Effects of specific interleukin-1β-converting enzyme inhibitor on ischemic acute renal failure in murine models1
Introduction
Acute renal failure (ARF) is a clinical syndrome with high morbidity and mortality. The incidence of ischemic ARF has been rising rapidly, especially with the rapid development of various difficult surgical operations and kidney transplantation[1,2]. Unfortunately, up to now, there was no really effective medicine available to treat this disease.
Several recent studies have reported that, aside from tubular injury directly caused by renal abnormal hemodynamic states, the recruitment and activation of numerous inflammatory cells after ischemia, and immune inflammatory responses mediated by the expression and secretion of inflammatory cytokines are the main causes of ischemic acute renal injury[3–5]. Among the various cytokines produced during renal ischemia, interleukin-18 (IL-18), interleukin-1β (IL-1β) and interferon-gamma (IFN-γ), a group of proinflam-matory cytokines with closely related function, play an important role in the inflammatory reaction and renal tubuloin-terstitial impairment[6–9]. Other studies have reported that applying antibodies or soluble receptors to block the roles of these cytokines can alleviate ischemic ARF[10,11]. It has been demonstrated that both IL-1β and IL-18 are members of the IL-1 family, which can be activated by interleukin-1β-converting enzyme (ICE) via cleaved precursor peptide, and furthermore, that IL-18 is the strongest inducing factor of IFN-γ[12,13]. We therefore postulated that it would be more effective to protect the kidney from acute ischemic injury by regulating the whole cytokine network, including IL-1β, IL-18, and IFN-γ, with selective ICE inhibitors, instead of only blocking any one of the cytokines using antibodies or soluble receptors. More significantly, several kinds of ICE inhibitors with simple structure and low antigenicity have been synthesized by chemical methods. The inhibitors are superior to cytokine antibodies and soluble receptors, which are difficult to produce, expensive, hypersensitive and require injection.
The present study aimed at exploring the role of selective ICE inhibitors in preventing the kidney from ischemic ARF by the combined inhibition of IL-1β, IL-18, and IFN-γ
Materials and methods
Materials All reagents were obtained from Sigma (St Louis, MO, USA), unless otherwise indicated.
Ischemic ARF model induction Male Kunming mice (SPF grade, Experimental Animal Center of Guangdong Medical College, Zhanjiang, China) weighing 22.1±1.3 g (20 g–25 g) were used. After being anesthetized with an injection of 0.3% sodium pentobarbital (5 mL/kg–10 mL/kg, ip), an abdominal mid-line incision was made and the renal pedicles were clamped bilaterally for 45 min with non-traumatic microaneurysm clamps. Restoration of blood flow was confirmed when the kidneys returned to their original color after the clamps were removed. Afterward, the abdomen was closed and the mice were allowed to recover. The mice were observed for 2 h after the operation was finished. A model was considered to be developed successfully if the animal’s behavior returned to normal. Those that failed to return to normal behavior after operation or those in which any 1 of the kidneys failed to return to normal color after the clamps were removed were regarded as unsuccessful models and were removed from the experiment. The sham-operated group consisted of the same surgical procedure except that clamps were not applied.
Experimental groups Animals were kept in a clean environment at 24 °C–29 °C with free access to standard food and water after model induction. The study was composed of 3 parts as follows. Experiment I: Thirty-nine mice were distributed randomly into the sham-operated group, the model control group or the therapy group. After post-operative elimination, these groups contained 13, 13, and 11 mice, respectively. The therapy group was administered AC-YVAD-CMK (6.25 mg/kg, ip; Calbiochem, Darmstadt, Germany), which was dissolved in 2% Me2SO saline, at 2 h, 8 h, and 16 h after surgery. The other 2 groups received the vehicle; an equal volume of 2% Me2SO saline at the same times as the therapy group. All of the mice were killed at 24 h after surgery. Experiment II: Forty-five mice were enrolled in this part of the experiment and were also distributed randomly into 3 groups as in Experiment I. Each group contained 13 mice after model construction and elimination. The mice underwent similar procedures as those in Experiment I, except that either AC-YVAD-CMK or the vehicle were administered to the mice at 2 h, 8 h, 14 h, 20 h, 28 h, and 36 h, and the mice were killed at 48 h after surgery. Experiment III: Thirty-nine mice were distributed randomly into the 3 groups as in Experiment I and Experiment II, and each group contained 12 mice. AC-YVAD-CMK or the vehicle was administered in the same way as in Experiment II, but the animals were not killed. Their clinical features were observed and recorded twice per day for 14 d after surgery.
Sample collection and pretreatment Blood samples were obtained via orbital cavity after removing eyeballs. The blood samples were then centrifuged to separate the serum. The kidneys were collected, each of which was divided into 4 equal sections. One section was fixed in 10% neutral formalin solution while the others were frozen immediately in liquid nitrogen and preserved at -72 °C until use.
Blood biochemical parameters assay The levels of blood urea nitrogen (BUN) and serum creatinine (Scr) were measured using an automatic biochemical analyzer (Beckman Instruments, Fullerton, CA, USA).
Renal histological examination The 4% paraformaldehyde-fixed and paraffin-embedded kidney samples were sectioned at 3 µm and stained with hematoxylin-eosin (HE) and periodic acid-Schiff (PAS) using standard methods. Histological examinations were carried out in a single blinded fashion. Histological changes due to acute renal tubulo-interstitial injury were quantitated using the method of Paller et al[14]. Five fields (×200) were reviewed for each slide. The scores included: diffuse tubular epithelial cell flattening and tubular lumen dilatation (1 point), lesion of brush border (1 point), loss of brush border (2 points), cell membrane bleb formation (1 point), cytoplasmic vacuolization (1 point), interstitial edema (1 point), necrotic cells in tubular lumen but no cast formation (1 point), and casts or cell pieces formation (2 points). Higher scores represented more severe damage.
Renal ICE activity assay Renal ICE activity was measured using a ICE fluorescent assay kit (Calbiochem), according to the manufacture’s protocols. Briefly, 1 mL of tissue lytic buffer (containing 1×phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate, 10 µg/mL phenylmethylsulfonylfluoride and 10 µL/mL Aprotinin) was added to approximately 100 mg renal tissue. The renal tissues were then homogenized artificially and incubated on ice for 1 h. The tissue lysate was then centrifuged at 4 °C at 12 000×g for 5 min. The supernatant was collected in a separate microfuge tube and stored immediately at -72 °C until use. The protein concentration of each tissue lysate was measured by spectrophotometry after being dyed with Coomassie brilliant blue G250. Supernatants were adjusted to a final protein concentration of 150µg/µL–200 µg/µL. The enzymatic activity was measured as follows: 50 µL of 2×ICE assay buffer, 5 µL of substrate (1 mmol/L YVAD-AFC) and 50 µL protein solution were added to a 96-well polyvinyl plate. The reaction system, kept in darkness, was incubated at 37 °C for 90 min. The absorbance of each well was then read by a DA620 fluorescence microplate reader (Bio-Tek, Winooski, Vermont, USA) at an excitation wavelength of 380 nm and an emission wavelength of 460 nm. Tissue lytic buffer was replaced with tissue lysate as a negative control. A ICE standard curve was determined for the experiment at the same time. ICE activity was expressed as units per mg protein.
Assay for expression of mature renal IL-18 protein The level of mature IL-18 protein expressed in renal tissue was measured using a mouse IL-18 enzyme-linked immunosorbent assay kit (MBL, Nagoga, Japan), which was only specific for mature IL-18. Renal tissue total protein was extracted as described above. The assay procedures strictly followed the manufacturer’s instructions. Every sample was measured by double parallel wells, and was remeasured when the intra-error was over 10%.
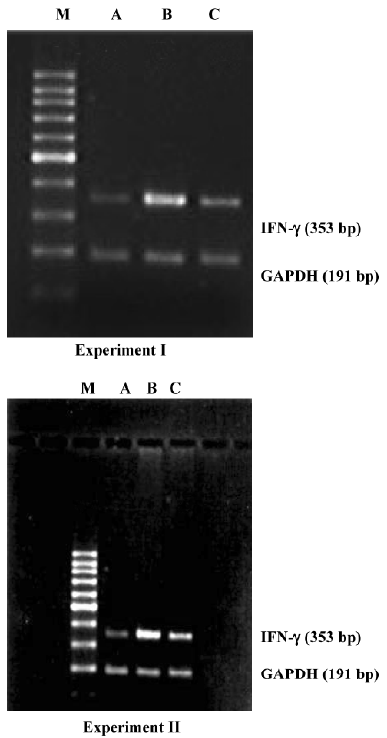
Assay for renal IFN-γ mRNA expression Approximately 100 mg of frozen kidney sample was homogenized in a muller by adding liquid nitrogen to crisp the tissue. Total RNA was extracted from kidney tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized with the SuperScriptionTM First-Strand Synthesis System (Invitrogen) according to the manufacturer’s protocol. The primers used for the IFN-γ polymerase chain reaction (PCR) were as follows: 5'-AGG AAC TGG CAA AAG GAT GGTG-3' (sense), and 5'-GTG CTG GCA GAA TTA TTC TTA TTG-3' (anti-sense), and the product was 353 bp. The primers used for the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) PCR were as follows: 5'-AAC GAC CCC TTC ATT GAC-3' (sense), and 5'-TCC ACG ACA TAC TCA GCAC-3' (anti-sense), and the product was 191 bp. Primers were synthesized and purified by Shanghai Biological Engineering (Shanghai, China). The PCR-amplified system included 1.2 µL of 25 mmol/L MgCl2, 0.4 µL of 10 mmol/L dNTP mix, 0.8 µL of 5 µmol/L sense and anti-sense primers, 2 U of Taq DNA polymerase, 1 µL of cDNA and sterile purified water to 20 µL. The IFN-γ PCR was carried out in a thermal cycler (Eppendorf, New York city, New York, USA) at 95 °C for 60 s, 58 °C for 60 s, and 72 °C for 60 s. The GAPDH PCR was carried out at 94 °C for 60 s, 58 °C for 60 s, and 72 °C for 60 s. IFN-γ was amplified for 35 cycles and GAPDH for 28 cycles. Equal volumes of each PCR product were loaded into gels and electrophoresis was carried out. The ethidium bromide-stained gels were analyzed semiquantitatively using a gel imaging system (UVP, Cambridge, UK). The quantity of IFN-γ expression in every renal tissue was the integral absorbency of the IFN-γ amplification band divided into GAPDH amplification band.
Statistical analysis SPSS version 11.0 software was used to obtain data statistics. Measurement data were expressed as mean±SD. One-way ANOVA was used for statistical analysis among multiple groups. Kaplan-Meier was used for statistical analysis of survival rates among multiple groups.
Results
Effect of AC-YVAD-CMK on renal function in ischemic ARF mice The levels of Scr and BUN in model controls were increased significantly compared with sham-operated groups both in experiment I and experiment II. The levels of Scr decreased significantly but that of BUN did not decrease significantly in the AC-YVAD-CMK therapy group compared with the vehicle-treated group in experiment I; the levels of both Scr and BUN decreased significantly in the therapy group in experiment II (Table 1).
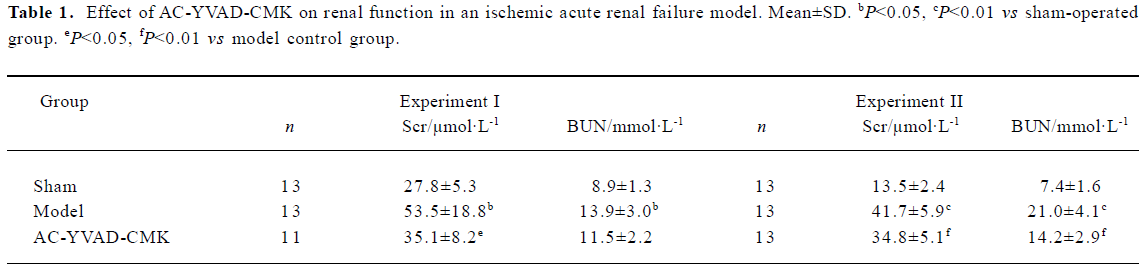
Full table
Effect of AC-YVAD-CMK on renal tubulointerstitial injury in ischemic ARF mice Kidneys from sham-operated groups were normal in form and rubicund color while those from model control groups were swollen with pale cortex and congested medulla. These anatomical changes were also observed but were significantly less in kidneys from AC-YVAD-CMK therapy groups. Renal tissues from sham-operated groups had a basically normal microcosmic structure except for focal vacuolation of tubular epithelial cells (TECs). Renal tissues from model control groups showed severe damage in microcosmic structure, including extensive TECs vacuolation, scattered TECs brush border flatting, shrinkage and loss as well as focal TECs nucleus nakedness and necrosis. Dilated lumens and cast formation were also found in parts of the tubules. Interstitial inflammatory cell infiltration was also found, especially with neutrophil cells. Renal tissue damage was relieved significantly in AC-YVAD-CMK therapy groups. The main histological changes included extensive TECs vacuolation, focal TECs nucleus nakedness and very rare TECs necrosis. The basement membranes were basically integrated; casts were seldom. Inflammatory infiltration in interstitial areas was slight. The mean histological score for the kidney tissue of model control groups was significantly higher than that of the sham-operated groups, both in experiment I and experiment II. The mean score for the AC-YVAD-CMK therapy groups was much lower than that for the vehicle-treated groups, again in both experiment I and experiment II (Figure 1, Table 2).
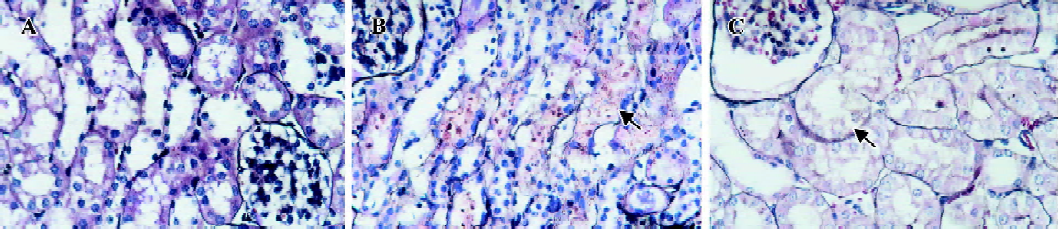
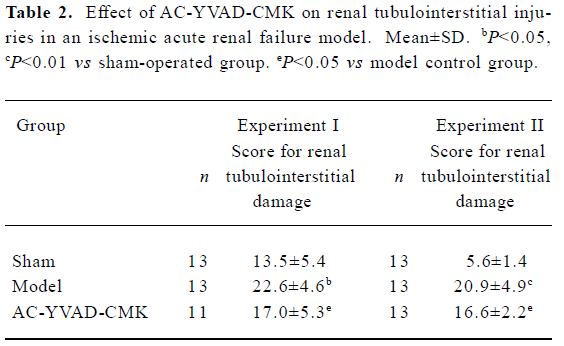
Full table
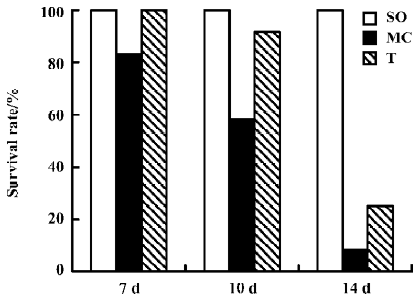
Effect of AC-YVAD-CMK on survival rate in ischemic ARF mice In Experiment III, Sham-operated groups had no apparent abnormal appearance, activity or ingestion during the whole observation period, and all of them had survived at the end of observation. Model controls were cachexia in different extents, with reduced activity and ingestion. Anasarca and dyspnea occurred gradually. The survival rate was 83.3% on d 7, 58.3% on d 10, and only 8.3% on d 14. However, the clinical features were relatively improved in the AC-YVAD-CMK therapy group during the period of observation compared with model controls. The survival rate was 100.0% on d 7, 91.6% on d 10, and 25.0% on d 14. All together, the 2-week accumulated survival rate in the AC-YVAD-CMK therapy group was higher than that of the vehicle-treated group by survival analysis for time (P<0.01, Figure 2).
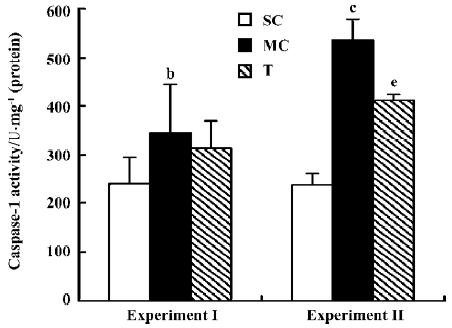
Effect of AC-YVAD-CMK on renal ICE activity in ischemia ARF mice ICE activity in renal tissue from model controls was 347.0±97.5 U/mg protein in experiment I and 536.1±43.0 U/mg protein in experiment II, both much higher than that for the sham-operated groups, which was 239.5±56.5 U/mg protein in experiment I and 237.2±27.4 U/mg protein in experiment II. The ICE activity for the therapy group was 314.0±56.0 U/mg protein in experiment I and 412.2±12.5 U/mg protein in experiment II. ICE activity was decreased significantly compared with the model control group in experiment II, but not in experiment I (Figure 3).
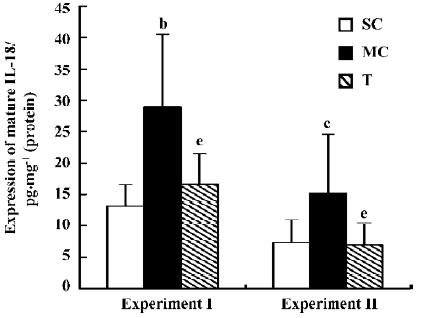
Effect of AC-YVAD-CMK on expression of renal mature IL-18 protein in ischemia ARF mice Interleukin-18 expression in renal tissue from model controls was 28.9±11.6 pg/mg protein in experiment I and 15.2±9.4 pg/mg protein in experiment II, both much higher than for the sham-operated groups, which was 13.1±3.5 pg/mg protein in experiment I and 7.3±3.5 pg/mg protein in experiment II. Mature IL-18 protein expression was 16.7±4.8 pg/mg protein in experiment I and 6.9±3.5 pg/mg protein in experiment II in AC-YVAD-CMK therapy groups; both were decreased significantly compared with model control groups (Figure 4).
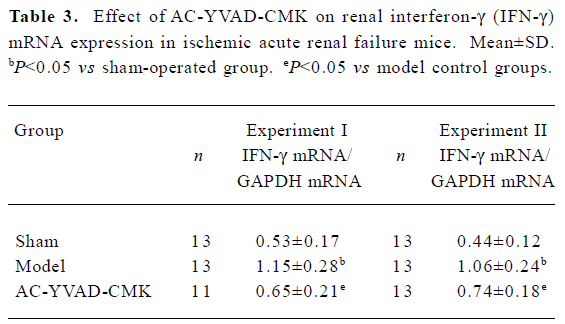
Effect of AC-YVAD-CMK on renal IFN-γ mRNA expression in ischemia ARF mice Model controls had a significantly higher expression of IFN-γ mRNA in renal tissues than sham-operated groups. The expression level in the AC-YVAD-CMK therapy group was decreased significantly compared with the vehicle-treated group (Table 3, Figure 5).
Full table
Discussion
The present study developed a successful ischemic ARF model according to the dynamic changes of the kidneys color during operation and the changes in renal function and morphological structure after operation. However, we also found that renal function parameters, especially the level of Scr, and renal histological scores in experiment I were higher than those in experiment II in the sham-operated group. This phenomenon may be caused by muscle lesion during peritoneotomy and incomple recovery from stress after anesthesia and surgical operation. The difference might also result from the variation between experiments at different times.
Based on a successful mouse model of ischemic ARF, we first studied the renal protective effects of AC-YVAD-CMK dynamically using a renal function assay and renal morphological study at different times. The results showed that renal function and morphological impairment were significantly improved in the ischemic ARF mouse model after being treated with specific ICE inhibitor. The findings demonstrate that a specific ICE inhibitor can exert remarkable renal-protective effects against acute ischemic lesion.
However, the ultimate objective of ischemic ARF therapy is to increase patient survival from the disease. In the past decade, different kinds of medicines, such as prostaglandins, “renal-dose” dopamine and atrial natriuretic factor, have been tried to treat patients with ischemic ARF and these animal models. Although some of these agents have improved renal function, urine output and even renal histological impairment in patients and animal models, few studies have demonstrated that these medicines can reduced the mortality of the disease[15–18]. In the present study, we found that applying this specific ICE inhibitor in the initial term of the disease remarkably increased the survival rate in this animal model. If it were applied continually for a longer period of time, the model’s survival rate might be further improved. Thus, we speculate that this medicine might be superior in some way to traditional medicines with regard to protection against ischemic ARF.
Caspases are a group of protein-cleaving enzymes, and play an important role in cell apoptosis and inflammatory responses. ICE, also known as interleukin-1β-converting enzyme, is a member of the inflammatory group in the caspase family. It is a key initiative factor and an important inducer of inflammatory chain responses secondary to an organ’s ischemic impairment[19]. Kaushal et al[20] reported that expression of the ICE gene and protein were up-regulated in an ischemic ARF rat model. In the present study, ICE activity was also increased in renal tissue of an ischemic ARF mouse model. All of the evidence above strongly revealed that gene transcription, protein synthesis and enzyme activation of ICE were up-regulated during the progression of ischemic ARF. Melnikov et al[11] reported that renal tubular impairment and renal interstitial inflammatory infiltration were much slighter in ICE gene knock-out mice than in wild-type mice after renal ischemia reperfusion. The present study also found that ICE inhibitor alleviated the impairment of renal tissue and down-regulated the activity of renal ICE. We conclude that excessive ICE activity prompts the development of ischemic ARF, and selectively inhibiting sham-operated groups ICE activity can exert renal-protective effects on the disease. ICE activity was not suppressed to normal levels by the dose of inhibitor used in the present study. We presume that a better protective effect would be achieved if a larger dose were administered.
Precursors of IL-18 and IL-1β are the main substrates of ICE[12,13], and many reports have proved these 2 cytokines can prompt acute ischemic organ impairment[21–23]. Recent reports seem to emphasize the role of IL-18. Mice with ischemic ARF have increased renal mature IL-18 expression, and IL-18 antiserum can significantly improve the state of the illness[11,22]. It was reported recently by Parikh et al[7] that urinary IL-18 level was increased almost 50-fold in patients with ARF, and the urinary IL-18 level in patients transplanted with corpse kidney was 10-fold that in patients transplanted with living kidney. These studies prove that IL-18 plays an important role in prompting renal impairment during acute ischemia. Does ICE mediate the acute renal impairment through prompting activation of IL-18 and IL-1β? In the present study we detected the expression of mature IL-18 in renal tissue. The results showed that expression of activated IL-18 increased remarkably in model controls and decreased significantly after the models were treated with ICE inhibitor, which demonstrates that the excessive activation of IL-18 resulting from ICE hyperactivity is one of the main mechanisms causing renal tissue impairment. We infer that IL-1β was also over-activated by ICE in this model. Specific ICE inhibitor indirectly inhibits activation of IL-18 and IL-1β through inhibiting the activity of ICE, and thereby protects against acute ischemic ARF.
Because IL-18 is the strongest IFN-γ inducer[13], we then detected the expression of the IFN-γ gene in renal tissue. We found that renal IFN-γ mRNA expression was up-regulated significantly in model controls, and decreased after the mice were treated with specific ICE inhibitor. The results again demonstrate that IFN-γ plays an important role in ischemic ARF, and ICE inhibitor suppresses the expression of IFN-γ mRNA through inhibiting activation of IL-18, thus further protecting against ischemic ARF.
In summary, the present study demonstrates that specific ICE inhibitors are promising agents in the treatment of ischemic ARF. It exerts renal-protective effects by inhibiting strong ICE activity, thereby regulating the activation of IL-18 and IL-1β, and further regulating abnormal expression of IFN-γ.
References
- Carmichael P, Carmichael AR. Acute renal failure in the surgical setting. ANZ J Surg 2003;73:144-53.
- Wiecek A, Nowicki M, Kokot F, Ritz E. Acute failure of the transplanted kidney – pathophysiology, diagnosis and prevention. Ann Transplant 1996;1:5-9.
- Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, et al. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int 2004;66:491-6.
- Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int 2004;66:480-5.
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int 2004;66:486-91.
- Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H, et al. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol 2003;285:F87-94.
- Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis 2004;43:405-14.
- Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, et al. Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J Clin Invest 2001;108:1283-90.
- Daemen MA, van’t Veer C, Wolfs TG, Buurman WA. Ischemia/reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and IL-18. J Immunol 1999;162:5506-10.
- Kitada H, Suitani A, Yamamoto H, Otomo N, Okabe Y, Inoue S, et al. Attenuation of renal ischemia-reperfusion injury by FR167653 in dogs. Surgery 2002;131:654-9.
- Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL, et al. Neutrophil-independent mechanisms of caspase-1 and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest 2002;110:1083-91.
- Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann NY Acad Sci 1998;856:1-11.
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995;378:88-91.
- Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest 1984;74:1156-64.
- Denton MD, Chertow GM, Brady HR. ‘Renal-dose’ dopamine for the treatment of acute renal failure: scientific rationale, experimental studies and clinical trials. Kidney Int 1996;50:4-14.
- Alkhunaizi AM, Schrier RW. Management of acute renal failure: new perspectives. Am J Kidney Dis 1996;28:315-28.
- Mitaka C, Hirata Y, Habuka K, Narumi Y, Yokoyama K, Makita K, et al. Atrial natriuretic peptide infusion improves ischemic renal failure after suprarenal abdominal aortic cross-clamping in dogs. Crit Care Med 2003;31:2205-10.
- Chatterjee PK, Patel NS, Cuzzocrea S, Brown PA, Stewart KN, Mota-Filipe H, et al. The cyclopentenone prostaglandin 15-deoxy-Delta(12,14)-prostaglandin J2 ameliorates ischemic acute renal failure. Cardiovasc Res 2004;61:630-43.
- Tilney NL, Guttmann RD. Effects of initial ischemia/reperfusion injury on the transplant kidney. J Transplant 1997;64:945-7.
- Kaushal GP, Singh AB, Shah SV. Identification of gene family of caspases in rat kidney and altered expression in ischemia–reperfusion injury. Am J Physiol 1998;274:587-95.
- Haq M, Norman J, Saba SR, Ramirez G, Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J Am Soc Nephrol 1998;9:614-9.
- Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, et al. Impaired IL-18 processing protects caspase-1 deficient mice from ischemic acute renal failure. J Clin Invest 2001;107:1145-52.
- Hedtjarn M, Leverin AL, Eriksson K, Blomgren K, Mallard C, Hagberg H, et al. Interleukin-18 involvement in hypoxic-ischemic brain injury. J Neurosci 2002;22:5910-9.
