High doses of bifendate elevate serum and hepatic triglyceride levels in rabbits and mice: animal models of acute hypertriglyceridemia1
Introduction
Atherosclerosis can lead to the development of coronary heart disease (CHD), cerebrovascular disease, and other peripheral vascular diseases. CHD, which causes more than twice as many deaths as do all forms of malignancy in USA, is currently the leading cause of death in many countries. As lipid deposition is a characteristic feature of athero-sclerosis, patients suffering from atherosclerosis often have plasma total cholesterol (TC) and/or triglyceride (TG) levels higher than those in non-affected populations[1–3]. In general, the mean plasma TC level is low in undeveloped/developing countries whose incidences of CHD are much lower than those in the developed countries, where the mean plasma TC level in the population is higher. Although many studies have shown that the pathogenesis of CHD involves multiple factors, high blood lipid levels frequently correlate with higher incidences of CHD. In recent years, much attention has been paid to the development of drugs used for the prevention and treatment of hyperlipidemia. In this regard, several animal models of hyperlipidemia, including diet-induced and transgenic models, have been developed for screening drug candidates that can lower blood TC/TG level[4–8].
Treatment with schisandrin B (Sch B), a dibenzocyclo-octadiene derivative isolated from Fructus Schisandrae, at high oral doses caused apparent increases in serum lipid levels in mice[9]. This observation has led to further investigations with an attempt to establish an animal model of hyperlipoproteinemia using Sch B[10]. Bifendate (biphenyl-dicarboxylate), a synthetic intermediate of schisandrin C (also a dibenzocyclooctadiene derivative) was found to protect against drug-induced liver injury and hepatitis in animal models[11,12], with the mode of action similar to that of Sch B and schisandrin C. Bifendate is now used clinically for the treatment of hepatitis. In the present study, the effects of bifendate treatment on serum and hepatic lipid levels as well as apolipoprotein levels are examined in rabbits and mice.
Materials and methods
Chemicals and reagents Bifendate (powdered pill suspended in 0.5% CMC) was purchased from Beijing Xiehe Pharmaceutical Factory (Beijing, China). Cholesterol (CHO) and bile salt were obtained from Beijing Chemical Reagent Company (Beijing, China). Inositol nicotinate and fenofibrate were bought from Beijing Yongkang Medical Company and Yimin Medical Company (Beijing, China), respectively. Both sodium carboxymethylcellulose (CMC) and polyethylene glycol 6000 (PEG) were obtained from Beijing Xudong Chemical Plant (Beijing, China). Assay kits for triglyceride (TG), total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C), apolipo-protein (Apo) A, and Apo B were bought from Zhong-sheng Beikong Bio-technology and Science Inc (Beijing, China).
Animal treatment Adult male ICR mice (26–28 g) [Grade II, Certificate N
Determination of lipid and apolipoprotein levels Serum samples were prepared by centrifuging the whole blood (obtained from the orbital vein in mice or ear vein in rabbits) for 8 min at 2000×g and then frozen at -20 oC until assay within 5 d. Liver tissue sample was homogenized in 9 volumes of 0.9% (w/v) NaCl solution by two 10-s bursts of a tissue disintegrator at 13 500 r/min, and the homogenate was then centrifuged at 2000×g for 15 min to obtain the supernatants. Hepatic supernatants 10 µL and 40 µL were used to determine the TG and TC concentrations, respectively. Both serum and hepatic TG and TC levels were artificially measured using 722 spectrophotometer (made in China), but serum LDL-C, HDL-C, Apo A-I, and Apo B were automatically determined using Olympus An 400 biochemical analysis apparatus (made in Japan).
Statistical analysis Data were analyzed using one-way ANOVA and expressed as mean±SEM. Significant difference between groups was detected by Duncan’s multiple range test using SPSS 12.0 software. Student’s t-test was used for comparison between two groups. The inter-group difference was considered as significant when P<0.05.
Results
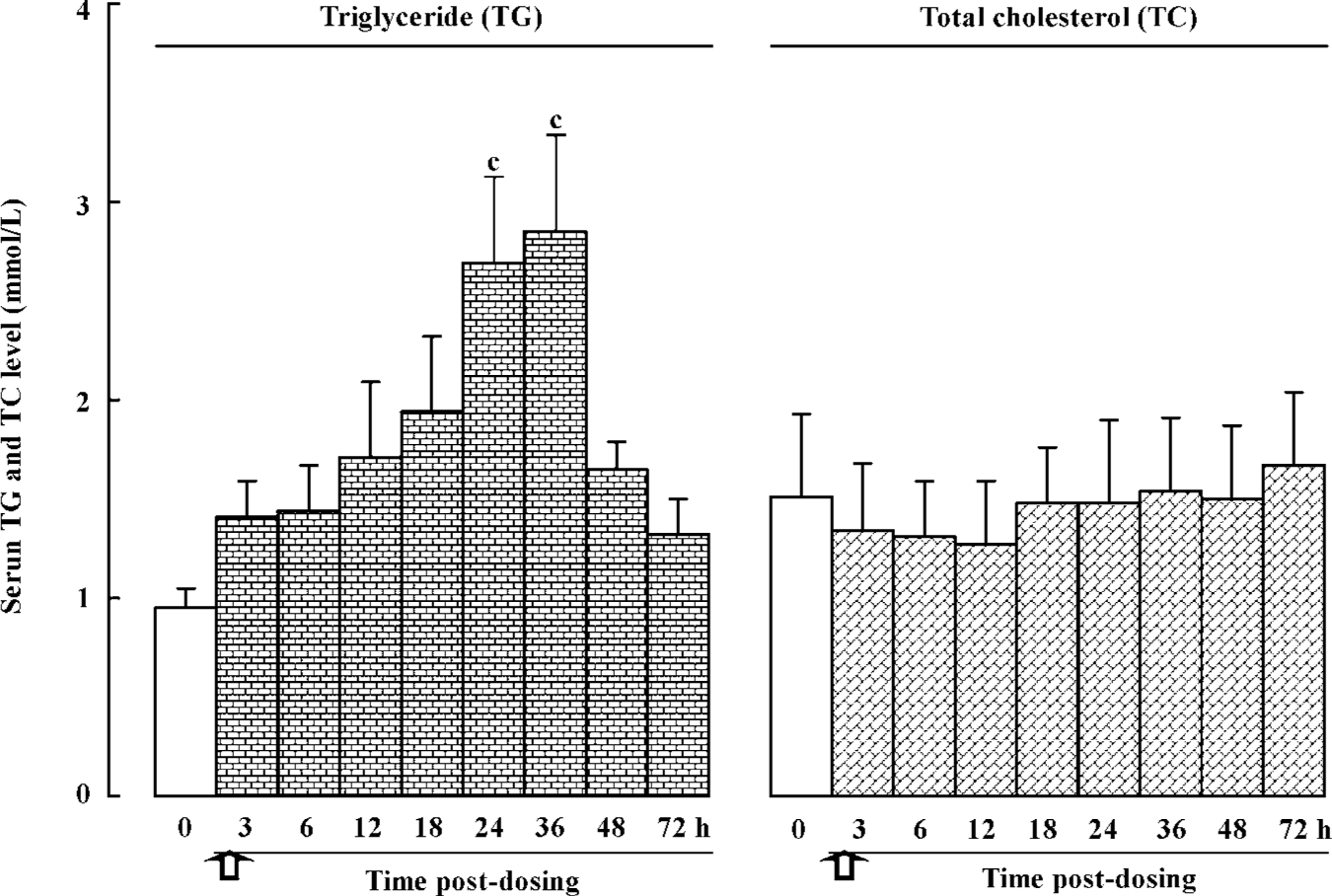
Effects of a single dose of bifendate on serum TG and TC levels in rabbits Prior to drug administration, a blood sample was obtained and serum TG and TC levels were measured in the rabbits. The values were used as baselines for comparison. Bifendate treatment (0.3 g/kg, ig) caused a time-dependent and biphasic change in serum TG level, with the extent of increase ranging from 39%–200% and the value reaching a maximum between 24–36 h post-dosing when compared with the baseline value. Serum TC levels in bifendate-treated rabbits was reduced (by 11%–15%) when compared with the baseline value, but the difference was not statistically significant (Figure 1).
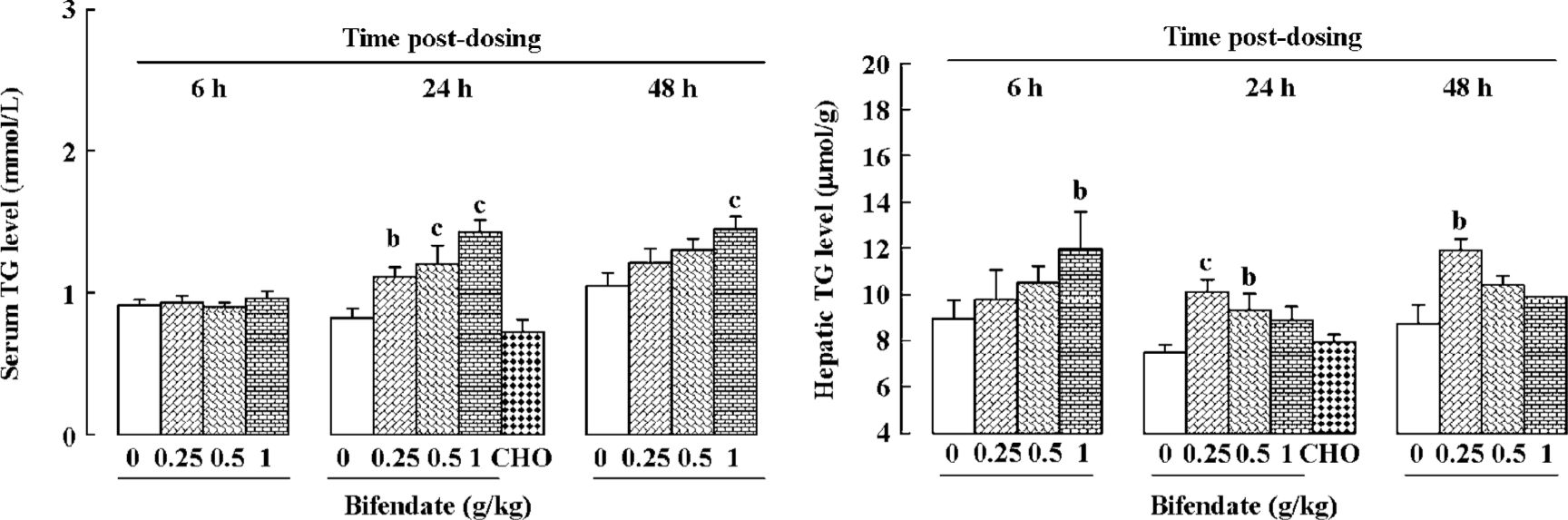
Effects of a single dose of bifendate on serum and hepatic TG levels in mice Bifendate treatment (0.25–1 g/kg) increased serum TG levels in a dose-dependent manner (39%–76% and 14%–39%, respectively) at 24 and 48 h post-dosing. Bifendate treatment (0.25–1 g/kg) also dose-dependently increased the hepatic TG level at 6 h post-dosing. However, the time-course of bifendate-induced change in hepatic TG level varied with doses, with the lower dose achieving a maximum increase in later time, and vice versa (Figure 2). CHO/bile salt treatment did not produce any detectable changes on serum and hepatic TG levels in mice. To exclude the possibility that the hypertriglyceridemic effect of the bifendate pill may be attributed to PEG (the main excipient present in the formulation), the effect of PEG treatment (10 g/kg, suspended in 0.5% CMC, ig) was examined along with mice treated with the vehicle (0.5% CMC, 20 mL/kg) and bifendate pill (1 g/kg, in 0.5% CMC). The result indicated that, while bifendate pill produced a significant increase in serum TG level at 24 h post-dosing when compared with the vehicle control, PEG treatment did not cause any detectable change (data not shown).
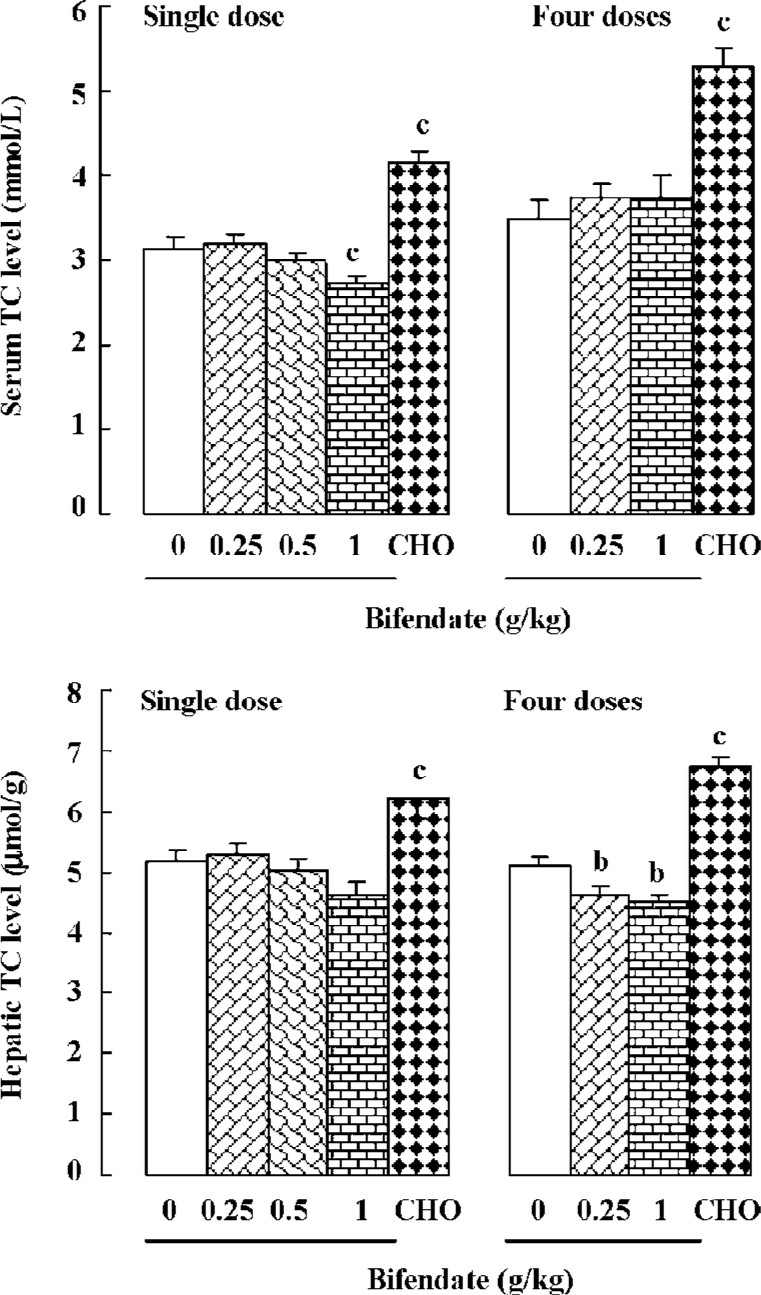
Effects of bifendate on serum and hepatic TC levels in mice Treating mice with bifendate at 1 g/kg decreased serum TC (13%) and hepatic TC (11%) levels at 24 h post-dosing. But the difference in hepatic TC was not statistically significant. When mice were treated with bifendate at daily doses of 0.25 and 1 g/kg for 4 d, hepatic TC level was slightly but significantly reduced (by 9%–10%) at 24 h after the last dosing. Single or multiple doses of CHO/bile salt caused significant increases in serum TC (14% or 48%, respectively) and hepatic TC (25% or 33%) in mice (Figure 3).
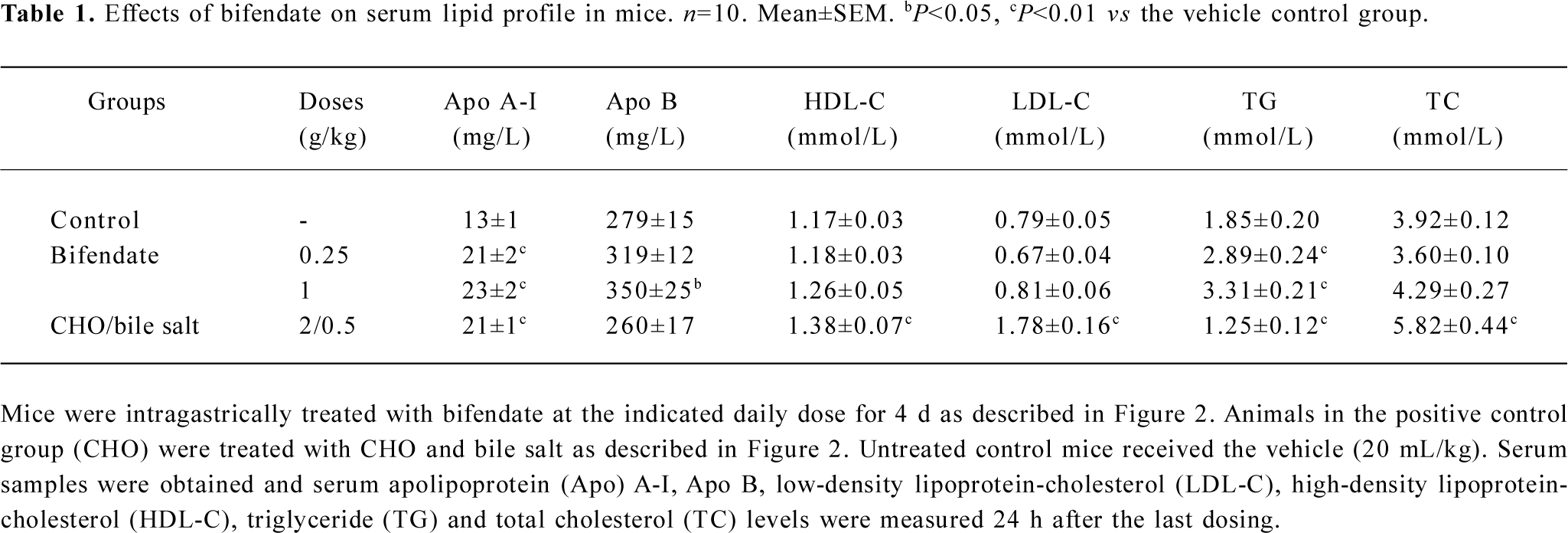
Effects of bifendate on serum lipid profile in mice Treatment with bifendate at oral daily doses of 0.25 and 1 g/kg for 4 d caused significant increases in serum levels of Apo A-I (38%–48%), Apo B (14%–25%) and TG (56%–79%) in a dose-dependent manner when compared with the vehicle control (Table 1). While bifendate treatment did not produce any detectable changes in serum TC, HDL-C and LDL-C levels. CHO/bile salt treatment (2/0.5 g/kg×4 d) significantly increased serum Apo A-I (38%), TC (48%), HDL-C (18%) and LDL-C (125%) levels. However, the serum TG level was decreased (by 32%) in CHO/bile salt-treated mice. The hepatic TG level was also significantly increased in bifendate-treated mice (data not shown).
Full table
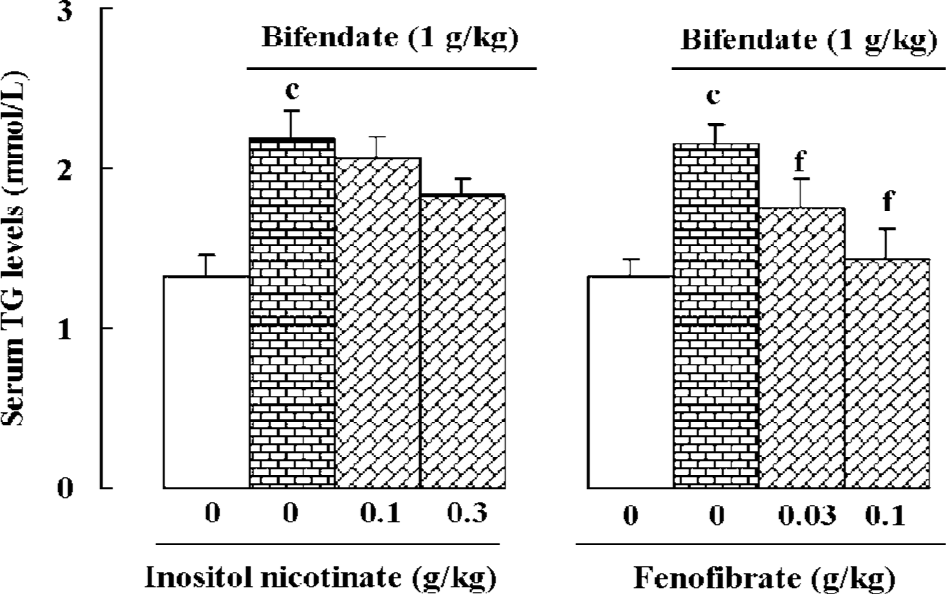
Effects of lipid-lowering agents on bifendate-induced hypertriglyceridemia Hypertriglyceridemia was induced by bifendate treatment (1 g/kg×4 d) in mice. Concurrent treatment with fenofibrate (0.03 and 0.1 g/kg×4 d) significantly reduced serum TG levels in a dose-dependent manner in bifendate-treated mice. Treatment with inositol nicotinate (0.1 and 0.3 g/kg×4 d) slightly decreased serum TG levels in bifendate-treated mice, but the differences did not show a statistical significance (Figure 4).
Discussion
Hyperlipidemia is characterized by elevated plasma/serum TC and TG levels, which are well over normal values in the population. Based on the lipoprotein profile, hyperlipidemia can be classified into various subtypes in that patients suffering from type I or IV hyperlipidemia exhibit a high plasma TG level, but little or no elevation in plasma TC level. In the present study, bifendate was found to increase serum TG but not TC levels and thus the resultant hyper-triglyceridemic state resembled that of type I or IV hyperlipidemia in humans. Experimental studies have demonstrated that the elevation of plasma TC and TG concentration may produce lesions similar to atherosclerosis in human blood vessels[13,14]. Whether or not hypertriglyceridemia induced by bifendate treatment can lead to the development of atherosclerosis remains to be determined. While high-fat and excessive carbohydrate intake caused hypertrigly-ceridemia, the inhibition of β-oxidation could lead to the production of excessive TG from the esterification of fatty acids[15,16]. Given the elevation of both serum and hepatic TG levels, it is possible that bifendate can stimulate the esterification of fatty acids and/or inhibit β-oxidation. Despite that fact that TG is just one type of lipid molecule for energy storage in the body, hypertriglyceridemia has been regarded as an independent risk factor of cardiovascular disease[17,18]. TG primarily exists in chylomicrons and very low density lipoproteins (VLDL). While the former is formed by intestinal mucosal cells during the absorption of dietary fat, the latter is manufactured in the liver in response to a high carbohydrate meal. Therefore, the amount of TG-rich lipoproteins, such as chylomicrons and VLDL, is increased in hypertriglyceridemic states[19]. Consistent with this, Apo A and Apo B, which are abundantly present in chylomicron and VLDL, were also increased by bifendate treatment.
Bifendate is available in a formulation of 1.5-mg pill for oral use at daily dosages ranging from 75 to 150 mg in adult (1.5−3 mg/kg for 50 kg body weight). However, the dosage (0.25–1 g/kg) adopted in the present animal study is about 83–667 fold higher than the human dosage. Pharmacokinetic studies indicate that orally administered bifendate (1 g/kg) reached the peak plasma level in 12 h in rats and the absorption efficiency was approximately 20%[20]. However, the formulated bifendate pill was found to be absorbed more efficiently from the gastrointestinal tract. This is consistent with the observation that the effective dosage of bifendate pill-induced hypertriglyceridemia is smaller than that of bifendate (unformulated compound) (data not shown). In the present study, the maximal degree of hypertriglyceridemia induced by bifendate pill was attained between 24 or 36 h following the intragastric administration of the drug.
Changes in plasma TG level reflect a dynamic process involving the removal of TG from the blood and synthesis/secretion of TG from the liver. If a drug enhances the hepatic synthesis and/or secretion of TG, the blood TG level will be increased. Results obtained from the present study indicate that, while bifendate treatment dose-dependently increased the serum TG level at all time intervals post-dosing, the bifendate-induced change in hepatic TG levels showed an inverse dose-response relationship at 24 h post-dosing and onwards. It is therefore conceivable that while low doses of bifendate stimulate hepatic TG synthesis, high doses might also enhance the secretion of TG from the liver into the blood.
Fibric acid derivatives are a class of lipid-modifying drugs mainly used in patients with elevated TG levels. Fenofibrate, one of the most widely used fibric acid derivatives, is a useful therapeutic option for patients with primary combined dyslipidemias or secondary dyslipidemias[21–23]. Fenofibrate, when administered at 0.3 g per day in adults, is especially good at lowering plasma TG. The drug increases lipolysis and the elimination of TG-rich particles from plasma by activating lipoprotein lipase and reducing production of apolipoprotein C-III, an inhibitor of lipoprotein lipase activity. In addition, fenofibrate was found to reduce body weight gain and adiposity in female sham-operated and ovariectomized mice[24]. Inositol nicotinate consists of six niacin molecules linked to inositol, and the niacin molecules are slowly cleaved and released from inositol in the body. Niacin was first reported to be a hypolipidemic agent in 1955, and it is now most frequently prescribed for patients suffering from dyslipidemia in an attempt to raise low HDL-C and to lower VLDL-C and LDL-C levels [25,26]. In addition, the blood vessel-widening effect of niacin may improve the circulation to the extremities, which is useful for the treatment of peripheral artery diseases[27,28]. Our finding that fenofibrate is more effective than inositol nicotinate in lowering serum TG levels in bifendate-treated mice is corroborated by a relatively higher clinical efficacy of fenofibrate than inositol nicotinate in lowering plasma TG levels.
In conclusion, our results help substantiate the suggestion for the development of animal models of acute hyper-triglyceridemia by oral administration of a single and high dose of bifendate in rabbits and mice. This will provide a convenient in vivo screen for novel TG-lowering agents.
References
- Chen ZM, Peto R, Collins R, MacMahon S, Lu J, Li WC. Continuous positive relationship between serum cholesterol and coronary heart disease in a population with low mean cholesterol. Br Med J 1991;303:276-82.
- Wakugami K, Iseki K, Kimura Y, Okumura K, Ikemiya Y, Muratani H, et al. Relationship between serum cholesterol and the risk of acute myocardial infarction in a screened cohort in Okinawa, Japan. Jpn Circ J 1998;62:7-14.
- Khoo KL, Tan H, Liew YM, Deslypere JP, Janus E. Lipids and coronary heart disease in Asia. Atherosclerosis 2003;169:1-10.
- Breslow JL. Mouse models of atherosclerosis. Science 1996;272:685-8.
- Breslow JL. Transgenic mouse models of lipoprotein metabolism and atherosclerosis. Proc Natl Acad Sci USA 1993;90:8314-8.
- Delawi D, Meijssen S, Castro CM. Intra-individual variations of fasting plasma lipids, apolipoproteins and postprandial lipemia in familial combined hyperlipidemia compared to controls. Clin Chim Acta 2003;328:139-45.
- Ueno T, Tremblay J, Kunes J, Zicha J, Dobesova Z, Pausova Z, et al. Rat model of familial combined hyperlipidemia as a result of comparative mapping. Physiol Genomics 2004;17:38-47.
- Vrana A, Kazdova L. The hereditary hypertriglyceridemic nonobese rat: an experimental model of human hypertriglyceridemia. Transplant Proc 1990;22:2579.
- Pan SY, Han YF, Carlier PR, Pang YP, Mak DHF, Lam BYH, et al. Schisandrin B protects against tacrine- and bis(7)-tacrine-induced hepatotoxicity and enhances cognitive function in mice. Planta Med 2002;68:217-20.
- Pan SY. New use of schisandrin. Chin Bull Inve Patent 2000; 16: 11. Chinese.
- Liu GT. From the study of Fructus Schizandrae to the discovery of biphenyl dimethyl-dicarboxylate. Acta Pharm Sin 1983;18:714-20. Chinese..
- Liu GT, Wei HL, Song ZY. Further studies on the protective action of biphenyl dimethyl-dicarboxylate (BDD) against experimental liver injury in mice. Acta Pharm Sin 1982;17:101-5. Chinese..
- Rekhter MD. How to evaluate plaque vulnerability in animal models of atherosclerosis? Cardiovas Res 2002;54:36-41.
- Joniken MP, Clarkson JB, Pritchard RW. Recent advances in molecular pathology: animal models in atherosclerosis. Exp Mol Pathol 1985;42:1-28.
- Klein CJ, Stanek GS, Wiles CE. Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc 1998;98:795-806.
- Eaton S, Record CO, Bartlett K. Multiple biochemical effects in the pathogenesis of alcoholic fatty liver. Eur J Clin Invest 1997;27:719-22.
- Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996;3:213-9.
- Fromenty B, Pessayre D. Impaired mitochondrial function in microvesicular steatosis. Effects of drugs, ethanol, hormones and cytokines. J Hepatol 1997;26 Suppl 2:43-53.
- Guerin M, Le Goff W, Lassel TS, Van Tol A, Steiner G, Chapman MJ. Proatherogenic role of elevated CE transfer from HDL to VLDL1 and dense LDL in type 2 diabetes: impact of the degree of triglyceridemia. Arterioscler Thromb Vasc Biol 2001;21:282-8.
- Wang XL, Yi MG, Liu ZM, Sung CY. The physiological dispositions of biphenyl dimethyl-dicarboxylate (BDD). Acta Pharm Sin 1983;18:892-9. Chinese..
- Ikewaki K, Tohyama J, Nakata Y, Wakikawa T, Kido T, Mochizuki S. Fenofibrate effectively reduces remnants, and small dense LDL, and increases HDL particle number in hyper-triglyceridemic men−a nuclear magnetic resonance study. J Atheroscler Thromb 2004;11:278-85.
- Saklamaz A, Comlekci A, Temiz A, Caliskan S, Ceylan C, Alacacioglu A, et al. The beneficial effects of lipid-lowering drugs beyond lipid-lowering effects: a comparative study with pravastatin, atorvastatin, and fenofibrate in patients with type IIa and type IIb hyperlipidemia. Metabolism 2005;54:677-81.
- Tsimihodimos V, Miltiadous G, Daskalopoulou SS, Mikhailidis DP, Elisaf MS. Fenofibrate: metabolic and pleiotropic effects. Cur Vasc Pharmacol 2005;3:87-98.
- Jeong S, Han M, Lee H, Kim M, Kim J, Nicol CJ, et al. Effects of fenofibrate on high-fat diet-induced body weight gain and adiposity in female C57BL/6J mice. Metabolism 2004;53:1284-9.
- Wink J, Giacoppe G, King J. Effect of very-low-dose niacin on high-density lipoprotein in patients undergoing long-term statin therapy. Am Heart J 2002;143:514-8.
- Grundy SM, Vega GL, McGovern ME, Tulloch BR, Kendall DM, Fitz-Patrick D, et al. Efficacy, safety, and tolerability of once-daily niacin for the treatment of dyslipidemia associated with type 2 diabetes: results of the assessment of diabetes control and evaluation of the efficacy of niaspan trial. Arch Intern Med 2002;162:1568-76.
- Sunderland GT, Belch JJ, Sturrock RD, Forbes CD, McKay AJ. A double blind randomised placebo controlled trial of hexopal in primary Raynaud’s disease. Clin Rheumatol 1988;7:6-49.
- Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, et al. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT Study: a randomized trial. Arterial Disease Multiple Intervention Trial. JAMA 2000;284:1263-70.
