Permeation-enhancing effects of chitosan formulations on recombinant hirudin-2 by nasal delivery in vitro and in vivo
Introduction
Hirudin, a 65–66 amino acid polypeptide (7 kDa), is one of the most potent inhibitors of thrombin and has proven to have outstanding anticoagulant and antithrombotic activities[1]. Recombinant hirudin (rHV), which can now be produced through DNA techniques, has a similar anticoagulative effect to natural hirudin[2]. Compared with other anticoagulants, including heparin, rHV possesses many advantages with respect to safety, antigenicity, and toxicity[3]. Currently, rHV has been used for the prophylaxis and treatment of heparin-induced thrombocytopenia (HIT), venous and arterial thrombosis, and shunt thrombosis, and the treatment of disseminated intravascular coagulation (DIC)[4,5].
However, because of its susceptibility to protease degradation and low mucosal permeability, only parenteral injection (iv or sc) is available for the delivery of rHV. Frequent injections, especially when rHV is indicated by chronic symptoms (and during prophylactic usage) would cause considerable discomfort to patients. Therefore, considerable effort has been directed towards developing alternative administration routes other than injection. Although recombinant hirudin-1 (rHV1) and rHV2 can be absorbed in the gastrointestinal tracts of rats after duodenal and oral administration, the absorption was limited or it varied depending on the analytical methods used, which indicated that the results were unreliable[6,7]. As a convenient method of administration, nasal delivery has many benefits relative to oral administration, including the avoidance of the liver first-pass effect and a higher bioavailability. Additionally, for polypeptides, nasal delivery is one of a few non-parenteral administrative routes that have gained regulatory approval so far. Nasal delivery formats for polypeptide drugs such as calcitonin, insulin, desmopressin and growth hormone are already commercially available or in clinical trials. However, there is limited information about intranasal delivery of rHV.
The main barriers to the nasal administration of hydrophilic peptides are mucosal penetration and mucociliary clearance. Chitosan, a positively charged bioadhesive polysaccharide, has been found to be able to improve the nasal absorption of peptides and reduce the clearance of liquid formulations from the nasal cavity through its bioadhesive characteristics, while causing negligible damage to the nasal mucosal membrane[8,9].
The present study was therefore intended to investigate the nasal administration of rHV using chitosan. To further improve the enhancing activity of chitosan, we studied the effects of chitosan with some enhancers on the permeation of rHV across excised rabbit nasal epithelium in vitro and the absorption of rHV by nasal delivery in rats. Furthermore, the mucosal ciliotoxicity of different formulations was also evaluated by using an in situ toad palate model[10]. In the present study, we chose rHV2 as the model drug. For the assays, the rHV2 was labeled with fluorescein isothiocyanate (FITC), which formed a stable covalent conjugate (FITC-rHV2) that could be assayed by fluorometry.
Materials and methods
Materials Recombinant hirudin-2 (rHV2, rHV-Lys47) was obtained from the College of Life Science (Peking University, Beijing, China), and chitosan (Mr 250 kDa, degree of deacetylation >85%) was from Yuhuan Ocean Biochemical Co (Zhejiang, China). FITC, Brij35, ethylenediamine tetraacetic acid (EDTA), lecithin and Sephadex G-25 were purchased from Sigma (St Louis, MO, USA), and sodium dodecylsulfate (SDS), hydroxyl-propyl-beta-cyclodextrin (HP-β-CD), menthol, l-dodecylazacycloheptan-2-one (Azone) and Tween 80 were from Beijing Chemical Co (Beijing, China). Glycyrrhizic acid monoammonium salt (GAM) was the product of Xinjiang Tianshan Pharmaceutical Industry Co (Wulumuqi, Xinjiang, China). All other chemicals were in analytical grade.
Animals Male rats (Sprague–Dawley, weighing 280–300 g) and male rabbits (Japanese White, weighing 2.5–3.0 kg) were obtained from the Experimental Animal Center of Weitonglihua (Beijing, China); toads (weighing 30–40 g) were from the Experimental Animal Center of the Health Science Center of Peking University (Beijing, China).
The care and handling of animals was performed with the approval of the Institutional Authority for Laboratory Animal Care.
Preparation and purification of FITC-rHV2 The synthesis of FITC-labeled rHV2 was based on the reaction between the isothiocyanate group of FITC and the tyrosine in rHV2[11]. FITC in 0.5 mol/L carbonate buffer (pH 9.5) was added into a 1/10 volume of rHV2 solution (20 g/L in 0.01 mol/L phosphate buffered saline [PBS]; pH 7.1); the molar rate of the two compounds was 3:1. After 4 h of reaction with magnetic mixture in the dark at 0–9 °C, FITC-labeled rHV2 was separated from unreacted FITC in a Sephadex G-25 column (2.0 cm ID×30 cm L) pre-washed with PBS (pH 7.4). A sample of approximately 1 mL was put on the top of the column and then washed with PBS as an elution solvent. The first yellow band was collected. After the collections were mixed, FITC-rHV2 was obtained by freeze-drying. All experiments were carried out under light exclusion conditions. The molecular weight of FITC-rHV2 was determined to be 7388.99 by mass spectrometry (data not shown). This result indicated that one rHV2 molecule had combined with one molecule of FITC. FITC-rHV2 and rHV2 were found to have similar activities when measured using the chromogenic thrombin substrate assay[12].
Preparation of FITC–rHV2 formulations For the in vitro studies, FITC-rHV2 was dissolved in 0.5% chitosan (pH 5.0, w/v) at a concentration of 400 mg/L for transport. In the in vivo studies, FITC-rHV2 was dissolved in 0.5% chitosan solution at a concentration of 36 g/L for intranasal administra-tion. When required, the enhancers were added into these formulations (menthol, Azone and lecithin were initially dissolved in propylene glycol). The concentration of FITC-rHV2 for transport control was 400 mg/L in Ringer’s solution and for subcutaneous administration was 0.5 g/L in 0.9% NaCl. All the formulations were prepared on the day of the experiments.
FITC-rHV2 assay The concentration of FITC-rHV2 was determined in a fluorescence spectrofluorometer (650–60; Hitachi, Japan) at an excitation wavelength of 495 nm and an emission wavelength of 515 nm. A standard curve was prepared using FITC-rHV2 at concentrations between 4 µg/L and 200 µg/L. The FITC-rHV2 concentrations of the test samples were estimated using the standard curve.
Nasal epithelium preparation Rabbit nasal epithelium was prepared as described by previous reports[13]. After each rabbit was killed, its nasal septum was surgically removed with a scalpel immediately, and then the epithelium was carefully excised from the septum and stored in ice-cold Ringer’s solution (pH 7.4; 125 mmol/L NaCl, 5 mmol/L KCl, 10 mmol/L NaHCO3, 1.2 mmol/L NaH2PO4, 1.4 mmol/L CaCl2 and 11 mmol/L D-glucose). The epithelium was used within 0.5 h of removal.
In vitro permeability experiments Prior to the experiment, Ringer’s solution was added to both sides of the horizontal diffusion chamber with a 4 mL volume in each side, and the excised rabbit nasal epithelium was mounted in the diffusion chamber at 37 °C. After an equilibration period of approximately 0.5 h, the buffer was replaced with the FITC-rHV2 solution on the donor side (mucosal), and fresh buffer on the receiver side (serosal). A 200 µL aliquot of sample was taken from the receiver side at particular times (0, 0.5, 1, 1.5, 2, 3, 4 and 6 h), and at the same time an equal volume of Ringer’s solution was added to the receiver side. The FITC-rHV2 concentration in the receiver side (Cr) was determined by FITC-rHV2 assay. All experiments were carried out under light exclusion conditions.
Calculation of the permeability coefficient The Cr values were plotted as a function of time (t) from 0 h to 6 h. The permeability coefficient (P) was calculated according to the following equation:
P = (dq/dt) / (C0 A)
where dq/dt (µg/s) represents the permeability rate, C0 (µg/mL) was the initial concentration in the donor chamber, and A (cm2) is the effective cross-sectional area available for diffusion (0.126 cm2). The transport enhancement ratios (ER) were calculated using the following equation:
ER = Penh / Pctrl
where Penh and Pctrl refer to the P values with added and no added enhancers, respectively. Student’s t-test was used to determine statistical significance.
In vivo studies The in vivo studies were performed as previously described[14]. The rats were fasted overnight before the study. Anesthesia was induced by intraperitoneal injection of 40 mg/kg sodium pentobarbital and maintained by additional 15 mg/kg doses as required. The rats were fixed on their backs on boards and were surgically prepared by cannulation of the trachea to enable breathing, cannulation of the carotid artery to facilitate blood sample collection, and ligation of the esophagus to prevent samples being swallowed. A 50 µL dose of the formulations was administered into the left nares via a flexible polyethylene tube attached to a microsyringe. In addition, 0.9% NaCl was administered as a control to ensure that there was no interference with FITC-rHV2. For the calculation of Fr, FITC-rHV2 solution (0.5 mg/kg) was subcutaneously administered by bolus injection. Blood samples (400 µL) were withdrawn from the carotid artery into the plastic microfuge tubes at particular times (0, 0.5, 1, 1.5, 2, 3, 4 and 6 h). The blood samples were anticoagulated with 3.8% (w/v) trisodium citrate solution at a ratio of 8.25:1.75 (v/v), and plasma was separated after centrifugation at 1 300×g for 5 min .
The fluorescence intensity in the plasma before and after trichloroacetic acid (TCA) precipitation was determined, and the intensity of the insoluble portion was used to calculate the intact FITC-rHV2 concentration[15].
Data analysis The area under the FITC-rHV2 concentration time curves (AUC) was calculated by using the trapezoidal method. The relative bioavailability (Fr) was calculated by comparing the area under the curve obtained after intranasal administration with that obtained after subcutaneous injection. Statistical analysis was performed using Student’s t-test. Differences were considered to be significant for values of P<0.05.
Ciliotoxicity evaluation An in situ toad palate model[10] was used. SDS (1%), Brij35 (5%), HP-β-CD (5%), Tween 80 (5%), EDTA (0.1%), and GAM (1%) were directly dissolved in a chitosan solution, and menthol (1.5%), Azone (4%), and lecithin (5%) were initially dissolved in propylene glycol and then in chitosan solution. The toads were fixed on their backs and their mouths were opened with pincers. The test formulations (0.5 mL) were applied to the upper palate of the toads for 30 min, and then the palates were rinsed twice with 0.9% NaCl. The palates were dissected out, and the mucocilia were examined with an optical microscope (Olympus, Japan). The duration of ciliary movement in different formulations was recorded and then the relative durations compared with 0.9% NaCl were calculated. Each group was duplicated 3 times and Student’s t-test was used to determine statistical significance.
Results
The time versus the amount of FITC-rHV2 that had permeated across the excised rabbit nasal epithelium after application of the chitosan solution with or without enhancers to the mucosal side is shown in Figure 1. The corresponding permeability coefficients and the transport enhancement ratios (ER) are listed in Table 1. When chitosan or chitosan with various enhancers was added into the FITC-rHV2 formulation, the amount of FITC-rHV2 moving across the epithelium increased, and there were significant increases in the permeability coefficient. We found that the permeability coefficient of FITC-rHV2 in various chitosan formulations was 1.7-fold to 33-fold higher than that without chitosan. In addition, there was a marked increase in the permeability coefficient of FITC-rHV2 in chitosan formulations with 1% SDS, 5% Brij35, 5% Tween 80, 1.5% menthol, 1% GAM or 4% Azone (P<0.05) compared with chitosan alone. No significant difference (P>0.05) in the permeability coefficient was observed between the chitosan formulations containing 5% HP-β-CD or 0.1% EDTA and chitosan alone, although there was a significant decrease in the permeability coefficient in the formulation containing 5% lecithin.
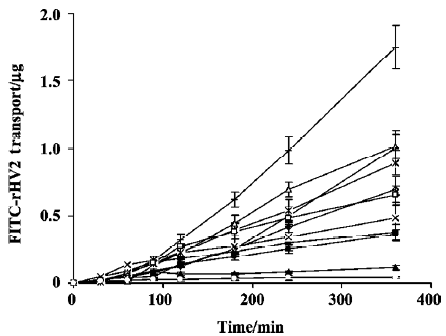
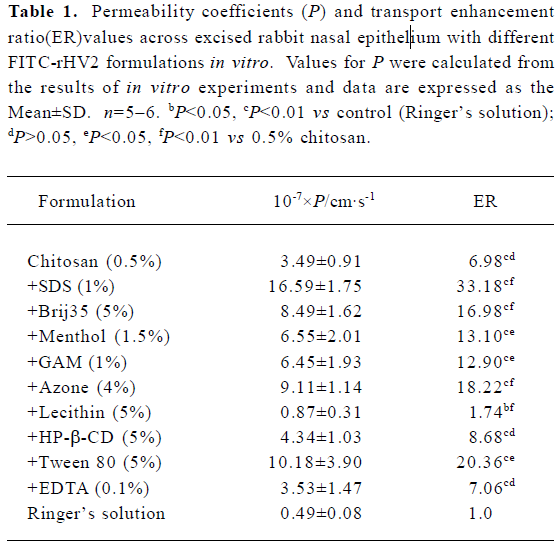
Full table
The mean plasma concentration of FITC-rHV2 versus time after nasal administration to rats using various formulations is shown in Figure 2, and the Fr values are given in Table 2. When administered intranasally, in a formulation containing neither chitosan nor enhancer (as a control), FITC-rHV2 was only poorly absorbed, with an Fr value of 1.86% compared with subcutaneous injection (Figure 2, Table 2). The addition of chitosan at a concentration of 0.5% resulted in a significant improvement, with an Fr value of 8.26%, which is 4-fold that of the control. After enhancers were added to the 0.5% chitosan solution, the Fr values of FITC-rHV2 changed markedly in some formulations. Compared with chitosan alone, the addition of 1% SDS, 5% Tween 80, 4% Azone, 1.5% menthol, 1% GAM or 5% Brij35 significantly increased the Fr of FITC-rHV2 (P<0.05), whereas the addition of 5% HP-β-CD, 5% lecithin or 0.1% EDTA did not increase Fr to any significant extent.
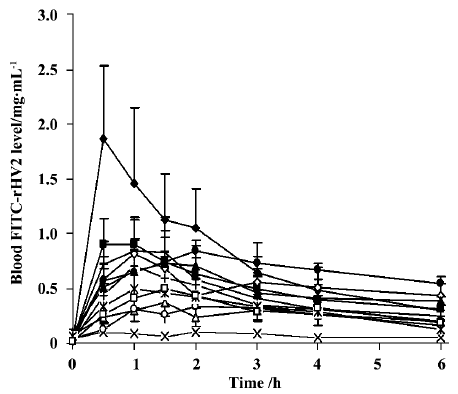
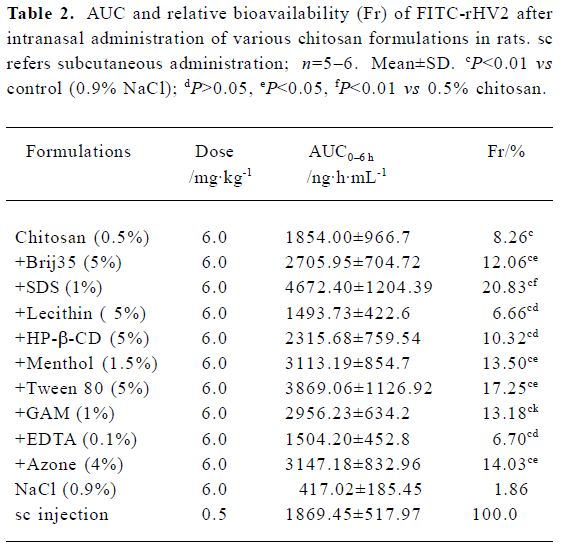
Full table
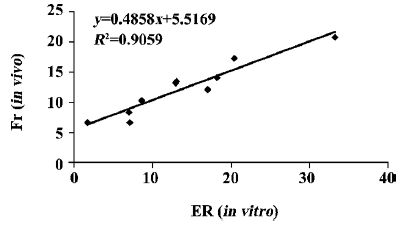
Regarding the permeability or absorption of FITC-rHV2 in chitosan formulations with or without enhancers, the results of the in vitro experiment (ER values) were in agreement with the in vivo results (Fr values), as shown in Figure 3 (R2=0.9059).
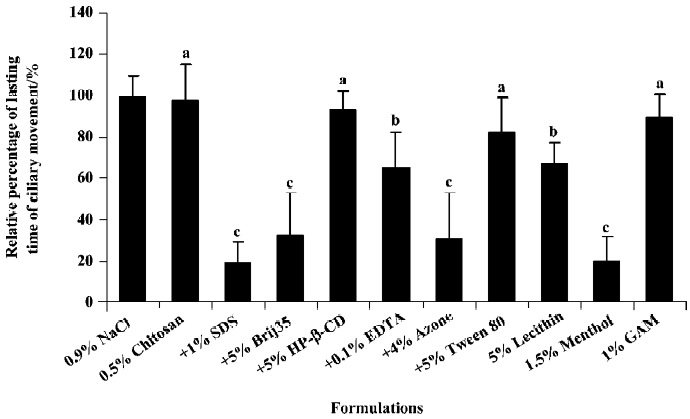
The effects of chitosan with or without enhancers on the ciliary movement duration in toad palate are shown in Figure 4. Ciliary movements were significantly inhibited by co-administration of chitosan with 1% SDS, 5% Brij35, 4% Azone, 5% lecithin, 0.1% EDTA or 1.5% menthol compared with the 0.9% NaCl control, but not significantly inhibited by 0.5% chitosan alone or 0.5% chitosan with 5% HP-β-CD, 5% tween80 or 1% GAM. Based on these data, the rank order of ciliotoxicity for different chitosan formulations based on the relative ciliary movement durations was as follows: no enhancer<0.5% chitosan<+5% HP-β-CD<+1% GAM<+5% Tween80<+5% lecithin<+0.1% EDTA<+5% Brij35<+4% Azone<+1.5% menthol<+1% SDS.
Discussion
For a nasal solution formulation, 0.5%–1.0% chitosan and a molecular weight greater than 100 kDa are preferred[16,17]. In the present study, we used 0.5% chitosan and found that at this concentration, the chitosan solution could significantly enhance the efflux as well as the nasal absorption of FITC-rHV2 both in vitro and in vivo. This result can be attributed to a combination of bioadhesion and a transient opening of the tight junctions in the cell membrane to allow hydrophilic macromolecules to pass through[8,9].
SDS, Brij35, Tween 80 and Azone are all percutaneous enhancers[18-20]. In our experiments, the addition of any of these agents into the chitosan solution significantly increased nasal absorption of FITC-rHV2 compared with chitosan alone. This result may be due to the fact that these compounds can all change the arrangement of the epithelial cell membrane phospholipids and increase the fluidity of the membrane lipid bilayers or interact with the membrane protein, therefore resulting in a transcellular pathway transport of FITC-rHV2. Chitosan was able to affect the paracellular pathway transport of FITC-rHV2 by its mucoadhesive properties and opening of the tight junction effect[8,9]. The combined effect of the two agents consequently increased the absorption of FITC-rHV2 compared with chitosan alone.
When EDTA was added to the FITC-rHV2 solution containing chitosan, EDTA activated protein kinase C by depletion of extracellular calcium via chelation, resulting in an expansion of the paracellular route[21], and chitosan interacted with the membrane protein, also causing the tight junctions to open. The combination of these two effects could potentially result in a marked enhancement of FITC-rHV2 absorption. However, the negatively charged carboxyl groups of EDTA interact with the positively charged amino groups of chitosan, which may inhibit the enhancing action of both agents. Therefore, in the present study we did not observe any absorption enhancement of FITC-rHV2 with EDTA added to chitosan compared with chitosan alone.
There exists a controversial explanation for the enhancing mechanism of cyclodextrins; that is, that the enhancing effects include the disaggregation of protein aggregates (insulin), an interaction with lipids and divalent cations on the membrane surface, and a direct effect on the paracellular pathway by a transient effect on tight junctions[22,23]. However, in most cases, cyclodextrin systems are used as a means of enhancing drug solubilisation[24]. In our experiment, when HP-β-CD was co-administered with chitosan, we did not observe the reported synergistic effect for FITC-rHV2 absorption[25]. The most likely reason is that FITC-rHV2 is not like insulin, which usually aggregates in hexamers in solution, and is more unstable in the presence of proteolytic enzymes in the nasal mucosa, so addition of HP-β-CD may lead to insulin deaggregation from hexamers to dimers and protect insulin from degradation by proteolytic enzymes in the nasal mucosa.
Phospholipids can bring about enhanced delivery of polar compounds administered nasally by inhibiting the apical membrane sodium channels and causing structural changes in tight junctions[26]. However, in our experiment a significant decrease in FITC-rHV2 absorption was observed when lecithin was added to the chitosan solution. This was probably because lecithin is a negatively charged compound, whereas chitosan has positively charged amino groups, so the two oppositely charged molecules might interact and produce no increase in FITC-rHV2 absorption.
Menthol is a monocyclic terpene. The mechanism of its enhancing effect is mainly due to it forming a eutectic with the penetrating compound, thereby increasing its solubility, and also enhancing the fluidity of the local lipid bilayers[27]. In the present study, the co-administration of menthol with chitosan markedly increased FITC-rHV2 absorption compared with chitosan solution alone, suggesting that the co-administration of the two agents cause the FITC-rHV2 molecule to more easily penetrate the cell membrane.
Sakai et al found that dipotassium glycyrrhizinate decreased intracellular calcium ion levels and did not induce any significant histomorphological changes in the actin filaments[28]. In addition, dipotassium glycyrrhizinate enhanced the cellular permeability of sodium fluorescein and fluorescein isothiocyanate dextran by enhancing the activation of a protein kinase C via sodium deoxycholate (an enhancer)[29]. Also, the combined use of the two enhancers had fewer toxic effects. In the present experiment we found that chitosan with GAM exerted an obvious enhancing effect on FITC-rHV2 absorption compared with chitosan alone, but it was not clear whether this effect was related to the mechanism described.
Because rat nasal epithelium covers an area that is too small to fit the device used in the in vitro experiment, we chose to use rabbit nasal epithelium instead. As shown in Figure 3, there is considerable correlation (R2=0.9059) between ER and Fr, suggesting a relationship between the in vitro and in vivo studies. Therefore, in the present study, it appears that using nasal mucosa from different animal species in the in vitro and in vivo experiments did not influence the correlation between the absorption-enhancing effects of formulations in vitro and in vivo.
For most absorption enhancers, a direct relationship may exist between the absorption-promoting effect and local toxicity, hence it is important to evaluate the local toxic effect for a prospective novel enhancer system. An in situ toad palate model was used in the present study to evaluate the ciliotoxicity of different formulations. Although recording the duration of ciliary movement may be less objective and less accurate than the ciliary beating frequency method, using a microscope it is possible to directly examine the quantity of fallen cilia, as well as the integrity of the mucosa at the same time. This method is especially suitable for the initial screening of drugs or formulations. Among the tested enhancers, chitosan alone and chitosan with HP-β-CD, GAM or Tween 80 were least ciliotoxic.
In conclusion, chitosan is an effective enhancer for increasing the nasal absorption of FITC-rHV2, and co-administration of chitosan with other enhancers can improve absorption further. Some chitosan formulations were less ciliotoxic than others. The chitosan formulation system could be a useful approach for improving nasal absorption.
Acknowledgement
The authors are very grateful to Prof Sheng-geng ZHU for kindly providing the rHV2.
References
- Walenga JM, Pifarre R, Fareed J. Recombinant hirudin as an antithrombotic agent. Drugs Future 1990;15:267-80.
- Markwardt F, Fink G, Kaiser B, Klocking HP, Nowak G, Richter M, et al. Pharmacological survey of recombinant hirudin. Pharmazie 1988;43:202-7.
- Doutremepuich C, Deharo E, Guyot M, Lalanne MC, Walenga JM, Fareed J. Antithrombotic activity of recombinant hirudin in rat: a comparative study with heparin. Thrombos Res 1989;54:435-45.
- Markwardt F. The development of hirudin as an antithrombotic drug. Thromb Res 1994;74:1-23.
- Markwardt F. Prospective clinical use of hirudin as an anticoa-gulant. Biomed Prog 1990;3:19-23.
- Han YM, Lu YF, Wang ZH. Studies on the effects of anticoagulant and anti-thrombin after duodenal administration of recombinant hirudin in rats. Chin J Hematol 1999;20:483-4.
- Yang XY, Wang XT, Zhang XN, Zhang Q. Gastrointestinal absorption of recombinant hirudin-2 in rats. J Pharmacol Exp Ther 2004;308:774-9.
- Schipper NG, Olsson S, Hoogstraate JA, deBoer AG, Varum KM, Artursson P. Chitosan as absorption enhancers for poorly absorbable drugs. 2: Mechanism of absorption enhancement. Pharm Res 1997;14:923-9.
- Aspden TJ, Mason JD, Jones NS, Lowe J, Skaugrud O, Illum L. Chitosan as a nasal delivery system: the effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J Pharm Sci 1997;86:509-13.
- Jiang XG, Cui JB, Fang XL, Wei Y, Xi NZ. Toxicity of drugs on nasal mucocilia and method of its evaluation. Acta Pharm Sin 1995;30:848-53.
- Wang HD, Zhou SH. Marking techniques of biomedicine. Beijing: People’s Medical Publishing House; 1995.
- Griessbach U, Stürzebecher J, Markwardt F. Assay of hirudin in plasma using a chromogenic thrombin substrate. Thromb Res 1985;37:347-50.
- Hosoya K, Kubo H, Natsume H, Sugibayashi K, Morimoto Y. Evaluation of enhancers to increase nasal absorption using chamber technique. Biol Pharm Bull 1994;17:316-22.
- Chandler SG, Illum L, Tomas NM. Nasal absorption in rats. II Effect of enhancers on insulin absorption and nasal histology. Int J Pharm 1991;76:61-70.
- Temesvari LA, Bush JM, Peterson MD, Novak KD, Titus MA, Cardelli JA. Examination of the endosomal and lysosomal pathways in Dictyostelium discoideum myosin I mutants. J Cell Sci 1996;109:663-73.
- Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res 1998;15:1326-31.
- Illum L, Farraj NF, Davis SD. Chitosan as a novel basal delivery system for peptide drugs. Pharm Res 1994;11:1186-9.
- Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci 1998;1:15-30.
- Rege BD, Kao JP, Polli JE. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharm Sci 2002;16:237-46.
- Sugibayashi K, Nakayama S, Seki T, Hosoya K, Morimoto YJ. Mechanism of skin penetration-enhancing effect by laurocapram. Pharm Sci 1992;81:58-64.
- Wang GS, Zabner J, Deering C, Launspach J, Shao JQ, Bodner M. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am J Respir Cell Mol Biol 2000;22:129-38.
- Marttin E, Verhoef JC, Cullander C, Romeijn SG, Nagelkerke JF, Merkus FW. Confocal laser scanning microscopic visualization of the transport of dextrans after nasal administration to rats: effects of absorption enhancer. Pharm Res 1997;14:631-7.
- Yang T, Hussain A, Paulson J, Abbruscato TJ, Ahsan F. Cyclodextrins in nasal delivery of low-molecular-weight heparins: in vivo and in vitro studies. Pharm Res 2004;21:1127-36.
- Merkus FW, Verhoef JC, Marttin E, Romeijn SG, van der Kuy PH, Hermens WA, et al. Cyclodextrins in nasal drug delivery. Adv Drug Deliv Rev 1999;36:41-57.
- Yu SY, Zhao Y, Wu FL, Zang X, Lü WL, Zhang H. Nasal insulin delivery in the chitosan solution: in vitro and in vivo studies. Int J Pharm 2004;281:11-23.
- Maitani Y, Nakamura K, Suenaga H, Kamata K, Takayama K, Nagai T. The effect of soybean-derived sterylglucoside and β-sitosterol β-D-glucoside on nasal absorption in rabbits. Int J Pharm 2000;200:17-26.
- Kaplun-Frischoff Y, Touitou E. Testosterone skin permeation enhancement by menthol through formation of eutectic with drug and interaction with skin lipids. J Pharm Sci 1997;86:1394-9.
- Sakai M, Imai T, Ohtake H, Azuma H, Otagiri M. Effects of absorption enhancers on cytoskeletal actin filaments in Caco-2 cell monolayers. Life Sci 1998;63:45-54.
- Sakai M, Imai T, Ohtake H, Azuma H, Otagiri M. Simultaneous use of sodium deoxycholate and dipotassium glycyrrhizinate enhances the cellular transport of poorly absorbed compounds across Caco-2 cell monolayers. J Pharm Biomed Anal 2004;36:915-9.
