hippocampus have different actions. We previously reported that the improvement of both working and reference memory induced by dorsal hippocampal histamine were antagonized by pyrilamine[18]. Our present data, at least in part, suggest that the histamine H1 receptors in the dorsal and ventral hippocampus participate in different components of memory. Histamine H1 receptors in the dorsal hippocampus are involved in both working and reference memory, but those in the ventral hippocampus are mainly related to reference memory.
Moreover, we were surprised to find that cimetidine, a histamine H2 antagonist, also blocked histamine-induced amelioration of reference memory. So far, most reports show that histamine H2 receptors are less involved in the learning and memory process[18,19,21,22]. For example, the ameliorating effect of histamine on scopolamine-induced learning deficit is antagonized by pyrilamine but not by the selective histamine H2 antagonists cimetidine and zolantidine[10,11]. Intra-cerebroventricular injection of the histamine H1 agonist, 2-thiazolylethylamine, but not the histamine H2 agonist, 4-methylhistamine, reverses the working memory deficits induced by MK-801 as evaluated by the 8-arm baited radial maze in rats[14]. However, Flood et al have recently reported that increased histamine H1 and H2 receptor activity in the septum facilitated memory retention, whereas decreased histamine receptor activity resulted in impaired memory processes as evaluated by T-maze behavior[23]. Giovannini et al also provided evidence that bilateral post-training injection of the histamine H2 agonist amthamine into the dorsal hippocampus improved memory consolidation[24]. These findings combined with our present data suggest that the histamine H2 receptor in certain brain regions may be involved in learning behavior. Further experiments are needed to elucidate the function of the histamine H2 receptor.
In conclusion, histamine in the ventral hippocampus is involved in spatial memory, and histamine ameliorates the memory deficits of both working memory and reference memory induced by MK-801. The ameliorating action on reference memory is mediated by postsynaptic histamine H1 and H2 receptors, whereas the effect of histamine on working memory may be mediated by other neuronal pathways.
Acknowledgement
We are very grateful to Dr Iain C BRUCE (University of Hong Kong) for reading the manuscript.
References
- Nadel L. Dorsal and ventral hippocampal lesions and behavior. Physiol Behav 1968;3:891-900.
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev 2001;38:247-89.
- Andersen P, Bliss TV, Skrede KK. Lamellar organization of hippocampal pathways. Exp Brain Res 1971;13:222-38.
- Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippo-campus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci 1999;113:1170-88.
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 1993;13:3916-25.
- Moser MB, Moser EI. Distributed encoding and retrieval of spatial memory in the hippocampus. J Neurosci 1998;18:7535-42.
- Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res 2003;139:197-213.
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci USA 2002;99:10825-30.
- Ferbinteanu J, Ray C, McDonald RJ. Both dorsal and ventral hippocampus contribute to spatial learning in Long-Evans rats. Neurosci Lett 2003;345:131-5.
- Chen Z. Effect of histamine H3-receptor antagonist clobenpropit on spatial memory of radial maze performance in rats. Acta Pharmacol Sin 2000;21:905-10.
- Chen Z, Kamei C. Facilitating effects of histamine on spatial memory deficit induced by scopolamine in rats. Acta Pharmacol Sin 2000;21:814-8.
- Kamei C, Chen Z, Nakamura S, Sugimoto Y. Effects of intracerebroventricular injection of histamine on memory deficits induced by hippocampal lesions in rats. Methods Find Exp Clin Pharmacol 1997;19:253-9.
- Chen Z, Sugimoto Y, Kamei C. Effects of intracerebroventricular injection of α−fluoromethylhistidine on radial maze performance in rats. Pharmacol Biochem Behav 1999;64:513-8.
- Chen Z, Zhao Q, Sugimoto Y, Fujii Y, Kamei C. Effects of histamine on MK-801-induced memory deficits in radial maze performance in rats. Brain Res 1999;839:186-9.
- Bekkers JM. Enhancement by histamine of NMDA-mediated synaptic transmission in the hippocampus. Science 1993;261:104-6.
- Pitkanen M, Sirvio J, MacDonald E, Niemi S, Ekonsalo T, Riekkinen P Sr. The effects of D-cycloserine and MK-801 on the performance of rats in two spatial learning and memory tasks. Eur Neuropharmacol 1995;5:457-63.
- Vorobjev VS, Sharonova IN, Walsh IB, Haas HL. Histamine potentiates N-methyl-D-aspartate responses in acutely isolated hippocampal neurons. Neuron 1993;11:837-44.
- Huang YW, Chen Z, Hu WW, Zhang LS, Wu W, Ying LY, et al. Facilitating effect of histamine on spatial memory deficits induced by dizocilpine as evaluated by 8-arm radial maze in SD rats. Acta Pharmacol Sin 2003;24:1270-6.
- Huang YW, Hu WW, Chen Z, Zhang LS, Shen HQ, Timmerman H, et al. Effect of the histamine H3-antagonist clobenpropit on spatial memory deficits induced by MK-801 as evaluated by radial maze in Sprague-Dawley rats. Behav Brain Res 2004;151:287-93.
- Poucet B, Herrmann T, Buhot MC. Effects of short-lasting inactivations of the ventral hippocampus and medial septum on long-term and short-term acquisition of spatial information in rats. Behav Brain Res 1991;44:53-65.
- Passani MB, Bacciottini LB, Mannaioni PF, Blandina P. Central histaminergic system and cognition. Neurosci Biobehav Rev 2000;24:107-13.
- Kamei C, Chung YH, Tasaka K. Influence of certain H1-blockers on the step-through active avoidance response in rats. Psychopharmacology (Berlin) 1990;102:312-8.
- Flood JF, Uezu K, Morley JE. Effect of histamine H2 and H3 receptor modulation in the septum on post-training memory processing. Psychopharmacology (Berlin) 1998;140:279-84.
- Giovannini MG, Efoudebe M, Passani MB, Baldi E, Bucherelli C, Giachi F, et al. Improvement in fear memory by histamine-elicited ERK2 activation in hippocampal CA3 cells. J Neurosci 2003;23:9016-23.
