Ginkgo biloba extract suppresses hypertrophy and extracellular matrix accumulation in rat mesangial cells1
Introduction
Diabetic nephropathy (DN) is one of the most common microangiopathies and ultimately leads to chronic renal failure. Hypertrophy of the mesangial cell and accumulation of the extracellular matrix (ECM) in the mesangial region is mainly composed of glomerulosclerosis. High glucose is presumed an initiating agent and increased transforming growth factor-β1 (TGF-β1) is thought the key cytokine involved in the progression of DN[1]. It has been found that the kidneys of DN rats exhibit oxidative stress[2]. Studies have found that high glucose can induce oxidative stress or reactive oxygen species (ROS) expressions and TGF-β1 synthesis in cultured mesangial cells[1,3]. The addition of antioxidants to high glucose caused a significant reversal of fibronectin and collagen IV gene expressions[3,4]. TGF-β1 plays an important role in cell hypertrophy and glomerular ECM accumulation by autocrine and paracrine methods. Interestingly, it has been found that ACE-inhibitors or Ang II receptor blockers can lower enhanced TGF-β1 and mesangial matrix accumulation[5]. This effect may be via the reduction of Ang II-stimulated TGF-β1 production[6]. With diabetes mellitus, high glucose and many factors including TGF-β1, Ang II and ROS may also activate various kinds of signals that arrest cells in the G1 phase inducing cell hypertrophy. It has also been found that hypertrophic cells could secrete more ECM[7]. Both create an infernal circle that leads to the aggravation of DN.
A central component of TGF-β1-stimulated ECM accumulation is the TGF-β1 family-specific Smad signal transduction pathway. TGF-β1 activates Smad2/3 by activating TGF-β1 receptors, after which it partners with Smad4 and translocates to the nucleus, where they act as transcriptional regulators of target genes including TGF-β1, tissue inhibitors of metalloproteinase (TIMP), collagen IV and laminin[8]. Smad6 and Smad7, the inhibitory Smads, appear to specifically inhibit Smad2/3 activation by blocking its access to TGF-β1 receptors.
Ginkgo biloba extract (EGb) is taken from the leaves of ginkgo biloba by modern extraction techniques. It is a mixture containing flavonoid glycosides (>24%) including quercetin, kaempferol, isorhamnethin, and terpene lactones (>8%) including bilobalide, ginkgolide. It has been found that EGb ameliorates hemodynamics, suppresses platelet-activating factor (PAF), scavenges ROS, relaxes vascular smooth muscles, and so on[9,10]. It has also been found that flavonoid glycosides have the effect of suppressing ACE activity and suppressing glycation end-products (AGE) expression[11,12]. All of these offer us a pharmacological foundation of EGb for DN therapy. Many scholars have explored the effects of EGb and ascertained its protective effects on DN in vivo. Most of EGb’s protective qualities were thought to be closely related to its hemodynamic action. On the cellular level, however, the protective mechanisms of EGb on glomerulosclerosis of DN have not been identified.
In our present work, using captopril as an antifibrotic control drug, we studied the possible influence of EGb in mesangial cells on the level of cell cycle, TGF-β1, Smad2/3, Smad7, collagen IV, laminin and antioxidases, which were all closely related to the glomerulosclerosis of DN.
Materials and methods
Materials Rat mesangial cells were provided by the China Center for Type Culture Collection (CCTCC) in Wuhan University (N
Considering that EGb can not dissolve in water completely, EGb and DMEM with D-glucose at 25 mmol/L were mixed with 1.0% ethanol in different concentrations.
Mesangial cell culture Taking the 5th–8th generation of mesangial cells, after incubating 24 h under normal conditions (containing 5.56 mmol/L glucose, 10% FBS, 100 IU/mL penicillin and 100 mg/mL streptomycin, 37 ºC, 5% CO2), we divided the cells into 7 groups, of which every group contained 6 bottles: normal glucose group (NS; DMEM), solvent control group (ET; 1.0% ethanol-DMEM), high glucose group (HG; 25 mmol/L glucose-DMEM), low dose of EGb group (GL; 10 µg/mL EGb-25 mmol/L glucose -DMEM), moderate dose of EGb group (GM; 20 µg/mL EGb-25 mmol/L glucose -DMEM), high dose of EGb group (GH; 40 µg/mL EGb-25 mmol/L glucose -DMEM), and captopril group (1 µmol/mL captopril-25 mmol/L glucose -DMEM). Cells were harvested after 48 h incubation. Adjusting the cell population to 1.5×106/mL, we took 1 mL of cell suspension from every group. The suspensions were then centrifuged three times at 100×g at 4 ºC for 8 min. The cell pellet was then added into 2 mL of cell lysis solution. After being shaken severely, the mixture was centrifuged at 650×g at 4 ºC for 8 min. The supernatants were stored at -20 ºC for analysis at a later stage.
Cell cycle analysis by flow cytometry[13] After incubation for 72 h, cells were harvested with 0.25% trypsin and washed three times with PBS buffer for 100×g at 4 ºC for 8 min. The cell pellets were fixed with 70% ethanol at 4 ºC for 24 h. And the ethanol was washed off with PBS buffer. The cell pellets were added into 0.5 mL propidium iodide-DNA fluorescein staining solution for 30 min at 4 ºC. Then the mixture was put in the sample chamber to be examined at 488 nm of excitation wave. 10 000 cells were detected in every sample and the cell cycle was analyzed by Mod Fit 2.0 package (Becton Dickinson, USA).
Determining the quantity of collagen IV and laminin[14] The stored supernatant at -20 ºC was tested by radioimmunoassay kits from Shanghai High Biotech Center (Lot N
Immunocytochemistry measurements of Smad2/3 and Smad7[15] The glass slides were pretreated with 10% polylysine, then placed on 24-well culture plates. The cells were transferred to the glass slides to incubate for 72 h. The supernatants were displaced and the cells were fixed by 4% paraformaldehyde (PFA) for 30 min. After permeation with 0.1% Triton-X-100 for 15 min, the cells were incubated with rabbit polyclonal anti-Smad2/3 at a dilution of 1:100 at 37 ºC for 2 h. After being washed, goat anti-rabbit IgG-horseradish peroxidase was added. To visualize Smad2/3, cells were stained with 3.3-diaminobenzidine (DAB) for 30 min and then examined by light microscopy (×400). All steps were performed at room temperature. Smad7 measurement was identical to Smad2/3. The stained Smad2/3 and Smad7 were quantified by gray scale analysis (Leica Qwin Standard V2.6; Leica Microsystems, Welzlar, Germany).
RT-PCR for the relative quantities of TGF-β1 mRNA of mesangial cells[16] A reverse transcription polymerase chain reaction (RT-PCR) procedure was performed to determine the relative quantities of TGF-β1 mRNA in mesangial cells, while β-actin mRNA, the house-keeping gene, was used as an internal control. Total RNA was extracted from mesangial cells with Promega Totle RNA Isolation system (Lot N
Measurement of oxidative stress in mesangial cells[14] Total antioxidative capability (T-AOC), catalase (CAT), total superoxidase dismutase (T-SOD) and glutathione-peroxidase (GSH-Px) activities of the stored supernatants were measured by spectrophotometry, using kits from Jiancheng Bioengineering Institute (Lot N
Statistical analysis Statistical analysis was performed to compare the effects of EGb on mesangial cells using one-way analysis of variance (ANOVA) and Dunnett’s t-test (2-side) for different groups using SPSS 10.0. Data were expressed as the mean±SD. P<0.05 was considered statistically significant.
Results
Effect of EGb on cell cycle of mesangial cells The cell cycle of ET group using 1.0% ethanol as solvent was of no significant difference to that of the NS group using normal sodium as solvent (P>0.05), which suggested that 1.0% ethanol had no significant effect on cell cycle and 1.0% ethanol could be used to incubate mesangial cells. The G0/G1 phase percentage of the HG group was significantly higher and the S phase percentage was lower than that of the ET group (P<0.05 or P<0.01). When compared with those of the HG group, the G0/G1 phase percentages of GL, GM, and GH were decreased and S phase percentages were increased in a concentration-dependent manner. The difference was significant (P<0.05 or P<0.01). The G0/G1 phase and S phase percentages of GM had no great difference to those of the ET group, indicating that the moderate dose of EGb could reverse the cell cycle changes in high glucose. The G0/G1 phase percentage of captopril was lowered and the S phase percentage was raised slightly, but they were not statistically significant (P>0.05) (Table 1).
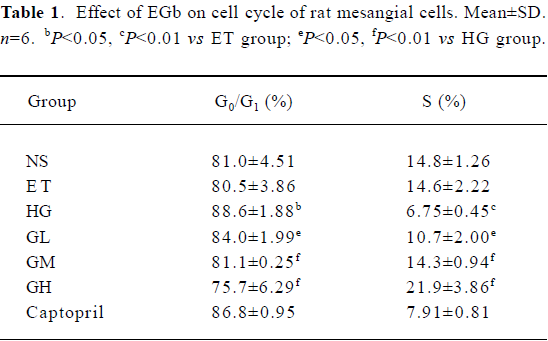
Full table
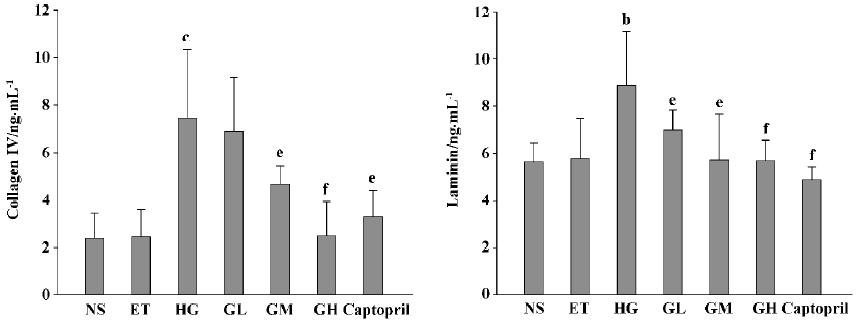
Effects of EGb on collagen IV and laminin of mesangial cells The levels of collagen IV and laminin of the ET group were of no significant difference to those of the NS group (P>0.05), suggesting that 1.0% ethanol had no significant effect on the cell expressions of collagen IV and laminin. The levels of collagen IV and laminin of the HG group were significant increased, when compared with those of the ET group (P<0.05 or P<0.01). Collagen IV levels of the GM, GH, and captopril group were strikingly lower than those of the HG group (P<0.05 or P<0.01). The level of the GH group was similar to that of the captopril group (P>0.05). Laminin levels of the GL, GM, GH, and captopril groups were all decreased (P<0.05 or P<0.01). These results suggested that EGb could decrease the expressions of collagen IV and laminin in mesangial cells and captopril’s capability of decreasing expressions of collagen IV and laminin was between that of EGb’s moderate and high dose (P>0.05) (Figure 1).
Immunocytochemistry analysis of Smad2/3 and Smad7 of mesangial cells Mesangial cells looked like an irregular star or a fusiform. The color of the stained Smad2/3 or Smad7 protein was brown. Smad2/3 or Smad7 was expressed in cytoplasm, but Smad2/3 could bind to Smad4 translocating into the nucleus. So Smad7 could be found only in the cytoplasm, whereas Smad2/3 could be seen in both the cytoplasm and nucleus (Figures 2 and 3). The staining intensity of Smad2/3 of the HG group was highly increased and Smad7 was markedly decreased, when compared with those of the ET group. After the exposure of mesangial cells to EGb or captopril, the intensity of Smad2/3 became scarce and Smad7 became intense.
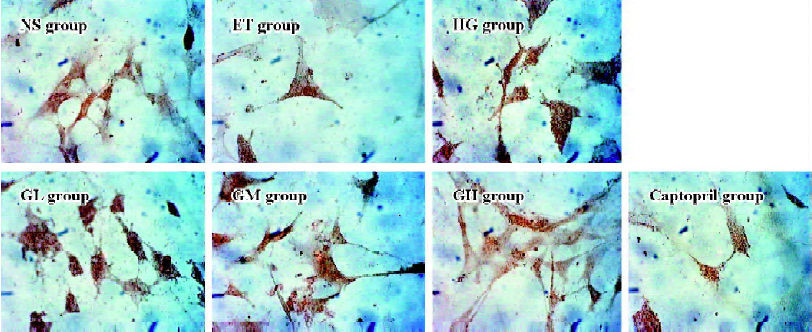
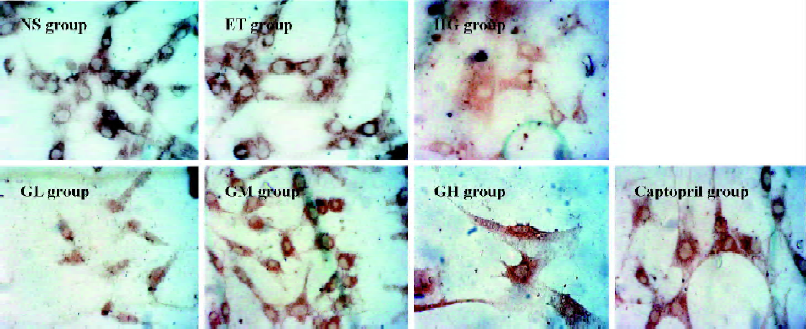
Using gray scale analysis to quantify Smad2/3 and Smad7 proteins, we found that the levels of Smad2/3 and Smad7 of the ET group were of no significant difference to those of the NS group (P>0.05), suggesting that 1.0% ethanol had no significant effect on the cell expressions of Smad2/3 and Smad7 (Figure 4).
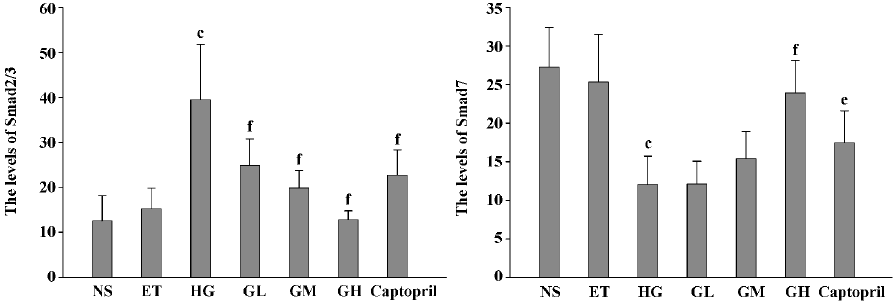
The level of Smad2/3 in the HG group was strikingly higher than that of the ET group and Smad7 was markedly lowered. They had significant difference when compared to those of the ET group (P<0.01). With the increasing concentration of EGb, the expressions of Smad2/3 in GL, GM, and GH were significantly decreased (P<0.01). The expressions in the captopril group were also decreased (P<0.01), and the level of Smad2/3 in the captopril group was between the GL group and GM group. The captopril group and the high dose of EGb could significantly increase the expression of Smad7 (P<0.01). The moderate dose of EGb also increased the expression of Smad7, but it had no significant difference when compared to that of the HG group. All of these results suggested that EGb had a potent influence on cell expressions of Smad2/3 and Smad7 and EGb could reverse the changes of Smad2/3 and Smad7 when mesangial cells were exposed to high glucose.
Effect of EGb on the relative quantity of TGF-β1 mRNA of mesangial cells The RT-PCR products of TGF-β1 were separated by 1% agarose electrophoresis, after which we could see distinct bands (Figure 5A). The relative quantity of TGF-β1 mRNA of the ET group was of no significant difference to that of the NS group (P>0.05), suggesting that 1.0% ethanol had no significant effect on the cell expression of TGF-β1 mRNA.
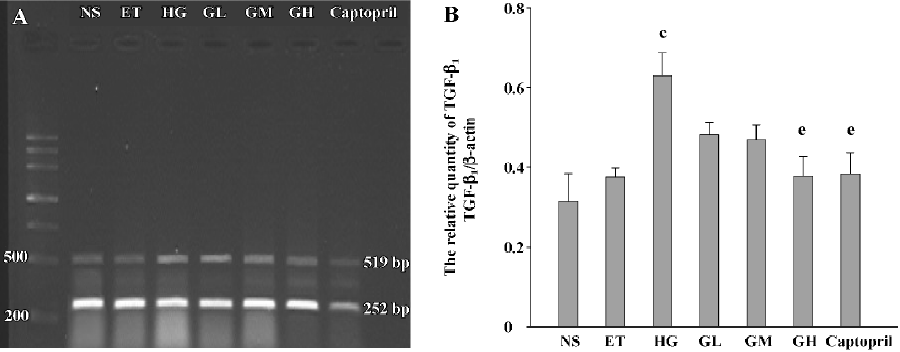
The relative quantity of TGF-β1 mRNA of the HG group was greatly higher than that of the ET group (P<0.01). The TGF-β1 mRNA level of the GH group and captopril group was strikingly decreased when compared with that of the HG group (P<0.05). The levels of the GL and GM group were also decreased, but the differences were not significant (P>0.05). The level of the GH group was similar to that of the captopril group. The results suggested that the high dose of EGb had a similar capability to captopril of decreasing the expression of TGF-β1 mRNA (Figure 5B).
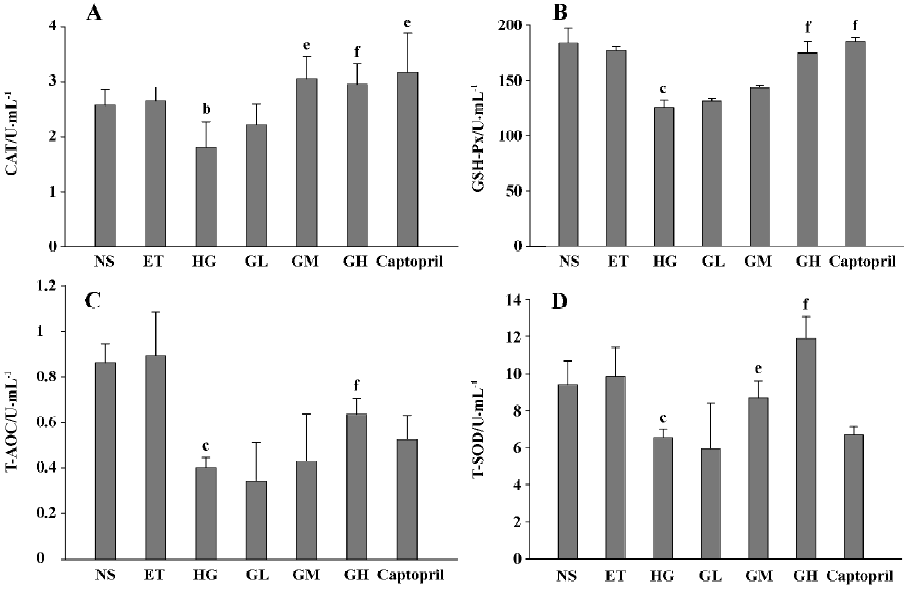
Effects of EGb on oxidative stress The CAT, GSH-Px, T-AOC, and T-SOD activities of the ET group were of no significant difference to those of the NS group (P>0.05), suggesting that 1.0% ethanol had no significant effect on the cell antioxidative indexes activities. The activities of the four antioxidases of HG were all lower than those of the ET group (P<0.05 or P<0.01), suggesting that mesangial cells in high glucose displayed oxidative stress. We also found that moderate and/or high doses of EGb increased the four antioxidases activities (P<0.05 or P<0.01). The activities of the GL group also increased but the differences were not significant. These results indicated that EGb could ameliorate the oxidative stress state of mesangial cells in high glucose. Captopril also significantly increased CAT and GSH-Px activities (P<0.05), but it had no evident effect on T-AOC and T-SOD (P>0.05) (Figure 6).
Discussion
Mesangial cells are a special kind of cell that can synthesize and secrete many protein factors regulating the structure and function of glomerulus. Alteration in mesangial cell function is central to the progression of glomerular disease in numerous models of chronic renal failure. It has been found that high glucose can stimulate the expression of Ang II, ROS, and TGF-β1[17]. Ang II may directly induce the irregularity of hemodynamics in the kidneys, and may also stimulate the expression of TGF-β1, ROS, and ECM. TGF-β1 itself can also induce accumulation of ECM mediated by signals such as MAPK, Smads, PKC, PKA, Ca2+, and so on, and the signals interact with each other constituting a complicated network which leads DN to aggravation[8]. The Smads protein is thought to be one of the most important factors in the process of ECM accumulation. Researchers have found that mesangial cell hypertrophy and the accumulation of ECM in the mesangial region consists mainly of glomerular sclerosis, while the autocrining cytokines of mesangial cells is a non-ignored element. The manifestations of diabetic nephropathy may be a consequence of the actions of certain cytokines and growth factors. Therefore, the research of mesangial cells has been a warm spot in DN research. In addition to blocking RAS and suppressing oxidative stress and TGF-β1 expression, the interference in Smads signals following TGF-β1 would be a new pathway to delay the progression of DN.
Cell cycle is an elementary process in vital movements of cells, and it has a close connection with the cell proliferation, differentiation and apoptosis. In the G1 phase cells synthesize RNA and protein. If the main protein or RNA is modified in the G1 phase cells can not step into the S phase. So G1/S transition is a restriction point in the cell cycle. If cells can progress to G1/S transition, cells will show proliferation; if cells can not progress to transition, cells will undergo cellular hypertrophy through stimulated protein synthesis. High glucose-mediated expression of TGF-β1 is pivotal for G1-phase arrest because neutralizing anti-TGF-β1 antibodies convert the G1-phase arrest into a proliferative phenotype[18]. This neutralization experiment clearly demonstrates that TGF-β1 is a necessary prerequisite for the development of cell hyper-trophy. G1 growth arrest is regulated by many elements of cell cycle machinery. Among regulators of G1 progression, the increased cyclin inhibitors p27 and p21 have been recognized as main regulators of TGF-β1-induced cell-cycle arrest in DN[19,20]. Flow cytometry is a technique for analyzing unicells rapidly. The cell cycle is analyzed acting on DNA contents. In our present study, we found that the percentage of G0/G1 in the HG group was increased and S phase percentage was decreased accompanied by increased TGF-β1, which is consistent with former reports[7,19]. When cells were cocultured with EGb, G0/G1 percentage was decreased, while the S percentage was increased correspondingly in a concentration-dependent manner. We also found that a middle dose of EGb could reverse the cell cycle changes in high glucose, which indicates that EGb has a potent effect on cytoprotective action, and that captopril do not significantly affect the cell cycle. All of these results demonstrate that high glucose/TGF-β1 induce the G1-phase arrest stimulating cell hypertrophy and that EGb could strikingly raise S phase percentage, therefore leading cells to surpass G1/S restriction. In this respect, EGb might have much more protection on mesangial cells than captopril. It has been found quercetin could suppress cell hypertrophy by degrading the expression of p27 in glomerulus[21]. But little is known about EGb’s cell protection, so it would be worthwhile to further investigate its potential mechanisms of manifold ingredients on cell cycle.
The accumulation of ECM is the result of imbalance between synthesis system such as plasminogen activator inhibitor-1 and TIMP and resolution system including matrix metalloproteinase. TGF-β1 is closely associated with the accumulation of mesangial ECM. The Smads protein following TGF-β1 is thought to be one of the most important factors in the process of ECM accumulation. In our experiment, the expressions of collagen IV and laminin of the HG group were strikingly increased. To trace back to their upstream signals, we found the expressions of Smad2/3 and the relative TGF-β1 level were also increased, while the inhibitory signal Smad7 was decreased. This indicates that it is high glucose that initially induces the increased expression of TGF-β1, ultimately resulting in the accumulation of collagen IV and laminin. In other words, it is the activated TGF-β1/Smads signal pathway that induces the accumulation of ECM. TGF-β1/Smads/ECM is obviously a linked response. Our results are in correspondence with other colleagues[22]. After cells were incubated with EGb, the expressions of TGF-β1 and Smad2/3 were lowered significantly, and Smad7 was raised greatly, suggesting that EGb suppress the TGF-β1/Smads/ECM response when mesangial cells are composed to high glucose. Recently Ang II blockade is rapidly becoming a standard antifibrotic therapy in renal diseases, and its mechanism has been a theme of research[23]. It is largely because ACE-inhibitors block up TGF-β1 induced by Ang II. Colleagues have proved that it is by suppressing TGF-β1/Smads signal pathway that ACE-inhibitors (N-acetyl-seryl-aspartyl-lysyl-proline, Ac-SDKP) decrease the accumulation of ECM in mesangial cells[24]. In our study we also found that an ACE-inhibitor (captopril) had a potent effect on suppressing ECM. The effect of EGb on ECM is similar to that of captopril. We know that the hypertrophic cells could express more ECM, so the potent effect of EGb suppressing ECM expression might be inseparable from its action of powerfully regulating cell cycle.
Oxidative stress has been known to play an important role in the development and progression of DN, and ROS is a direct consequence of hyperglycemia. It has been found that TGF-β1 and AGE could also activate ROS[25]. ROS activates other signaling molecules, such as PKC and MAPK and transcription factors including NF-kappa B, activator protein-1 and specificity protein 1 leading to a transcription of genes encoding cytokines, growth factors, and ECM proteins, all of which are closely relative to DN[26]. Various antioxidants inhibit mesangial cell activation by high glucose and ameliorate features of DN. It has been reported that the glucose-induced collagen IV expression can be partially reversed by the addition of two structurally unrelated antioxidants, trolox and α-lipoic acid, in porcine mesangial cells[3]. So antioxidant treatment is a potential antifibrotic therapy. N-acetylcysteine, a classic antioxidant, was used as an effective antifibrotic drug by some scholars[27]. In our study, after the cells were incubated with EGb, we found that CAT, T-AOC, T-SOD, and GSH-Px activities (common indicators for changes in the antioxidation system) were all increased significantly accompanied by decreased collagen IV and laminin expressions, strongly suggesting that EGb has a potent antioxidative capability and antifibrotic capability in vitro. This result is consistent with a former report, in which EGb suppressed oxidized LDL-stimulated fibronectin production through an antioxidant action in rat mesangial cells[28]. In another report EGb was used as a free radical scavenger[10]. The control drug captopril also had significant effects on enhancing CAT and GSH-Px activities, but had no significant effect on T-AOC and T-SOD. These data suggest that EGb may have a more profound effect than captopril on ameliorating the oxidative stress state of mesangial cells and antifibrotic action.
More recently, scholars have paid more attention to the interaction between ROS and TGF-β1/Smads in DN. It has been found that AGE-RAGE-mediated ROS generation activates TGF-β1-Smads signals and subsequently induces mesangial cell hypertrophy and fibronectin synthesis by autocrine production of Ang II[25]. It has also been found that H2O2 mediated the activation of ERK with the Smads pathway in TGF-β1 induction of p21[29]. Therefore, we could conclude that ROS and TGF-β1/Smads signals interact mutually and upregulate each other in the pathogenesis of DN. In our present study, we could see that EGb could act as an antioxidant suppressing TGF-β1-Smads signals and could also suppress TGF-β1 expression ameliorating the oxidative stress state. Both observations could suppress ECM accumulation and cell hypertrophy in mesangial cells. Therefore, the non-hemodynamic effects of EGb on DN are also significant.
In conclusion, EGb can regulate the mesangial cell cycle, ameliorate oxidative stress state, suppress the accumulation of collagen IV and laminin, decrease TGF-β1 and Smad2/3, and increase Smad7 expressions in cultured cells. That is to say, EGb can suppress cell hypertrophy and the accumulation of ECM mediated by TGF-β1/Smads and ROS signals on cell levels, which means it could vitally postpone glomerulosclerosis of DN.
References
- Sharma K, McGowan TA. TGF-β in diabetic kidney disease: role of novel signaling pathways. Cytokine Growth Factor Rev 2000;11:115-23.
- Xu Y, Osborne BW, Stanton RC. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol 2005;289:F1940-7.
- Catherwood MA, Powell LA, Anderson P, McMaster D, Sharpe PC, Trimble ER. Glucose-induced oxidative stress in mesangial cells. Kidney Int 2002;61:599-608.
- Kobayashi T, Uehara S, Ikeda T, Itadani H, Kotani H. Vitamin D3 up-regulates collagen expression in mesangial cells. Kidney Int 2003;65:1632-42.
- Liang XB, Zhang H, Zhou AY, Wang HY. AngRem104, an angiotensin II-induced novel upregulated gene in human mesangial cells, is potentially involved in the regulation of fibronection expression. J Am Soc Nephrol 2003;14:1443-51.
- Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol 2004;286:F1039-45.
- Wolf G, Schroeder R, Zahner G, Stahl RA, Shankland SJ. High glucose-induced hypertrophy of mesangial cells requires p27(Kip 1), an inhibitor of cyclin-independent kinases. Am J Pathol 2001;158:1091-101.
- Wang JY, Yin XX. Relationship between transforming growth factor-β1 signal transduction and renal fibrosis of diabetes. Chin J Clin Pharmacol Ther 2005;10:371-6. Chinese..
- Akisu M, Kultursay N, Coker I, Huseyinov A. Flunarizine and Ginkgo biloba extract reduce PAF concentration in the brain. Biol Neonate 1998;74:439-44.
- Haines DD, Bak I, Ferdinandy P, Mahmoud FF. Cardioprotective effects of the calcineurin inhibitor FK506 and the PAF receptor antagonist and free radical scavenger, EGb761, in isolated ischemic/reperfused rat hears. J Cardiovasc Pharmacol 2000;35:37-44.
- Bormann H, Melzig MF. Inhibition of metallopeptidases by flavonoids and related compounds. Pharmazie 2000;55:129-32.
- Wu CH, Yen GC. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J Agric Food Chem 2005;53:3167-73.
- Mou S, Zhang QY, Ni ZH, Luo HY, Shen GF. Expression of HGF and TGF-β in high glucose influences cell cycle regulation of human fibroblasts. Acta Univ Med Sec Shanghai 2002;22:201-4. Chinese..
- Yin XX, Zhang YD, Shen JP, Wu HW, Zhu X, Li LM, et al. Protective effects of bendazac lysine on early experimental diabetic nephropathy in rats. Acta Pharmacol Sin 2005;26:721-28.
- Zhang JH, Huang YJ, Cai WQ. Location and changes of expressions of Smad2, Smad3, Smad4, and Smad7 proteins in the 5/6 subtotally nephrectomized rat kidney. Acta Acad Med Mil 2004;26:1141-4. Chinese..
- Xu HQ, Hao HP. Effects of iridoid total glycoside from Cornus officinalis on prevention of glomerular overexpression of transforming growth factor beta 1 and matrixes in an experimental diabetes model. Biol Pharm Bull 2004;27:1014-8.
- Fukami K, Ueda S, Yamagishi S, Kato S, Inagaki Y, Takeuchi M, et al. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int 2004;66:2137-47.
- Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 1996;45:522-30.
- Wolf G, Schroeder R, Ziyadeh FN, Thaiss F, Zahner G, Stahl RAH. High glucose stimulates expression of p27 in cultured mouse mesangial cells: Relationship to hypertrophy. Am J Physiol 1997;273:348-56.
- Kuan CJ, Al-Douahji M, Shankland SJ. The cyclin kinase inhibitor p21 is increased in experiment diabetic nephropathy: Potential role in glomerular hypertrophy. J Am Soc Nehprol 1998;9:986-1003.
- Mei XB, Gao CR, Cui RL, Yuan WJ, Ye ZB, Fu P, et al. Quercetin protected the diabetic glomerulus through decreasing the level of cyclin-kinase inhibitor p27. Shanghai Med J 2003;26:246-8. Chinese..
- Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int 2003;63:2010-9.
- Yu L, Border WA, Anderson I, McCourt M, Huang Y, Noble NA. Combining TGF-beta inhibition and angiotensin II blockade results in enhanced antifibrotic effect. Kidney Int 2004;66:1774-84.
- Kanasaki K, Koya D, Suqimoto T, Isono M, Kashiwaqi A, Haneda M. N-Acetyl-seryl-aspartyl-lysyl-proline inhibits TGF-beta plasminogen activator inhibitor-1 expression via inhibition of Smad pathway in human mesangial cells. J Am Soc Nephrol 2003;14:863-72.
- Fukami K, Ueda S, Yamagishi S, Kato S, Inagaki Y, Takeuchi M, et al. AGEs activate mesangial TGF-beta-Smad signaling via an angiotensin II type I receptor interaction. Kidney Int 2004;66:2137-47.
- Ha H, Lee HB. Oxidative stress in diabete nephropathy: basic and clinical information. Curr Diab Rep 2001;1:282-7.
- Meurer SK, Lahme B, Tihaa L, Weiskirchen R, Gressner AM. N-acetyl-L-cysteine suppresses TGF-beta signaling at distinct molecular steps: the biochemical and biological efficacy of a multifunctional, antifibrotic drug. Biochem Pharmacol 2005;70:1026-34.
- Akiba S, Chiba M, Mukaida Y, Tamura A, Sato T. The leaf extract of Ginkgo biloba L suppress oxidized LDL-stimulated fibronection production through an antioxidant action in rat mesangial cells. Br J Pharmacol 2004;142:419-24.
- Kim YK, Bae GU, Kang JK, Park JW, Lee EK, Lee HY, et al. Cooperation of H2O2-mediated ERK activation with Smad pathway in TGF-β1 induction of p21 (WAF1/Cip1). Cell Signal 2006;18:236-43.
