Conformational re-analysis of (+)-meptazinol: an opioid with mixed analgesic pharmacophores1
Introduction
Meptazinol [its (+)-isomer is shown in Figure 1] is an opioid with similar analgesic activity to pethidine (also known as meperidine). Since it entered into the market in the 1980s as a racemic mixture, it has been recognized and recommended as a mixed agonist-antagonist opioid[1]. It was found to antagonize morphine induced physical dependence in vivo[2], however, its analgesic mechanism remains unclear. For a long time meptazinol was suggested to perform its analgesic function through the m1 opioid receptor[3], which was believed to mediate analgesia while reducing the addition and respiratory depression potential.
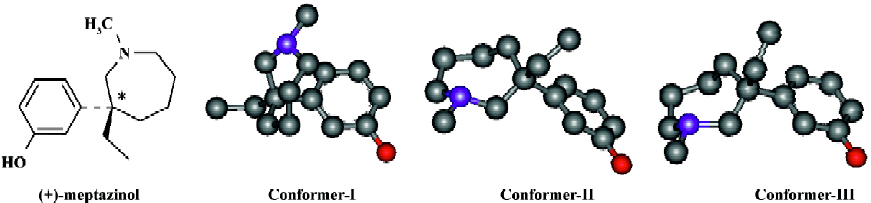
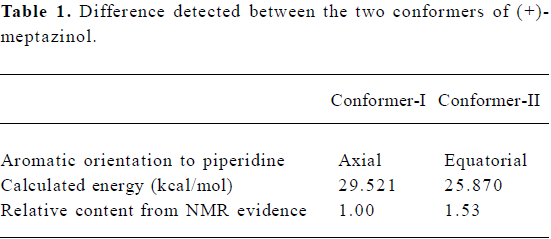
In our previous studies, the X-ray determined enatiomers of meptazinol were compared with typical µ opiate pharma-cophore, but they failed to fit that pharmacophore well[4]. Meanwhile, during the investigation of (+)-meptazinol hydrochloride in solution, two conformers (Conformer-I and -II; Figure 1) were elucidated with NMR spectroscopy, in which the phenyl group of meptazinol could take ax- or eq- orientations, respectively, to the azepine ring. Conformer-II was very similar to Conformer-III (Figure 1), which was determined by X-ray crystallography. Here only the two NMR conformers of (+)-meptazinol were discussed. It is worthy to note that the relative quantity of the two conformers in solution were almost the same amount (approximately 3:2), although their calculated energy was somewhat different (Table 1).
Full table
As Conformer-III (very similar to Conformer-II) failed to fit the typical opiate pharmacophore[4], it was reasonable to consider that Conformer-I, with higher energy, was the bio-active conformer while Conformer-II (or Conformer-III) was inactive. After all, a conformer with the lowest energy is not always the biological active conformer for a compound with certain biological activities.
In this study, two typical pharmacophores from different opioids were established and validated. And both conformers of (+)-meptazinol were used for structural comparisons to analyze their specific properties. This study provides new insights into understanding the nature of mixed pharmacophores to (+)-meptazinol and the complicated pharmacology of meptazinol.
Materials and methods
Material preparation All calculations were carried out on a R14000 SGI Fuel workstation using the molecular modeling software package SYBYL version 6.9 (Tripos, St Louis, MO, USA).
The initial pharmacophore of morphine analogs were modeled in our previously published paper[4]. The conformer of meperidine was well examined by X-ray crystallographic[5] and NMR[6] techniques and thus was modeled according to literature. The two different conformers of (+)-meptazinol (Conformer-I and -II) were obtained by molecular dynamics from NOESY and TOCSY spectra. Several conformationally constrained aromatic substituted piperidines[6-11] were sketched in SYBYL according to the reported preferred conformers with all basic nitrogen atoms protonated. The initial structures were firstly minimized by Tripos force field with Gasteiger-Huckel charges, followed by random searches to ensure their stable conformations by default settings. Finally, all structures were optimized with the semi-empirical quantum chemical method Austin Model 1 (AM1; available in SYBYL)[12], and the geometrically optimized structures were used for further structural comparisons.
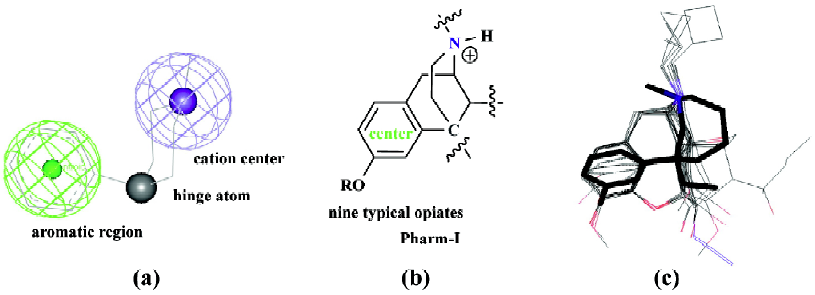
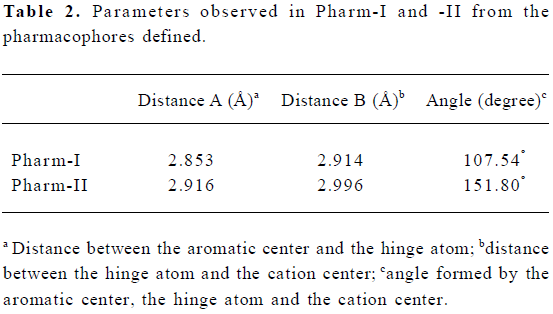
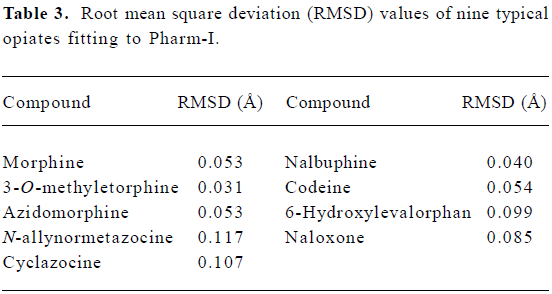
Establishment and validation of two opioid pharmaco-phores Two opioid pharmacophores, namely Pharm-I and -II, were defined from structures of nine typical opiates and meperidine, separately, (Figure 2a and 3a). Each pharma-cophore contains one aromatic region (defined by the center of phenyl rings), one cation center and a hinge atom. In addition, two distance and one angle parameter were also calculated to constrain these components’ distribution in space (Table 2). Pharm-I was modeled from the mean values of nine typical opiates (Figure 2b). And the root mean square deviations (RMSD) fitting values of nine opiates were listed in Table 3. Pharm-II was extracted from the well-established meperidine conformer (Figure 3b).
Full table
Full table
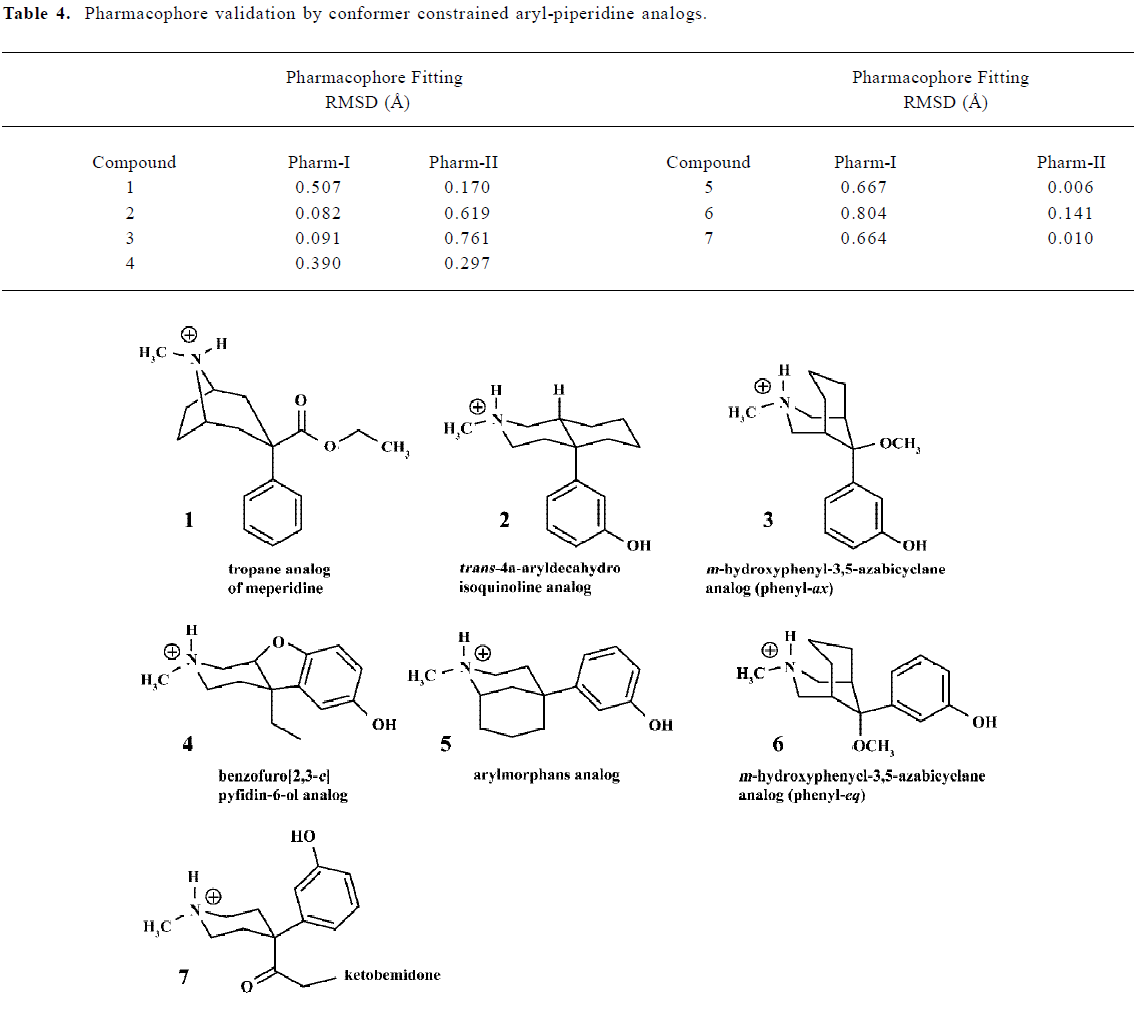
With the defined pharmacophores, all structural comparisons were made by Fit Atoms facilities available in SYBYL. And the established pharmacophores were validated by fitting a set of constrained arylpiperidines (Table 4).
Full table
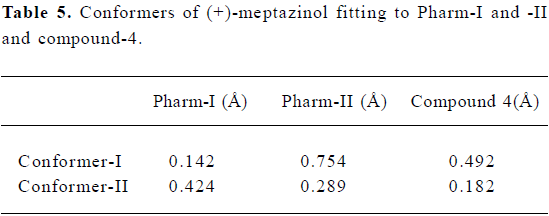
Fitting conformers of (+)-meptazinol to the pharmaco-phores Both Conformer-I and -II of (+)-meptazinol were fitted into both opioid pharmacophores separately to see if they share similar properties. Furthermore, both conformers were superimposed with the structure of compound-4 (Table 5).
Full table
Results and discussion
Establishment and validation of opioid pharmacophores It was suggested that arylpiperidines and structural related analgesics could be divided into two categories according to the orientation of the aryl group[13]: ax- and eq- phenyl models. The prototype analgesics of ax- and eq- phenyl models were morphine and meperidine, respectively. And arylpiperidines with phenyl ax-conformer could also be classified as ax- phenyl models not only for similar phenyl orientation but also for similar structure activity relationships.
Although there was evidence that analgesics in these categories were both µ opioid ligands[14], these two models bear different structure activity relationships properties based on the following facts: 1) N-allyl or cyclopropyl methyl analogs in ax- phenyl conformers induced antagonist activity, but those in eq- phenyl conformers did not[13]; 2) introduction of a 3R-methyl group into the piperidine ring in analogs of eq- phenyl conformers mediates pure antagonist potency[15] but not in those of ax- phenyl conformers; 3) in reversed esters of meperidine with preferred eq- phenyl conformers, phenolic hydroxyl insertion virtually abolishes activity[16,17]. However, in most other analogs, an m-placed phenolic hydroxyl group enhances analgesic activity. The major structural difference between these two categories was the different orientations of their aryl groups which mainly account for different pharmacological properties. Thus Portoghese et al proposed different binding models to μ opioid receptor for compounds in these categories[16].
Here, typical opioids of ax-phenyl (nine opiates) and eq-phenyl (meperidine) were used to establish their corresponding pharmacophores. Although the tyramine fragment was widely used as typical opioid pharmacophore in the published literature[18] as well as in our previous studies[4], the phenyl ring of ax- arylpiperidines actually bears a relationship orthogonal to that obtained in the rigid skeleton of morphine[19,20]. Thus not only the phenyl plane but also the hydroxyl group on the aromatic ring between opiates and ax- arylpiperidines were quite different. Superimposing ax- arylpiperidines to morphine derived tyramine fragments will lead to poor overlaps. Considering phenyl groups could form either parallel or T-shape π-π interactions with the hydrophobic site, and hydroxyl groups in a given region could also interact with the hydrogen acceptor on the receptor, it seemed unnecessary to consider the overall fit between the phenolic groups between ax- arylpiperidine and opiates. Here, we excised the tyramine fragment down to an aromatic center, a cation center and a hinge atom connecting piperidine and phenyl rings to unify these structurally diverse opioids with ax- phenyl conformers. The hydroxyl group was excluded from our pharmacophore not only for its ambiguous function in arylpiperidines, but for poor fitting results among different analgesics, although it was also used for structural reference in this study. The relative orientation of the phenyl ring toward the piperidine group could be simply determined by the angle values defined in Table 2.
Common model definitions for these two pharmacophores were as follows: 1) The center of aryl rings to form hydrophobic interactions with the opioid receptor; 2) the protonated nitrogen atom to form salt bridges with anion center on the receptor; 3) the hinge atom that held the relative orientation of phenyl to piperidine ring. Although the two pharmacophores shared similar distance parameters (Table 2), the angle parameters were quite different. The angle was 107.54º in Pharm-I (with ax- phenyl conformer) and 151.8º in Pharm-II (with eq- phenyl conformer), which could also be regarded as the main difference between the two pharmaco-phores.
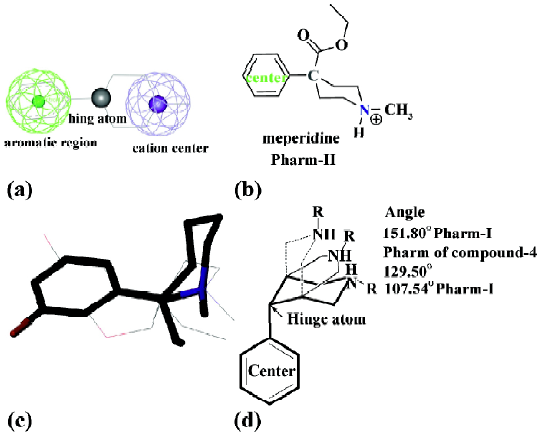
Some typical constrained arylpiperidines with known potent analgesic potencies were used for validation of the modeled pharmacophores. And all of these compounds could be identified to their correct pharmacophores by RMSD fitting values below 0.2Å, as well as by determining their phenyl orientations except for compound-1 and 4. Although compound-1 took ax- phenyl conformer, it seemed more reasonable to be classified to Pharm-II because the framework of the tropane ring constrained the relative position of the defined three components to Pharm-II (with eq- phenyl conformer). However, compound 4 could be classified to neither Pharm-I nor Pharm-II and its special pharmacophore will be discussed in the next section of this paper, although its phenyl ring took eq- conformer.
Structural comparison of (+)-meptazinol with opioid pharmacophores As a ring expanded the analog of 4-arylpiperidine, both (+)-meptazinol conformers were fitted to the established opioid pharmacophores. Conformer-I was found to fit Pharm-I well but not Pharm-II (Figure 2c and Table 5).
However, Conformer-II fit none of these pharmacophores (Table 5). Similar conclusions were made in our previous studies based on tyramine fragment derived opiate pharmacophore[4]. Furthermore, Conformer-II was energy favored with calculated energy 3.651 kcal/mol lower than Conformer-I. Should Conformer-II also be considered as an inactive conformer?
Surprisingly, conformer-II showed similar fitting values to both pharmacophores (0.424 for Pharm-I and 0.289 for Pharm-II) as compound-4 (0.390 for Pharm-I and 0.297 for Pharm-II), although they fit neither pharmacophore (Table 4 and Table 5). Limited studies were carried out on these analogs[10,11], but compound-4 was a potent µ opioid agonist. Another question raised was whether these two structures shared similar properties. Considering its rigid structure and potency, compound-4 could also be employed as an eligible template for structural comparison. With the same components of pharmacophore defined previously, fitting Conformer-II to compound-4 led to good overlap results with a RMSD value of 0.182 (Figure 3c and Table 5).
In addition, the angle parameter of compound-4 was 129.50º, a halfway transition state between Pharm-I and -II (Figure 3d). And it was 136.56º for Conformer-II of (+)-meptazinol. Typical arylpiperidines generally took Pharm-I (ax- phenyl) or Pharm-II (eq- phenyl) conformers to avoid disfavored steric clashes. But the furan ring constrained compound-4 took such a distorted conformer that it belonged to neither of these two conformers. Instead, it held a transition conformer between the two conformers. Considering other arylpiperidines with ring constrains lacked analgesic potencies[21], compound-4 may display specific pharmacological properties other than Pharm-I and -II. Though conformer-II of (+)-meptazinol failed to fit Pharm-I and Pharm-II, it fit compound-4 well. So conformer-II was also suggested to be an active conformer as analgesics.
Although both conformers of (+)-meptazinol were suggested to be active conformers against the µ opioid receptor, their pharmacophores were different to each other. Conformer-I was suggested to fit typical opiate pharmacophore (Pharm-I) and conformer-II shared similar properties with benzofuro[2,3-c]pyridin-6-ol analogs. These conformers exist in similar amounts in solution and could not transform to each other freely in ordinary conditions. Although it is also possible that (-)-meptazinol may also account for analgesics and reduced side-effects as meptazinol was marketed as a racemic mixture. The existence of multiple analgesic mechanisms in (+)-meptazinol may provide further insight into meptazinol’s complex pharmacological behavior.
References
- Hoskin PJ, Hanks GW. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs 1991;41:326-44.
- Green D. Current concepts concerning the mode of action of meptazinol as an analgesic. Postgrad Med J 1983; 59: 9–12. Supp1.
- Spiegel K, Pasternak GW. Meptazinol: a novel mu-1 selective opioid analgesic. J Pharmacol Exp Ther 1984;228:414B.
- Li W, Hao JL, Tang Y, Chen Y, Qiu ZB. Structural comparisons of meptazinol with opioid analgesics. Acta Pharmacol Sin 2005;26:334-8.
- Tillack JV, Seccombe RC, Kennard CHL, Oh PWT., Analgetics I. Crystal structure of pethidine hydrochloride, 4-carbethoxy-1-4-phenylpiperidine hydrochloride, C15H21NO2·HCl. Recl Trav Chim Pays-Bas 1974;93:164-5.
- Casy AF, Dewar GH, Al-Deeb OAA. Conformational equilibra of hydrochloride salts of pethidine, ketobemidone, and related central analgesics of the 4-arylpiperidine class. J Chem Soc Perkin Trans 2 1989;2:1243-7.
- Daum SJ, Martini CM, Kullnig RK, Clarke RL. Analgesic activity of the epimeric tropane analogs of meperidine. Physical and pharmacological study. J Med Chem 1975;18:496-501.
- Zimmerman DM, Cantrell BE, Swartzendruber JK, Jones ND, Mendelsohn LG, Leander JD, et al. Synthesis and analgesic properties of N-substituted trans-4a-aryldecahydro isoquinolines. J Med Chem 1988;31:555-60.
- Froimowitz M, Salva P, Hite GJ, Gianntsos G, Suzdak P, Heyman R. Conformational properties of α- and β-azabicyclane opiates. The effect of conformation on pharmacological activity. J Comput Chem 1984;5:291-8.
- Hutchison AJ, de Jesus R, Wiliams M, Simke JP, Neale RF, Jackson RH, et al. Benzofuro[2,3-c]pyridine-6-ols: synthesis, affinity for opioid subtypes, and antinociceptive activity. J Med Chem 1989;32:2221-6.
- Froimowitz M, Pick CG, Pasternak GW. Phenylmorphans and analogs-opioid receptor subtype selectivity and effect of conformation on activity. J Med Chem 1992;35:1521-5.
- Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP. AM1: a new general purpose quantum mechanical model. J Am Chem Soc 1985;107:3902-9.
- Casy A F, Parfitt RT. Opioid analgesics: Chemistry and receptor. Plenum Press: New York and London, 1986, p485.
- Tyers MB. A classification of opiate receptors that mediate antinociception in animals. Br J Pharmacol 1980;69:503-12.
- Zimmerman DM, Nickander R, Horng JS, Wong DT. New structural concepts for narcotic-antagonists defined in a 4-phenylpi-peridine series. Nature 1978;275:332-4.
- Portoghese PS, Alreja BD, Larson DL. Allylprodine analogs as receptor probes - evidence that phenolic and nonphenolic ligands interact with different subsites on identical opioid Receptors. J Med Chem 1981;24:782-7.
- Casy AF, Ogungbamila FO. Phenolic analogs of reversed esters of pethidine. J Pharm Pharmacol 1985;37:121-3.
- Horn AS, Rodgers JR. The enkephalins and opiates: structure-activity relations. J Pharm Pharmacol 1977;29:257-65.
- Hodgson DJ, Rychlewska U, Eliel EL, Manoharan M, Knox DE, Olefirowicz EM. Rotational conformation of the phenyl moiety in geminally substituted phenylcyclohexanes with equatorial phenyl. J Org Chem 1985;50:4838-43.
- Allinger NL, Tribble MT. Conformational analysis LXXVIII. The conformation of phenyl cyclohexane and related molecules. Tetrahedron Lett 1971;12:3259-62.
- Burke TRJ, Bajwa BS, Jacobson AE, Rice KC, Streaty RA, Klee WA. Probes for narcotic receptor mediated phenomena 7. Synthesis and pharmacological properties of irreversible ligands specific for mu and delta opiate receptors. J Med Chem 1984;27:1570-4.
